Histopathological Evaluation of Gastric Mucosal Atrophy for Predicting Gastric Cancer Risk: Problems and Solutions
Abstract
1. Introduction
2. Who Should Be Examined? Or in Other Words—Who Is at Risk for Chronic Atrophic Gastritis
3. The Etiological Factor of Gastritis and the Atrophy Risk
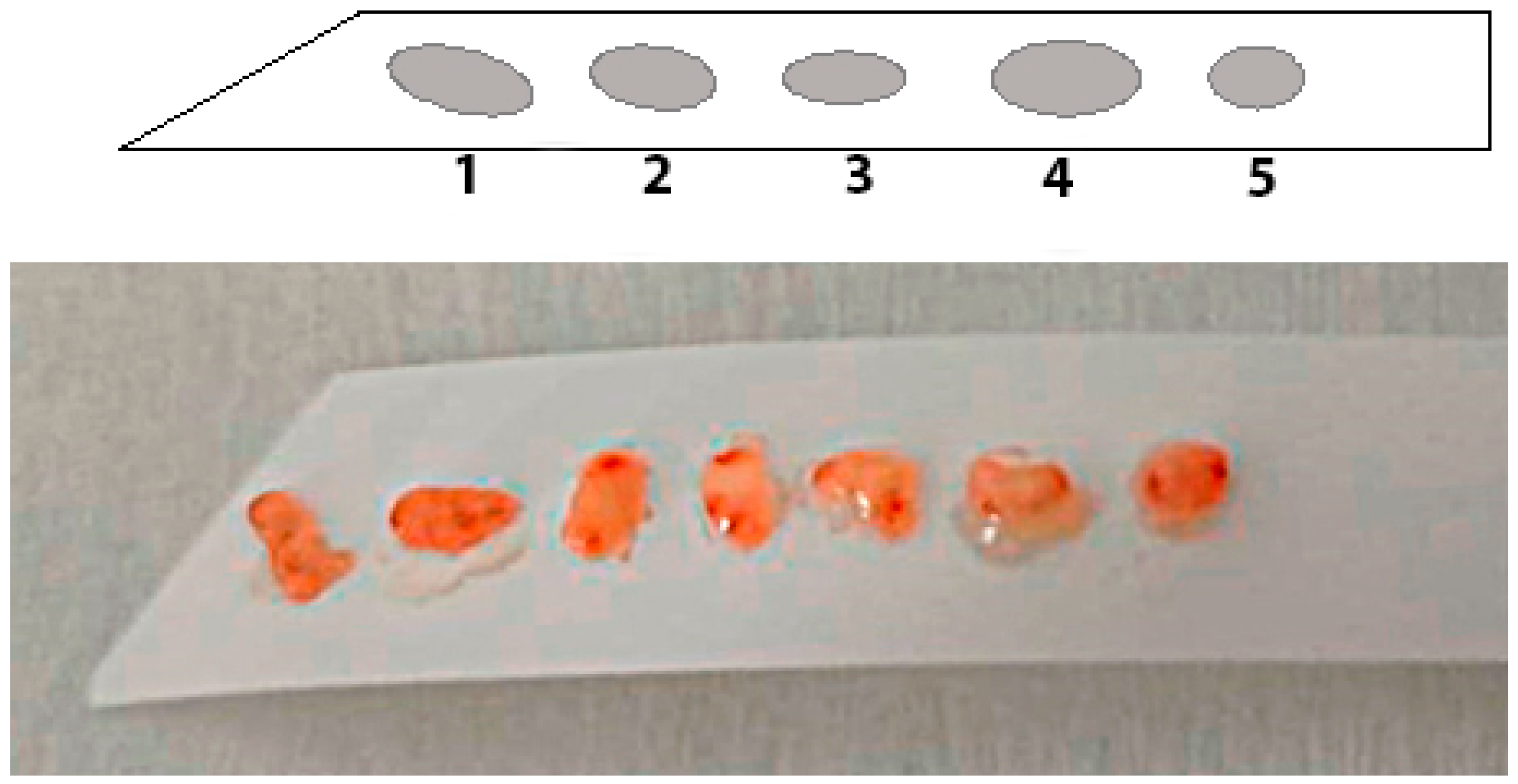
4. Possibilities of Endoscopic Examination and Sampling of Biopsy Specimens for the Diagnosis of Gastric Mucosa Atrophy
5. Histological Examination—“Pitfalls” of a Standard Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Correa, P. Human gastric carcinogenesis: A multistep and multifactorial process—First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992, 52, 6735–6740. [Google Scholar] [PubMed]
- Banks, M.; Graham, D.; Jansen, M.; Gotoda, T.; Coda, S.; di Pietro, M.; Uedo, N.; Bhandari, P.; Pritchard, D.M.; Kuipers, E.J.; et al. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut 2019, 68, 1545–1575. [Google Scholar] [CrossRef] [PubMed]
- Luu, M.N.; Quach, D.T.; Hiyama, T. Screening and surveillance for gastric cancer: Does family history play an important role in shaping our strategy? Asia Pac. J. Clin. Oncol. 2022, 18, 353–362. [Google Scholar] [CrossRef]
- Shin, C.M.; Kim, N.; Yang, H.J.; Cho, S.I.; Lee, H.S.; Kim, J.S.; Jung, H.C.; Song, I.S. Stomach cancer risk in gastric cancer relatives: Interaction between Helicobacter pylori infection and family history of gastric cancer for the risk of stomach cancer. J Clin. Gastroenterol. 2010, 44, e34–e39. [Google Scholar] [CrossRef] [PubMed]
- Lissowska, J.; Groves, F.D.; Sobin, L.H.; Fraumeni, J.F., Jr.; Nasierowska-Guttmejer, A.; Radziszewski, J.; Regula, J.; Hsing, A.W.; Zatonski, W.; Blot, W.J.; et al. Family history and risk of stomach cancer in Warsaw, Poland. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. ECP 1999, 8, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Ogino, K.; Yoshida, I.; Matsuda, H.; Yoshida, M.; Nakamura, H.; Dan, S.; Ishimaru, M. Family history-related risk of gastric cancer in Japan: A hospital-based case-control study. Jpn. J. Cancer Res. 1996, 87, 1025–1028. [Google Scholar] [CrossRef] [PubMed]
- Baek, D.H.; Kim, G.H.; Park, D.Y.; Lee, B.E.; Jeon, H.K.; Lim, W.; Song, G.A. Gastric epithelial dysplasia: Characteristics and long-term follow-up results after endoscopic resection according to morphological categorization. BMC Gastroenterol. 2015, 15, 17. [Google Scholar] [CrossRef]
- Lichtenstein, P.; Holm, N.V.; Verkasalo, P.K.; Iliadou, A.; Kaprio, J.; Koskenvuo, M.; Pukkala, E.; Skytthe, A.; Hemminki, K. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N. Engl. J. Med. 2000, 343, 78–85. [Google Scholar] [CrossRef]
- de Vries, A.C.; van Grieken, N.C.; Looman, C.W.; Casparie, M.K.; de Vries, E.; Meijer, G.A.; Kuipers, E.J. Gastric cancer risk in patients with premalignant gastric lesions: A nationwide cohort study in the Netherlands. Gastroenterology 2008, 134, 945–952. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, M.; Partida-Rodríguez, O.; Camorlinga-Ponce, M.; Flores-Luna, L.; Lazcano, E.; Gómez, A.; Herrera-Goepfert, R.; Medrano-Guzmán, R.; Torres, J. Polymorphisms in HLA-DQ genes, together with age, sex, and Helicobacter pylori infection, as potential biomarkers for the early diagnosis of gastric cancer. Helicobacter 2017, 22, 12326. [Google Scholar] [CrossRef]
