A Review of Machine Learning Techniques for the Classification and Detection of Breast Cancer from Medical Images
Abstract
1. Introduction
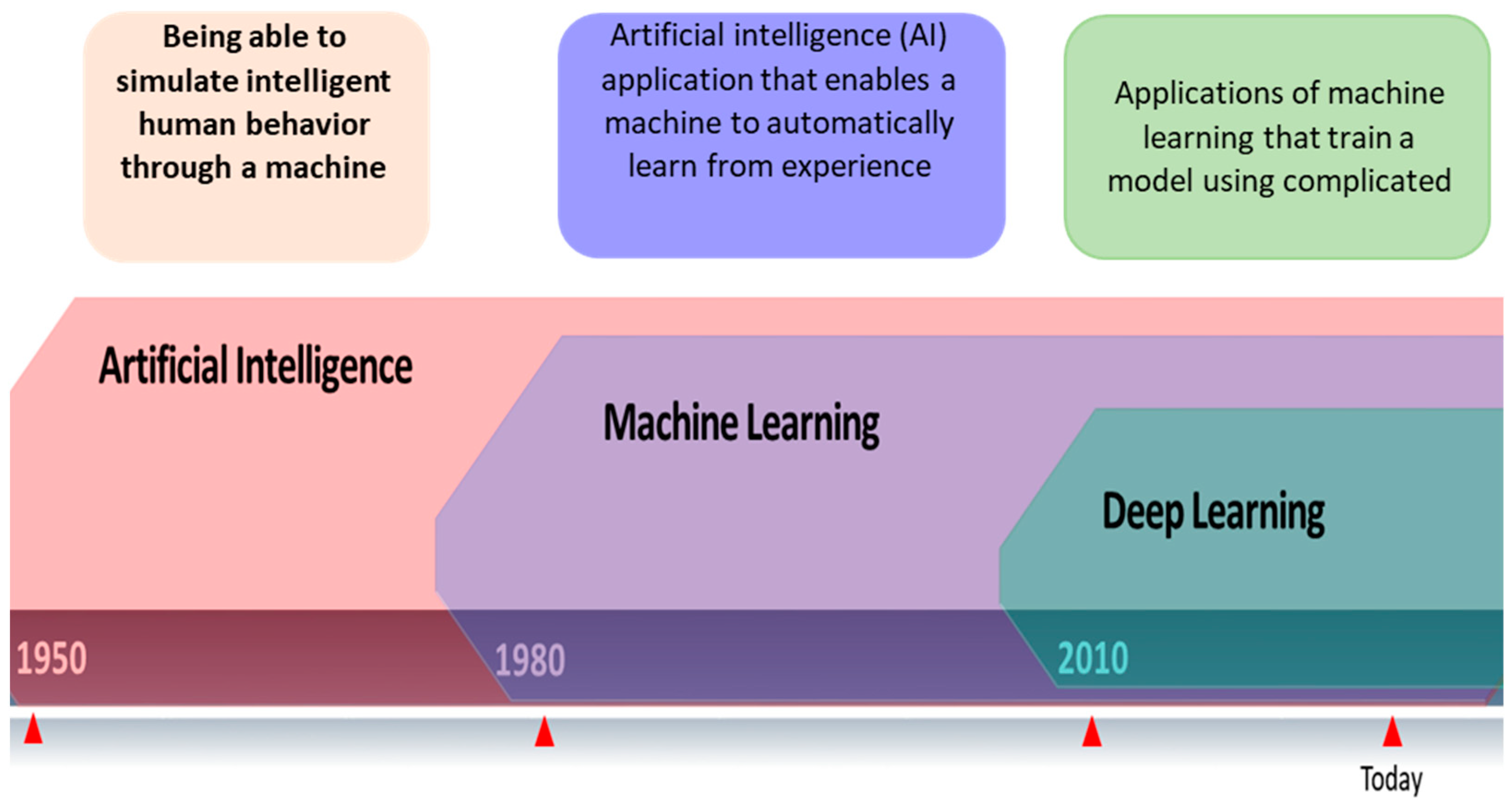
2. Artificial Intelligence and Machine Learning Techniques
- ▪
- Supervised learning: In supervised machine learning (ML), every issue may be viewed as the learning of a parametrized function, also referred to as a “model”, that maps inputs (i.e., predictor variables) to outputs (i.e., “target variables”). The purpose of supervised learning is to utilize an algorithm to extract the parameters of those functions from the given data. It is possible to think of supervised learning as using logistic and linear regressions. The majority of ML approaches fall within this category. SVM, DT, Clustering-NN, and K-means are examples of supervised machine learning methods.