- Lee, T.Y.; Wang, R.C.; Lee, Y.C.; Lin, J.T.; Ho, H.J.; Hsieh, M.C.; Wu, C.Y. The Incidence of Gastric Adenocarcinoma Among Patients With Gastric Intestinal Metaplasia: A Long-term Cohort Study. J. Clin. Gastroenterol. 2016, 50, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Leung, W.K.; Lin, S.R.; Ching, J.Y.; To, K.F.; Ng, E.K.; Chan, F.K.; Lau, J.Y.; Sung, J.J. Factors predicting progression of gastric intestinal metaplasia: Results of a randomised trial on Helicobacter pylori eradication. Gut 2004, 53, 1244–1249. [Google Scholar] [CrossRef]
- You, W.C.; Li, J.Y.; Blot, W.J.; Chang, Y.S.; Jin, M.L.; Gail, M.H.; Zhang, L.; Liu, W.D.; Ma, J.L.; Hu, Y.R.; et al. Evolution of precancerous lesions in a rural Chinese population at high risk of gastric cancer. Int. J. Cancer 1999, 83, 615–619. [Google Scholar] [CrossRef]
- Watari, J.; Chen, N.; Amenta, P.S.; Fukui, H.; Oshima, T.; Tomita, T.; Miwa, H.; Lim, K.J.; Das, K.M. Helicobacter pylori associated chronic gastritis, clinical syndromes, precancerous lesions, and pathogenesis of gastric cancer development. World J. Gastroenterol. 2014, 20, 5461–5473. [Google Scholar] [CrossRef] [PubMed]
- Livzan, M.A.; Gaus, O.V.; Mozgovoi, S.I.; Bordin, D.S. Chronic Autoimmune Gastritis: Modern Diagnostic Principles. Diagnostics 2021, 11, 2113. [Google Scholar] [CrossRef] [PubMed]
- Rustgi, S.D.; Bijlani, P.; Shah, S.C. Autoimmune gastritis, with or without pernicious anemia: Epidemiology, risk factors, and clinical management. Therap. Adv. Gastroenterol. 2021, 14, 17562848211038771. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Metz, D.C.; Ellenberg, S.; Kaplan, D.E.; Goldberg, D.S. Risk Factors and Incidence of Gastric Cancer After Detection of Helicobacter pylori Infection: A Large Cohort Study. Gastroenterology 2020, 158, 527–536.e7. [Google Scholar] [CrossRef]
- Shah, S.C.; Piazuelo, M.B.; Kuipers, E.J.; Li, D. AGA Clinical Practice Update on the Diagnosis and Management of Atrophic Gastritis: Expert Review. Gastroenterology 2021, 161, 1325–1332.e7. [Google Scholar] [CrossRef]
- Suerbaum, S.; Michetti, P. Helicobacter pylori infection. N. Engl. J. Med. 2022, 347, 1175–1186. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Camargo, M.C.; El-Omar, E.; Liou, J.M.; Peek, R.; Schulz, C.; Smith, S.I.; Suerbaum, S. Helicobacter pylori infection. Nat. Rev. Dis. Primers 2023, 9, 19. [Google Scholar] [CrossRef]
- Ailloud, F.; Estibariz, I.; Suerbaum, S. Evolved to vary: Genome and epigenome variation in the human pathogen Helicobacter pylori. FEMS Microbiol. Rev. 2021, 45, fuaa042. [Google Scholar] [CrossRef] [PubMed]
- Moodley, Y.; Linz, B.; Yamaoka, Y.; Windsor, H.M.; Breurec, S.; Wu, J.Y.; Maady, A.; Bernhöft, S.; Thiberge, J.M.; Phuanukoonnon, S.; et al. The peopling of the Pacific from a bacterial perspective. Science 2009, 323, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Censini, S.; Lange, C.; Xiang, Z.; Crabtree, J.E.; Ghiara, P.; Borodovsky, M.; Rappuoli, R.; Covacci, A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA 1996, 93, 14648–14653. [Google Scholar] [CrossRef] [PubMed]
- Olbermann, P.; Josenhans, C.; Moodley, Y.; Uhr, M.; Stamer, C.; Vauterin, M.; Suerbaum, S.; Achtman, M.; Linz, B. A global overview of the genetic and functional diversity in the Helicobacter pylori cag pathogenicity island. PLoS Genet. 2010, 6, e1001069. [Google Scholar] [CrossRef] [PubMed]
- Gu, H. Role of Flagella in the Pathogenesis of Helicobacter pylori. Curr. Microbiol. 2017, 74, 863–869. [Google Scholar] [CrossRef]
- Sharndama, H.C.; Mba, I.E. Helicobacter pylori: An up-to-date overview on the virulence and pathogenesis mechanisms. Braz. J. Microbiol. 2022, 53, 33–50. [Google Scholar] [CrossRef]
- Ansari, S.; Yamaoka, Y. Helicobacter pylori Virulence Factors Exploiting Gastric Colonization and its Pathogenicity. Toxins 2019, 11, 677. [Google Scholar] [CrossRef]
- Cook, K.W.; Letley, D.P.; Ingram, R.J.; Staples, E.; Skjoldmose, H.; Atherton, J.C.; Robinson, K. CCL20/CCR6-mediated migration of regulatory T cells to the Helicobacter pylori-infected human gastric mucosa. Gut 2014, 63, 1550–1559. [Google Scholar] [CrossRef]
- Robinson, K.; Kenefeck, R.; Pidgeon, E.L.; Shakib, S.; Patel, S.; Polson, R.J.; Zaitoun, A.M.; Atherton, J.C. Helicobacter pylori-induced peptic ulcer disease is associated with inadequate regulatory T cell responses. Gut 2008, 57, 1375–1385. [Google Scholar] [CrossRef]
- Kao, C.Y.; Sheu, B.S.; Wu, J.J. Helicobacter pylori infection: An overview of bacterial virulence factors and pathogenesis. Biomed. J. 2016, 39, 14–23. [Google Scholar] [CrossRef]
- Backert, S.; Haas, R.; Gerhard, M.; Naumann, M. The Helicobacter pylori Type IV Secretion System Encoded by the cag Pathogenicity Island: Architecture, Function, and Signaling. Curr. Top. Microbiol. Immunol. 2017, 413, 187–220. [Google Scholar]
- Chen, B.; Zhang, J.; Ma, Q. The relationship between the simultaneity present of cagA and hopQI genes in Helicobacter pylori and the risk of gastric cancer. Cell Mol. Biol. 2021, 67, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Chmiela, M.; Kupcinskas, J. Review: Pathogenesis of Helicobacter pylori infection. Helicobacter 2019, 24 (Suppl. S1), e12638. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.M.; Malfertheiner, P.; Lee, Y.C.; Sheu, B.S.; Sugano, K.; Cheng, H.C.; Yeoh, K.G.; Hsu, P.I.; Goh, K.L.; Mahachai, V.; et al. Screening and eradication of Helicobacter pylori for gastric cancer prevention: The Taipei global consensus. Gut 2020, 69, 2093–2112. [Google Scholar] [CrossRef] [PubMed]
- Plummer, M.; Franceschi, S.; Vignat, J.; Forman, D.; de Martel, C. Global burden of gastric cancer attributable to Helicobacter pylori. Int. J. Cancer 2015, 136, 487–490. [Google Scholar] [CrossRef]
- Adamu, M.A.; Weck, M.N.; Gao, L.; Brenner, H. Incidence of chronic atrophic gastritis: Systematic review and meta-analysis of follow-up studies. Eur. J. Epidemiol. 2010, 25, 439–448. [Google Scholar] [CrossRef]
- Moss, S.F. The Clinical Evidence Linking Helicobacter pylori to Gastric Cancer. Cell. Mol. Gastroenterol. Hepatol. 2016, 3, 183–191. [Google Scholar] [CrossRef]
- Fann, J.C.; Chiang, T.H.; Yen, A.M.; Lee, Y.C.; Wu, M.S.; Chen, H.H. Personalized risk assessment for dynamic transition of gastric neoplasms. J. Biomed. Sci. 2018, 25, 84. [Google Scholar] [CrossRef]