- ▪
- Unsupervised learning: ML issues are often far more difficult to solve if the target variables are unavailable. Unsupervised learning uses the common dimensionality reduction and clustering tasks to find correlations or patterns in the data without providing any direction for the “correct” answer.
- ▪
- Agent-based learning: Between guided and unguided learning: It is a collection of machine learning techniques where learning occurs by replicating the activities and communications of a single autonomous agent or a group of autonomous agents. To effectively learn, carefully determine values (or preferences), and employ inquiry procedures, one must deal with problems that regularly arise in real life. It is necessary to develop generalizable models because these general unsupervised approaches rely on target obtain variables for which there is little information. Only by experimenting can one detect essential parts of the surroundings. In this context, a specific example of a problem with decision-making over time is reinforcement learning.
2.1. Decision Tree
2.2. Random Forests
2.3. K-Nearest Neighbor
2.4. Support Vector Machine: (SVM)
2.5. Artificial Neural Networks (ANN) and Deep Learning (DL)
2.5.1. Convolutional Neural Network (CNN)
- ▪
- Convolutional Layer: Convolutional layers are utilized to produce feature maps using the weight distribution theory and the local connection concept. Local connectivity and weight distribution objectives are used to reduce the number of parameters while maximizing the advantages of the strongly connected local pixel neighborhood and location-independent local image statistics. The weight distribution model looks like this. Each unit (neuron) in a feature map only has a “local connection” to surrounding patches of the feature map at the previous stage thanks to a weight group called a “filter bank”. Each unit has a filter row they share on a feature map. Other feature maps employ different filter banks as well. The weighted sum of each unit serves as the input to the activation function, a nonlinear transformation function. The weighted total of each succeeding unit is sent to the activation function, a nonlinear transformation function. According to [17], the activation function enables the transmitted data to change nonlinearly for subsequent processing steps.
- ▪
- Pooling layer: The pooling layer combines (semantically) linked convolutional layer features into one using a subsampling technique. A unit within a pooling layer uses a local patch as input from a previous entity map (convolutional layer) to calculate the maximum or average patch value at the output. Reduced representation size and increased robustness lower the parameters needed in later stages.
- ▪
- Fully connected layer: According to a multilayer perceptron, a classic neural network, the units in this layer are fully connected to all the units in the layer above.
- LeNet-5: LeNet-5 [13] has two convolutional layers and three fully linked layers.
- According to [15], AlexNet includes five convolutional and three fully linked layers.
- VGG-16 [18] uses three fully connected layers and thirteen convolutional layers, taking the ReLU from Alex Net.
- According to [19], Inception-v1 has a 22-layer architecture with 5 M parameters.
- According to [20], Inception-v3 is a version of Inception-v1 with parameters of 24 M.
- ResNet-50: A network with 50 layers [21].
- Thirty-six convolutional layers, according to Xception [22].
- Inception-V4: According to [23] Inception-V4 consists of a feature extractor and fully connected layers.
- One hundred and sixty-four layers are deep in Inception-ResNet.