- Venneman, K.; Huybrechts, I.; Gunter, M.J.; Vandendaele, L.; Herrero, R.; Van Herck, K. The epidemiology of Helicobacter pylori infection in Europe and the impact of lifestyle on its natural evolution toward stomach cancer after infection: A systematic review. Helicobacter 2018, 23, e12483. [Google Scholar] [CrossRef]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef]
- Park, J.S.; Jun, J.S.; Seo, J.H.; Youn, H.S.; Rhee, K. Changing prevalence of Helicobacter pylori infection in children and adolescents. Clin. Exp. Pediatr. 2021, 64, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Chen, Y.; Chen, F.; Tao, T.; Hu, Z.; Wang, J.; You, J.; Wong, B.C.Y.; Chen, J.; Ye, W. Effect of Helicobacter pylori Eradication on Gastric Cancer Prevention: Updated Report from a Randomized Controlled Trial with 26.5 Years of Follow-up. Gastroenterology 2022, 163, 154–162.e3. [Google Scholar] [CrossRef]
- Shichijo, S.; Hirata, Y.; Niikura, R.; Hayakawa, Y.; Yamada, A.; Ushiku, T.; Fukayama, M.; Koike, K. Histologic intestinal metaplasia and endoscopic atrophy are predictors of gastric cancer development after Helicobacter pylori eradication. Gastrointest. Endosc. 2016, 84, 618–624. [Google Scholar] [CrossRef]
- Khan, M.Y.; Aslam, A.; Mihali, A.B.; Shabbir Rawala, M.; Dirweesh, A.; Khan, S.; Adler, D.G.; Siddiqui, A. Effectiveness of Helicobacter pylori eradication in preventing metachronous gastric cancer and preneoplastic lesions. A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2020, 32, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Livzan, M.A.; Mozgovoy, S.I.; Kononov, A.V. Gastritis aft er eradication of Helicobacter pylori—Simple traces or serious consequences? Lechaschi Vrach. 2011, 7, 7. [Google Scholar]
- Shibata, W.; Sue, S.; Tsumura, S.; Ishii, Y.; Sato, T.; Kameta, E.; Sugimori, M.; Yamada, H.; Kaneko, H.; Sasaki, T.; et al. Correction to: Helicobacter-induced gastric inflammation alters the properties of gastric tissue stem/progenitor cells. BMC Gastroenterol. 2018, 18, 4. [Google Scholar] [CrossRef]
- Meyer, A.R.; Goldenring, J.R. Injury, repair, inflammation and metaplasia in the stomach. J. Physiol. 2018, 596, 3861–3867. [Google Scholar] [CrossRef]
- Nakajima, T.; Enomoto, S.; Yamashita, S.; Ando, T.; Nakanishi, Y.; Nakazawa, K.; Oda, I.; Gotoda, T.; Ushijima, T. Persistence of a component of DNA methylation in gastric mucosae after Helicobacter pylori eradication. J. Gastroenterol. 2010, 45, 37–44. [Google Scholar] [CrossRef]
- Cheung, K.S.; Leung, W.K. Risk of gastric cancer development after eradication of Helicobacter pylori. World J. Gastrointest. Oncol. 2018, 10, 115–123. [Google Scholar] [CrossRef]
- Sung, J.J.Y.; Coker, O.O.; Chu, E.; Szeto, C.H.; Luk, S.T.Y.; Lau, H.C.H.; Yu, J. Gastric microbes associated with gastric inflammation, atrophy and intestinal metaplasia 1 year after Helicobacter pylori eradication. Gut 2020, 69, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Shiota, S.; Thrift, A.P.; Green, L.; Shah, R.; Verstovsek, G.; Rugge, M.; Graham, D.Y.; El-Serag, H.B. Clinical Manifestations of Helicobacter pylori-Negative Gastritis. Clin. Gastroenterol. Hepatol. 2017, 15, 1037–1046.e3. [Google Scholar] [CrossRef] [PubMed]