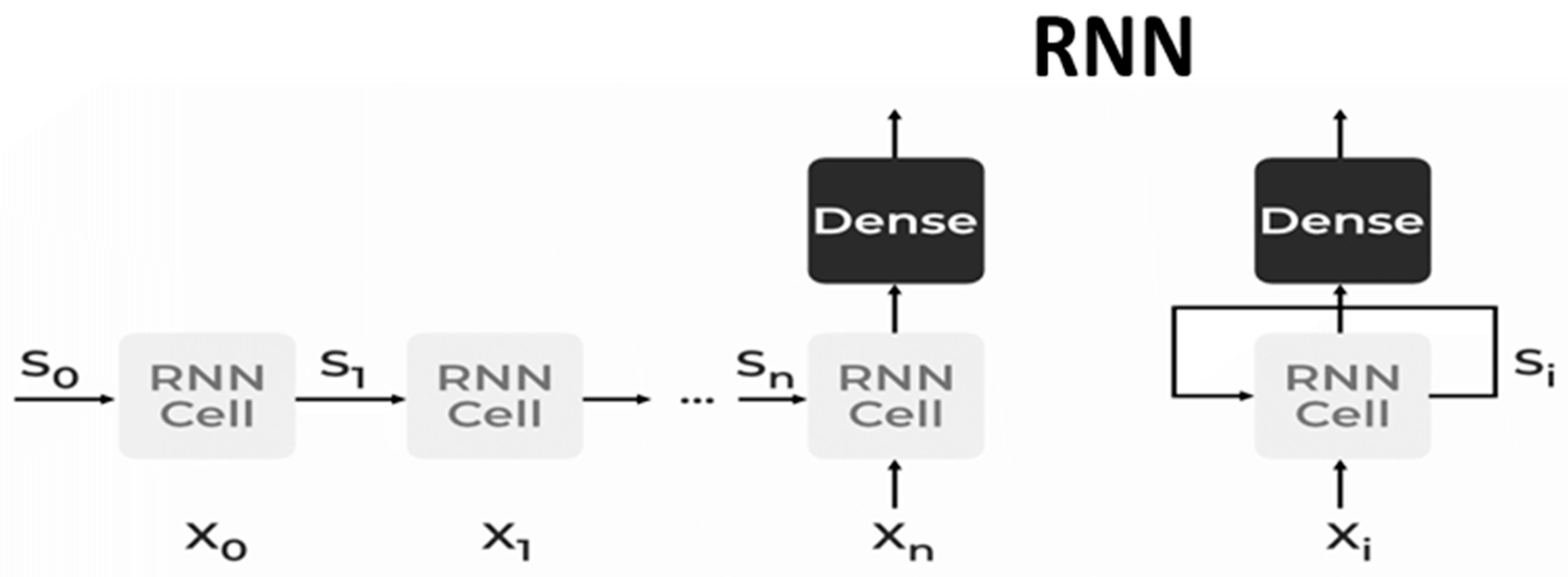
2.5.2. Recurrent Neural Network (RNN)
- ▪
- Bidirectional Neural Network (BiNN): A BiNN is a type of recurrent neural network in which data are input in both directions and output from both directions is combined to create the input. In cases such as NLP tasks and time-series analysis issues, where the context of the input is more crucial, BiNN is helpful.
- ▪
- Long Short-Term Memory (LSTM): based on the read–write–forget principle, which states that given an input of information, a network should read and write just the information that will be most valuable in forecasting the outcome and ignore the rest. Three new gates are added to the RNN to accomplish this. Only the chosen information is transmitted via the network in this way [25].
2.5.3. Deep Convolutional Neural Network
- (1)
- Convolutional layer: The main component of the DCNN is the convolutional layer, filter or kernel weights represent the layer parameters. The output feature map is created by multiplying each of the receptive fields, which are the small areas formed by the input feature map. If the stride hyperparameter is smaller than the filter size, the convolution is performed in overlapping windows. The stride is the distance between the applications of filters.
- (2)
- Pooling layer: Down sampling the input’s spatial dimension is performed by the pooling layer. The primary goals of this layer type are the gradual reduction of the representation’s spatial dimension and the reduction of the parameters and calculations needed by the network. Although many different pooling functions are available, such as average pooling and L2-norm pooling, max pooling is the most popular since it computes the maximum in the input patch.
- (3)
- Fully connected layer: A conventional multi-layer perceptron with a SoftMax activation function in the output layer makes up the completely connected layer. Neuronal cells link to every activation in the preceding layer. To categorize the input image using high-level features extracted from convolutional and pooling layers is the goal of the fully connected layer [26].
3. Types of Breast Cancer Imaging
3.1. Mammography Images
Mammography Datasets
- The digital database for screening mammography (DDSM) comprises 2620 mammograms scanned from film which were then separated into 43 volumes (Figure 3). For each example, there are four breast mammograms since the Mediolateral Oblique (MLO) and Cranio-Caudal projections were used to photograph each breast side. The dataset includes pixel-level annotations for the suspicious regions and the ground truth. The breast density score for each patient was calculated using the ACR BI-RADS (American College of Radiology Breast Imaging Reporting and Data System). The file for each case also contains information about the size and resolution of each scanned image. JPEG (Joint Photographic Experts Group) format, available in various formats and resolutions, was used for the images.