- Kishikawa, H.; Ojiro, K.; Nakamura, K.; Katayama, T.; Arahata, K.; Takarabe, S.; Miura, S.; Kanai, T.; Nishida, J. Helicobacter pylori infection-induced atrophic gastritis: A distinct disease entity in an understudied population without a history of eradication. Helicobacter 2020, 25, e12669. [Google Scholar] [CrossRef] [PubMed]
- Toh, B.H. Diagnosis and classification of autoimmune gastritis. Autoimmun. Rev. 2014, 13, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Castro, K.I.; Franceschi, M.; Miraglia, C.; Russo, M.; Nouvenne, A.; Leandro, G.; Meschi, T.; De’Angelis, G.L.; Di Mario, F. Autoimmune diseases in autoimmune atrophic gastritis. Acta Biomed. 2018, 89, 100–103. [Google Scholar]
- Mahmud, N.; Stashek, K.; Katona, B.W.; Tondon, R.; Shroff, S.G.; Roses, R.; Furth, E.E.; Metz, D.C. The incidence of neoplasia in patients with autoimmune metaplastic atrophic gastritis: A renewed call for surveillance. Ann. Gastroenterol. 2019, 32, 67–72. [Google Scholar] [CrossRef]
- Hsing, A.W.; Hansson, L.E.; McLaughlin, J.K.; Nyren, O.; Blot, W.J.; Ekbom, A.; Fraumeni, J.F., Jr. Pernicious anemia and subsequent cancer. A population-based cohort study. Cancer 1993, 71, 745–750. [Google Scholar] [CrossRef]
- Kokkola, A.; Sjöblom, S.M.; Haapiainen, R.; Sipponen, P.; Puolakkainen, P.; Järvinen, H. The risk of gastric carcinoma and carcinoid tumours in patients with pernicious anaemia. A prospective follow-up study. Scand. J. Gastroenterol. 1998, 33, 88–92. [Google Scholar]
- Săftoiu, A.; Hassan, C.; Areia, M.; Bhutani, M.S.; Bisschops, R.; Bories, E.; Cazacu, I.M.; Dekker, E.; Deprez, P.H.; Pereira, S.P.; et al. Role of gastrointestinal endoscopy in the screening of digestive tract cancers in Europe: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2020, 52, 293–304. [Google Scholar] [CrossRef]
- Martins, B.C.; Moura, R.N.; Kum, A.S.T.; Matsubayashi, C.O.; Marques, S.B.; Safatle-Ribeiro, A.V. Endoscopic Imaging for the Diagnosis of Neoplastic and Pre-Neoplastic Conditions of the Stomach. Cancers 2023, 15, 2445. [Google Scholar] [CrossRef]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 1996, 20, 1161–1181. [Google Scholar] [CrossRef]
- Correa, P.; Yardley, J.H. Grading and classification of chronic gastritis: One American response to the Sydney system. Gastroenterology 1992, 102, 355–359. [Google Scholar] [CrossRef]
- Yagi, K.; Nakamura, A.; Sekine, A.; Graham, D. Features of the atrophic corpus mucosa in three cases of autoimmune gastritis revealed by magnifying endoscopy. Case Rep. Med. 2012, 2012, 368160. [Google Scholar] [CrossRef]
- Pimentel-Nunes, P.; Libânio, D.; Marcos-Pinto, R.; Areia, M.; Leja, M.; Esposito, G.; Garrido, M.; Kikuste, I.; Megraud, F.; Matysiak-Budnik, T.; et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 2019, 51, 365–388. [Google Scholar]
- Zhang, Q.; Wang, F.; Chen, Z.Y.; Wang, Z.; Zhi, F.C.; Liu, S.D.; Bai, Y. Comparison of the diagnostic efficacy of white light endoscopy and magnifying endoscopy with narrow band imaging for early gastric cancer: A meta-analysis. Gastric Cancer 2016, 19, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Pimenta-Melo, A.R.; Monteiro-Soares, M.; Libânio, D.; Dinis-Ribeiro, M. Missing rate for gastric cancer during upper gastrointestinal endoscopy: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1041–1049. [Google Scholar] [CrossRef]