- The Curated Breast Imaging Subset of the DDSM (CBIS-DDSM) is an upgraded version of the DDSM that includes bounding boxes for the region of interest (ROI), updated mass segmentation, and decompressed pictures. The data were picked and reviewed by mammographers with the necessary training, and the images are in the Digital Imaging and Communication in Medicine (DICOM) format. The collection is 163.6 GB in size and contains 6775 studies. There were 10,239 images in total, all mammography scans with associated mask images. CSV files are associated with the dataset that includes the patients’ pathological data. A mass training set, a mass testing set, a training set for calcification, and a testing set for calcification make up the dataset’s four CSV files. The mass testing set only includes images for 378 cancers, whereas the dataset consists of images of 1318 tumors. Images for 1622 calcifications are included in the calcification training set, whereas photos for 326 calcifications are included in the calcification testing set.
- IN Breast: Breast consists of 410 images and 115 cases. In 90 of the 115 cases, there was malignancy in both breasts. The dataset represents the four types of breast illnesses: breast bulk, breast calcification, breast asymmetries, and breast distortions. Images of (CC) and (MLO) views, stored in DICOM format, are included in the dataset. The dataset also offers the breast density score from the Breast Imaging-Reporting and Data System (BI-RADS).
- Mini-MIAS: The dataset includes ground truth indicators for potential abnormalities and 322 digital films. The collection contains five types of abnormalities: masses, architectural distortion, asymmetry, and normal. Ultimately, 1024 by 1024 pixels) were used as the final resolution for the images. The images are accessible to everyone on the University of Essex’s Pilot European Image Processing Archive (PEIPA).
- BCD: The BCDR consists of two mammographic repositories:
- ○
- The BCDR-FM and the Film Mammography-based Repository.
- ○
- The BCDR-DM, or Full Field Digital Mammography-based Repository.
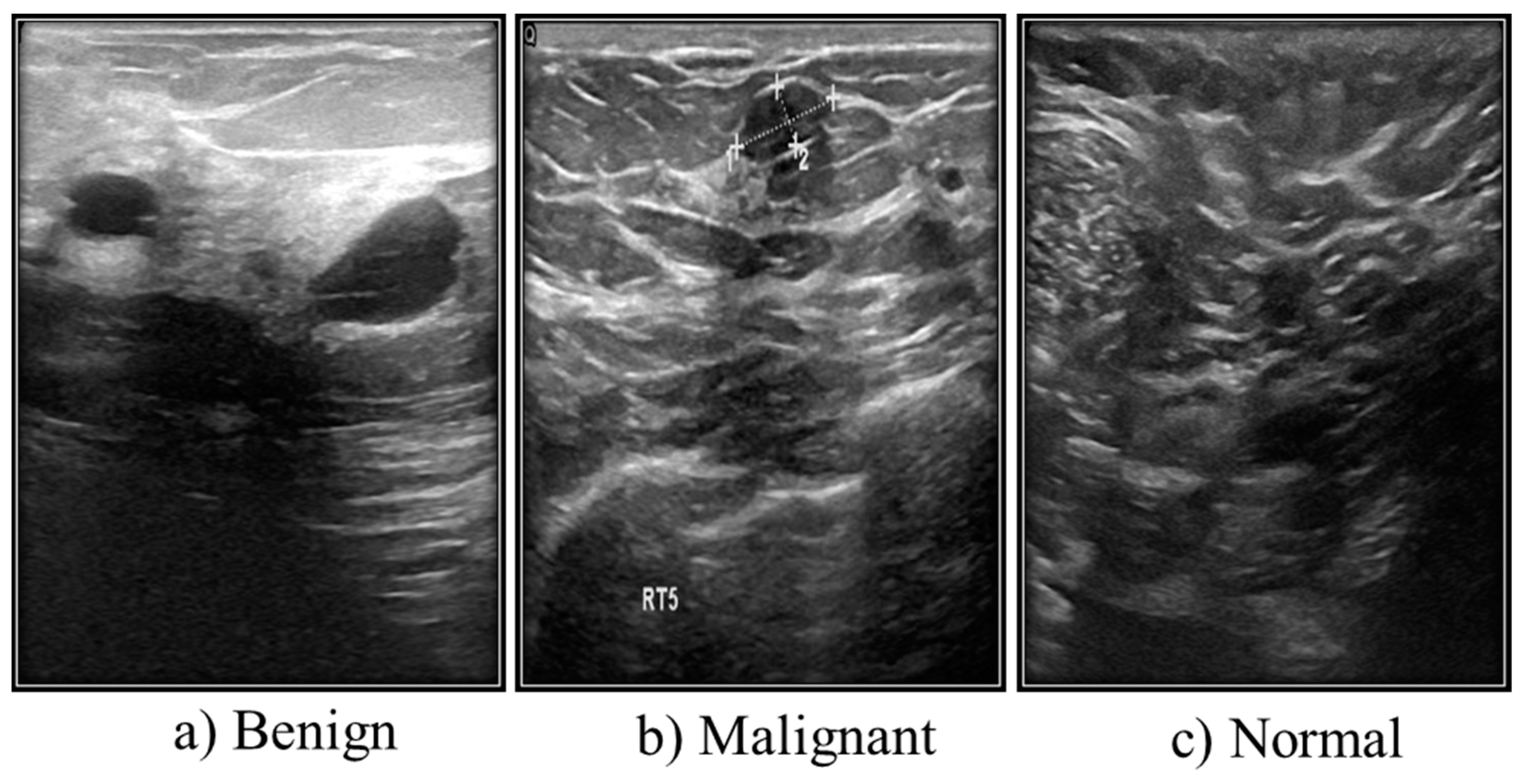
3.2. Ultrasound Images
Ultrasound Dataset
3.3. Magnetic Resonance Imaging (MRI)
- High risk of developing breast cancer.
- Evaluation of the staging period.
- Neoadjuvant chemotherapy (NAC) follow-up.
- Evaluation of an auxiliary lymph node region when mammography could not identify the primary location.
MRI Datasets
- Breast–MRI–NACT–Pilot dataset: The database for this dataset is 19.5 GB in size and contains 99,058 MRI images for 64 patients.
- Mouse–Mammary: This dataset has 23,487 Images, the database is 8.6 GB, and there are 32 patients.
3.4. Histopathological Images
Dataset for Breast Cancer Histopathological Images
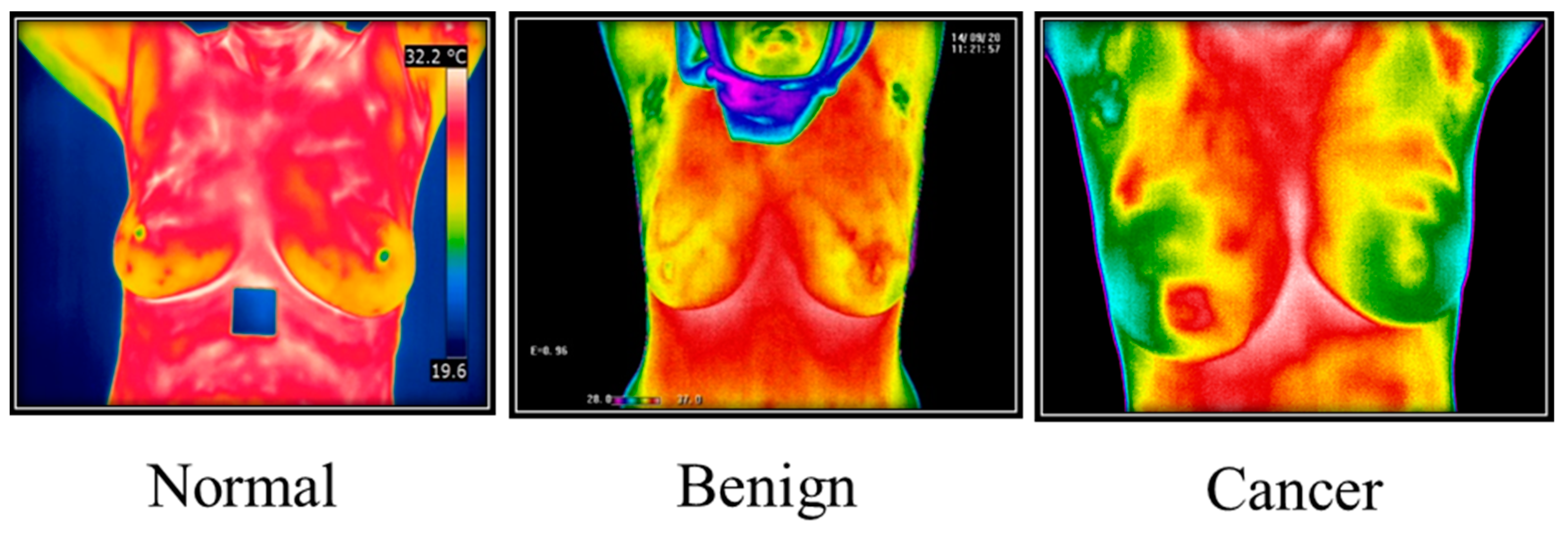
3.5. Thermography Images
3.5.1. Thermal Camera
3.5.2. Thermography Datasets
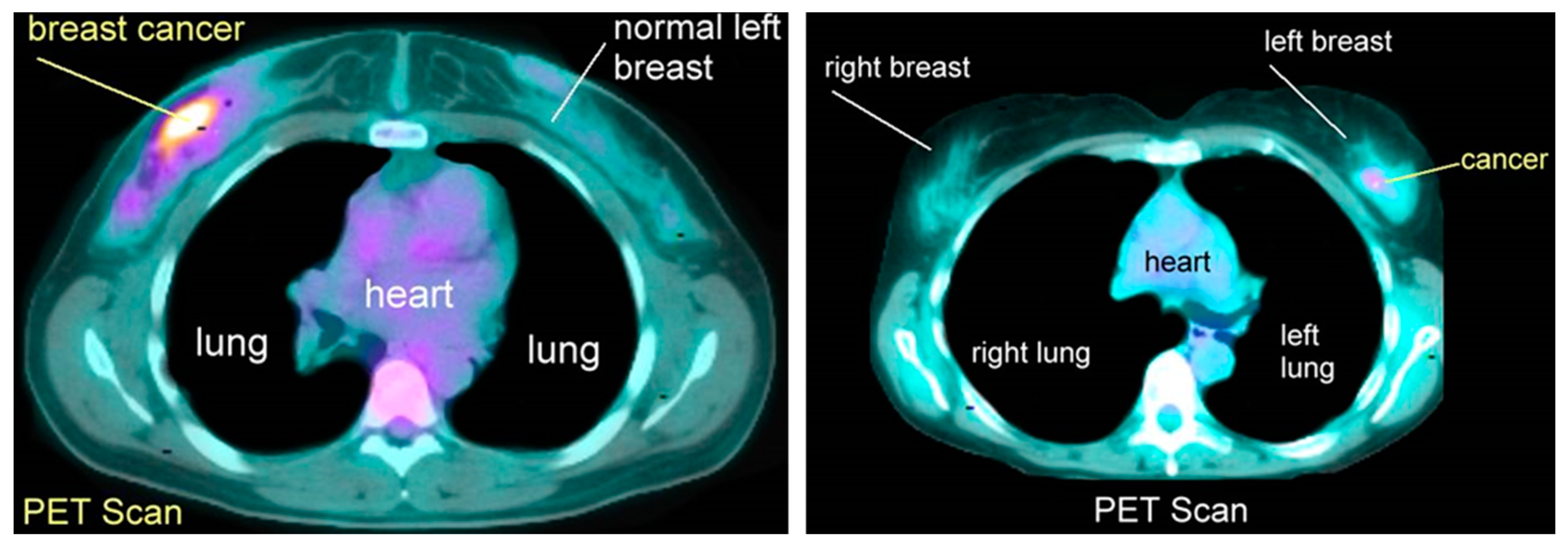
3.6. Positron Emission Tomography (PET)
4. Review on Machine Learning in Breast Cancer
4.1. Machine Techniques for Mammogram Images
| References | Dataset | ML Method | Results |
|---|---|---|---|
| [36] | Mini-MIAS INBreast | SVM Classifier | Accuracy of 99% AUC value is 0.933 |