- Zhao, Z.; Yin, Z.; Wang, S.; Wang, J.; Bai, B.; Qiu, Z.; Zhao, Q. Meta-Analysis: The Diagnostic Efficacy of Chromoendoscopy for Early Gastric Cancer and Premalignant Gastric Lesions. J. Gastroenterol. Hepatol. 2016, 31, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Fiuza, F.; Maluf-Filho, F.; Ide, E.; Furuya, C.K., Jr.; Fylyk, S.N.; Ruas, J.N.; Stabach, L.; Araujo, G.A.; Matuguma, S.E.; Uemura, R.S.; et al. Association between Mucosal Surface Pattern under near Focus Technology and Helicobacter Pylori Infection. World J. Gastrointest. Endosc. 2021, 13, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Vu, N.T.H.; Quach, D.T.; Dang, N.L.B.; Le, Q.D.; Nguyen, D.T.N.; Le, H.M.; Le, N.Q.; Hiyama, T. Performance of chromoendoscopy and narrow-band imaging in the diagnosis of gastric intestinal metaplasia. Scand. J. Gastroenterol. 2022, 57, 1005–1010. [Google Scholar] [CrossRef]
- Sha, J.; Wang, P.; Zhu, B.; Zhu, M.; Li, X.; Gao, F. Acetic Acid Enhanced Narrow Band Imaging for the Diagnosis of Gastric Intestinal Metaplasia. PLoS ONE 2017, 12, e0170957. [Google Scholar] [CrossRef]
- Pal, P.; Singh, A.P.; Kanuri, N.D.; Banerjee, R. Electronic chromo-endoscopy: Technical details and a clinical perspective. Transl. Gastroenterol. Hepatol. 2022, 7, 6. [Google Scholar] [CrossRef]
- Pimentel-Nunes, P.; Libânio, D.; Lage, J.; Abrantes, D.; Coimbra, M.; Esposito, G.; Hormozdi, D.; Pepper, M.; Drasovean, S.; White, J.R.; et al. A multicenter prospective study of the real-time use of narrow-band imaging in the diagnosis of premalignant gastric conditions and lesions. Endoscopy 2016, 48, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Kikuste, I.; Marques-Pereira, R.; Monteiro-Soares, M.; Pimentel-Nunes, P.; Areia, M.; Leja, M.; Dinis-Ribeiro, M. Systematic review of the diagnosis of gastric premalignant conditions and neoplasia with high-resolution endoscopic technologies. Scand. J. Gastroenterol. 2023, 48, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lim, L.G.; Yeoh, K.G. Advanced endoscopic imaging in gastric neoplasia and preneoplasia. BMJ Open Gastroenterol. 2017, 4, e000105. [Google Scholar] [CrossRef]
- Bacha, D.; Walha, M.; Ben Slama, S.; Ben Romdhane, H.; Bouraoui, S.; Bellil, K.; Lahmar, A. Chronic gastritis classifications. Tunis Med. 2018, 96, 405–410. [Google Scholar] [PubMed]
- Rugge, M.; Savarino, E.; Sbaraglia, M.; Bricca, L.; Malfertheiner, P. Gastritis: The clinico-pathological spectrum. Dig. Liver Dis. 2021, 53, 1237–1246. [Google Scholar] [CrossRef]
- Dinis-Ribeiro, M.; Areia, M.; de Vries, A.C.; Marcos-Pinto, R.; Monteiro-Soares, M.; O’Connor, A.; Pereira, C.; Pimentel-Nunes, P.; Correia, R.; Ensari, A.; et al. Management of precancerous conditions and lesions in the stomach (MAPS): Guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy 2012, 44, 74–94. [Google Scholar] [PubMed]
- Rugge, M.; Genta, R.M.; Fassan, M.; Valentini, E.; Coati, I.; Guzzinati, S.; Savarino, E.; Zorzi, M.; Farinati, F.; Malfertheiner, P. OLGA Gastritis Staging for the Prediction of Gastric Cancer Risk: A Long-term Follow-up Study of 7436 Patients. Am. J. Gastroenterol. 2018, 113, 1621–1628. [Google Scholar] [CrossRef]
- Rugge, M.; Correa, P.; Di Mario, F.; El-Omar, E.; Fiocca, R.; Geboes, K.; Genta, R.M.; Graham, D.Y.; Hattori, T.; Malfertheiner, P.; et al. OLGA staging for gastritis: A tutorial. Dig. Liver Dis. 2008, 40, 650–658. [Google Scholar] [CrossRef]