| [52] | DDSM | SVM Classifier | Sn value is 82.4% |
| [37] | DDSM | SVM Classifier | Accuracy is 98.9% |
| [38] | MIAS INBreast | SVM Classifier | Accuracy is 99% ± 0.50 AUC value 0.99 ± 0.005 |
| [39] | MIAS | SVM Classifier | Accuracy 93.17% |
| [40] | MIAS: 109 cases | SVM Classifier | Accuracy value from 68% to 100% |
| [41] | MIAS | RBFNN classifier | RBF (normal/abnormal) Accuracy is 93.9% Sn value is 97.2% RBF (benign/malignant) Accuracy is 94.3% Sn value is 100% |
| [53] | Private-1896 cases | GLCM SFFS (sequential floating forward selection) the bilateral CC and MLO view images | Sn-value is 68.8% Sp value is 95.0% The AUC value is 0.85 ± 0.046 |
| [45] | MIAS: 57 images 37 benign and 20 malignant | CNN classifier | Accuracy is 90.9% AUC value is 96.9% |
| [46] | MIAS -BancoWeb: 100 images | CNN and hybrid of K-means a | Accuracy 96% |
| [42] | DDSM | Fuzzy C-Means (FCM) | Accuracy is 87% Sn value is 90 to 47% Sp value is 84 to 84% |
| [49] | 252 images from Mini-MIAS -DDSM | KNN | Abnormality detecting: Accuracy is 91.2% AUC value is 0.98 Malignancy detecting: Accuracy is 81.4% AUC value is 0.84 |
| [50] | 300 images from DDSM | Fuzzy Gaussian Mixture Model (FGMM) | Accuracy is 93% Sn value is 90% Sp value is 96% |
| [44] | IRMA-MIAS | k-NN | Accuracy is 92.8% ± 0.009 Sn value is 92.85% ± 0.01 AUC value is 0.971 |
| [54] | DDSM | CNN and transfer learning | Sensitivity of the mass 89.9% |
| [55] | DDSM, MIAS | LS SVM, KNN, Random Forest, and Naive Bayes | Accuracy 92% |
| [56] | (Mini-MIAS) DDSM | CNN | The accuracy of 0.936, 0.890, 0.871 on the DDSM, 0.944, 0.915, 0.892 on the Mini-MIAS for normal, benign, and malignant regions |