- Cotruta, B.; Gheorghe, C.; Iacob, R.; Dumbrava, M.; Radu, C.; Bancila, I.; Becheanu, G. The Orientation of Gastric Biopsy Samples Improves the Inter-observer Agreement of the OLGA Staging System. J. Gastrointestin. Liver Dis. 2017, 26, 351–356. [Google Scholar] [CrossRef]
- Rugge, M.; Genta, R.M. OLGA Group Staging gastritis: An international proposal. Gastroenterology 2005, 129, 1807–1808. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Mendoza, P.; González-Angulo, J.; Angeles-Garay, U.; Segovia-Cueva, G.A. Evaluaciónhistopatológica de gastritis atrófica. Comparación de lossistemas Sidney y OLGA [Evaluation of Gastric Atrophy. Comparison between Sidney and OLGA Systems]. Rev. Med. Inst. Mex Seguro Soc. 2008, 46, 135–139. [Google Scholar] [PubMed]
- Yue, H.; Shan, L.; Bin, L. The significance of OLGA and OLGIM staging systems in the risk assessment of gastric cancer: A systematic review and meta-analysis. Gastric Cancer 2018, 21, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Aruin, L.I.; Kononov, A.V.; Mozgovoy, S.I. New classification of chronic gastritis. Actual problems of pathological anatomy: Proceedings of the III Congress of the Russian Academy of Sciences. Total pathologists. Samara 2009, 1, 5–8. [Google Scholar]
- Satoh, K.; Osawa, H.; Yoshizawa, M.; Nakano, H.; Hirasawa, T.; Kihira, K.; Sugano, K. Assessment of atrophic gastritis using the OLGA system. Helicobacter 2008, 13, 225–229. [Google Scholar]
- Isajevs, S.; Liepniece-Karele, I.; Janciauskas, D.; Moisejevs, G.; Putnins, V.; Funka, K.; Kikuste, I.; Vanags, A.; Tolmanis, I.; Leja, M. Gastritis staging: Interobserver agreement by applying OLGA and OLGIM systems. Virchows Arch. 2014, 464, 403–407. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, N.; Yun, C.Y.; Lee, H.S. The clinical meaning of the “indefinite for atrophy” lesions within gastric mucosa biopsy specimens in a region with a high prevalence of gastric cancer. Helicobacter 2019, 24, e12605. [Google Scholar] [CrossRef]
- Rugge, M.; Sacchi, D.; Genta, R.M.; Zanco, F.; Guzzinati, S.; Pizzi, M.; Fassan, M.; Di Sabatino, A.; El-Serag, H. Histological assessment of gastric pseudopyloric metaplasia: Intra- and inter-observer consistency. Dig. Liver Dis. 2021, 53, 61–65. [Google Scholar]
- Cho, S.J.; Choi, I.J.; Kook, M.C.; Nam, B.H.; Kim, C.G.; Lee, J.Y.; Ryu, K.W.; Kim, Y.W. Staging of intestinal- and diffuse-type gastric cancers with the OLGA and OLGIM staging systems. Aliment. Pharmacol. Ther. 2013, 38, 1292–1302. [Google Scholar] [CrossRef]
- Cheng, H.C.; Tsai, Y.C.; Yang, H.B.; Yeh, Y.C.; Chang, W.L.; Kuo, H.Y.; Lu, C.C.; Sheu, B.S. The corpus-predominant gastritis index can be an early and reversible marker to identify the gastric cancer risk of Helicobacter pylori-infected nonulcer dyspepsia. Helicobacter 2017, 22, e12385. [Google Scholar] [CrossRef]
- Genta, R.M.; Rugge, M. Assessing risks for gastric cancer: New tools for pathologists. World J. Gastroenterol. 2006, 12, 5622–5627. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.K.; Li, C.; Zhou, Y.M.; Zeng, L.; Li, Y.Y.; Huang, S.L.; Zhu, C.Y.; Wang, Y.; Wang, S.N.; Chen, X.D. Histopathological Features of Helicobacter pylori Infection in Gastric Mucosa. J. Inflamm. Res. 2022, 15, 6231–6243. [Google Scholar] [CrossRef] [PubMed]
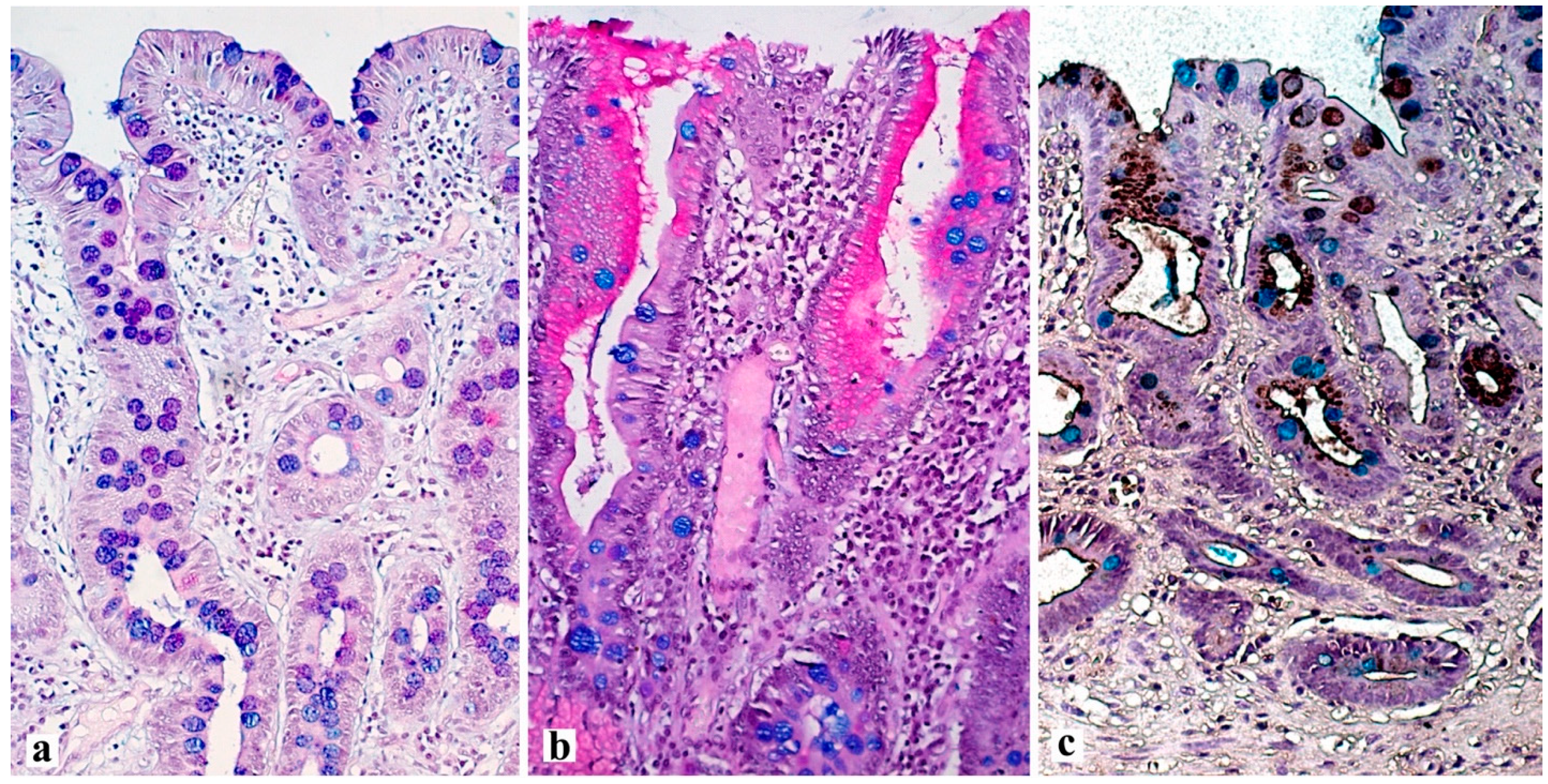

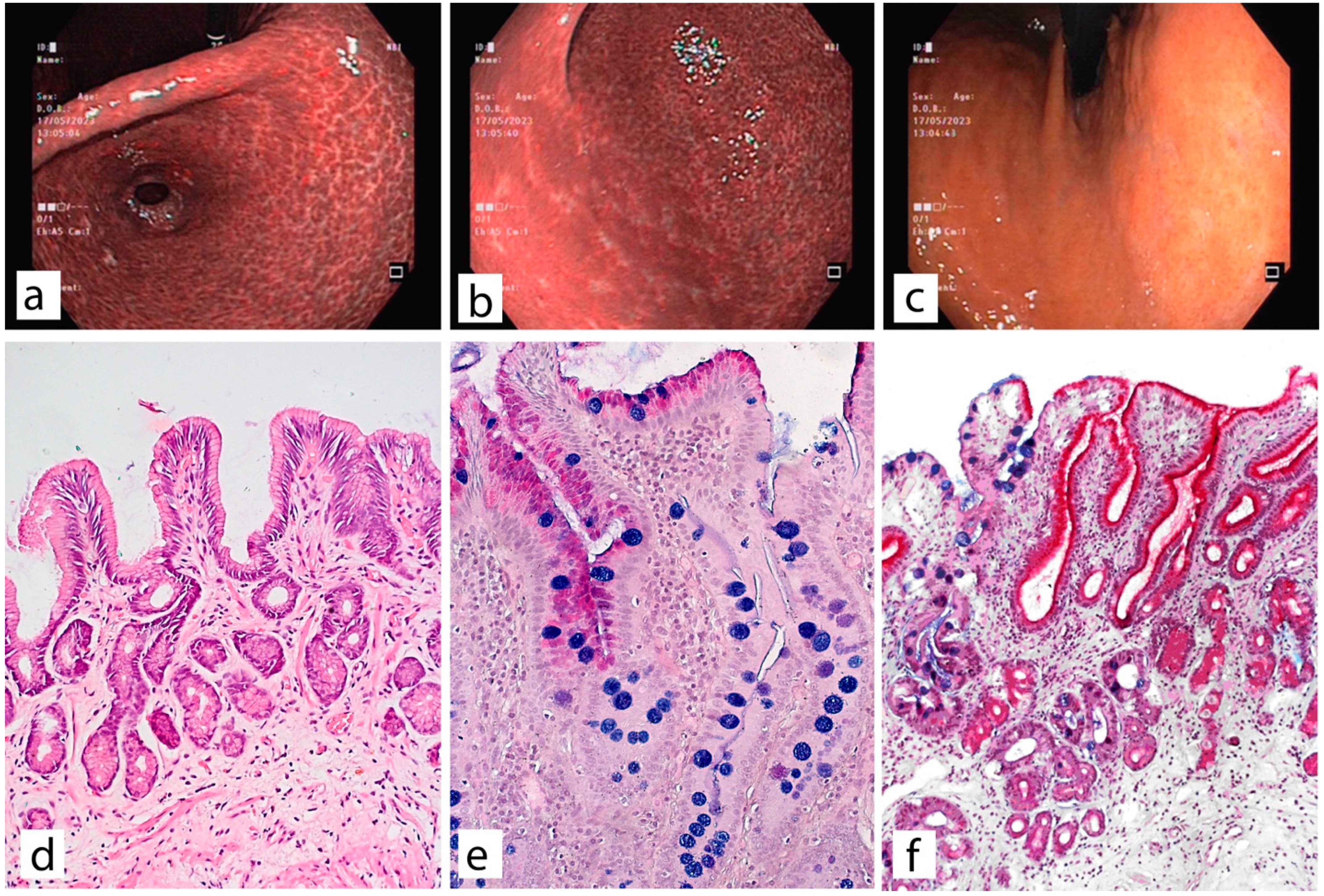
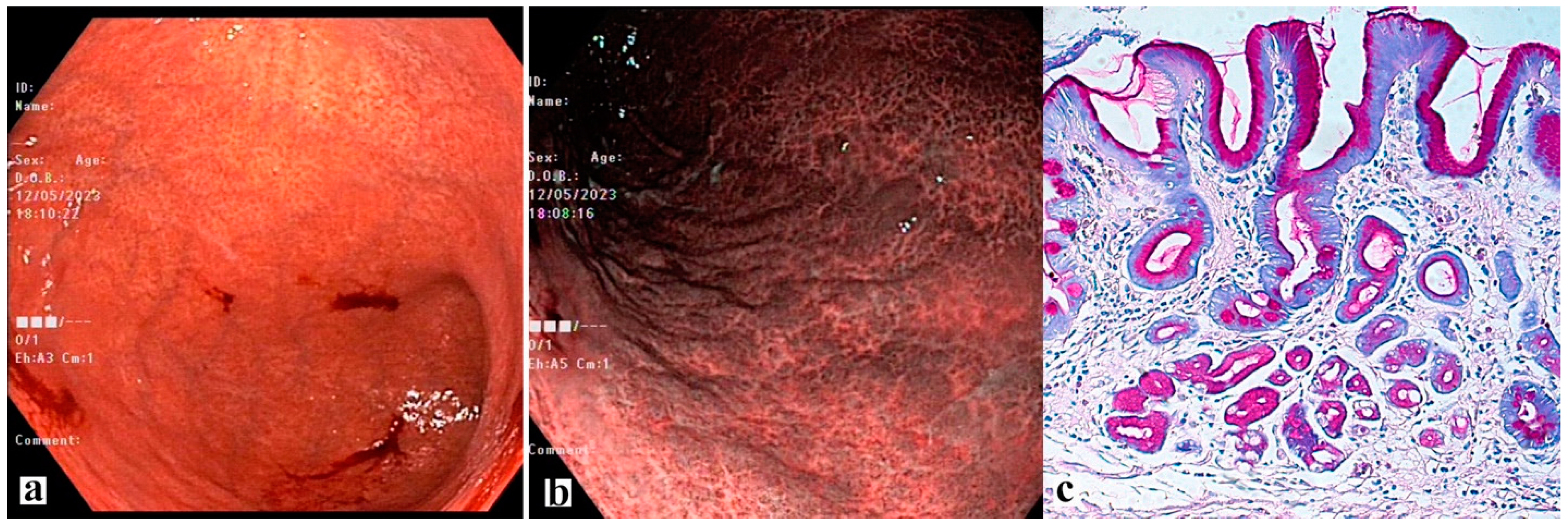

| Marker | Functional Role | Normal Expression | Expression in Pathological Condition | Associated Metaplasia |
|---|---|---|---|---|
| MUC1 | formation of mucosal protective barrier | foveolar epithelium of the body and antrum of the stomach | lack of expression | positive expression in incomplete intestinal metaplasia |
| MUC2 | formation of mucosal protective barrier | goblet cells | goblet cells of metaplastic epithelium | complete intestinal metaplasia |
| MUC5AC | formation of mucosal protective barrier | foveolar epithelium of the body and antrum of the stomach | lack of expression | pseudopyloric metaplasia positive expression in incomplete intestinal metaplasia |
| MUC6 | formation of mucosal protective barrier | lower part of antral glands and neck cells | similar to TFF2 | pseudopyloric and incomplete intestinal metaplasia |
| CDX2 | intestinal transcription factor | absent | cell nuclei in glands, which are transformed to intestinal metaplasia | intestinal metaplasia |
| Hep | urea metabolism | hepatocytes, small intestinal epithelium | cells of metaplastic glands | incomplete intestinal metaplasia |
| CD44v9 | cell adhesion factor | absent | cytoplasm and membrane of damaged epithelial cells | SPEM |
| SOX9 | transcription factor | neck of the gastric glands of the antrum | basal part of metaplastic glands | intestinal metaplasia, SPEM |
| TFF2 | Formation of mucosal barrier of the stomach | mucocytes of neck of the gastric glands of the body of the stomach, lower part of of antral glands | cytoplasm of mucocytes of metaplastic glands | SPEM |
| TFF3 | formation of mucosal protective barrier | goblet cells | in goblet cells of metaplastic glands | intestinal metaplasia |
| AQP5 | water-channel protein | lower part of antral glands, stem cells | increase expression | pseudopyloric metaplasia, SPEM, intestinal metaplasia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Livzan, M.A.; Mozgovoi, S.I.; Gaus, O.V.; Shimanskaya, A.G.; Kononov, A.V. Histopathological Evaluation of Gastric Mucosal Atrophy for Predicting Gastric Cancer Risk: Problems and Solutions. Diagnostics 2023, 13, 2478. https://doi.org/10.3390/diagnostics13152478
Livzan MA, Mozgovoi SI, Gaus OV, Shimanskaya AG, Kononov AV. Histopathological Evaluation of Gastric Mucosal Atrophy for Predicting Gastric Cancer Risk: Problems and Solutions. Diagnostics. 2023; 13(15):2478. https://doi.org/10.3390/diagnostics13152478
Chicago/Turabian StyleLivzan, Maria A., Sergei I. Mozgovoi, Olga V. Gaus, Anna G. Shimanskaya, and Alexei V. Kononov. 2023. "Histopathological Evaluation of Gastric Mucosal Atrophy for Predicting Gastric Cancer Risk: Problems and Solutions" Diagnostics 13, no. 15: 2478. https://doi.org/10.3390/diagnostics13152478
APA StyleLivzan, M. A., Mozgovoi, S. I., Gaus, O. V., Shimanskaya, A. G., & Kononov, A. V. (2023). Histopathological Evaluation of Gastric Mucosal Atrophy for Predicting Gastric Cancer Risk: Problems and Solutions. Diagnostics, 13(15), 2478. https://doi.org/10.3390/diagnostics13152478