| [48] | MIAS | CNN a pre-trained architecture such as Inception V3, ResNet50, Visual Geometry Group networks (VGG)-19, VGG-16, and Inception-V2 ResNet | Overall Accuracy, Sn, Sp, precision, F-score, and AUC of 98.96%, 97.8%, 99.1%, 97.4%, 97.7%, and 0.995, respectively, for the 80–20 method and 98.87%, 97.3%, 98.2%, 98.84%, 98.04%, and 0.993 for the 10-fold cross-validation method, the TL of the VGG16 model is adequate for diagnosis. |
| [57] | DDSM | CNN | Accuracy 71.4% |
4.2. Machine Learning Techniques for Ultrasound Images
4.3. Machine Learning Techniques (MLT) for Thermography
| References | Dataset | ML Method | Performance Evaluation |
|---|---|---|---|
| [82] | 50 breast images | SVM | Accuracy is 88.1% Sn value is 85.7% Sp value is 90.5% |
| [83] | 40 images | SVM Naive Bayes K-Nearest Neighbor | Accuracy is 92.5% |
| [75] | 306 images | DWT | Accuracy is 90.5% Sn value is 87.6% Sp value is 89.7% |
| [84] | 63 images | Fuzzy c-means ROI SVM | Accuracy is 100% |
| [85] | 63 thermography Images | Bio-inspired Swarm Techniques | Accuracy: 85.71%, 84.12%, 85.71%, and 96.83% for each swarm |
| [77] | Mastology Research -Dataset | ROI ANN | Accuracy is 90.2% Sn value is 89.34% Sp value is 91% |
| [78] | Mastology Research Dataset | CNN | Accuracy is 98.95% |
| [86] | Mastology Research Dataset | DWAN | Sn value: 0.95 |
| [87] | 63 thermographic (35 normal and 28 abnormal) | CNN TRF MLP BN | CNN presents better results than TRF, MLP, and BN and the accuracy between (80–100% for CNN) |
| [88] | DMR-IR | ANN SVM | SVM sensitivity of 76% and specificity of 84% ANN sensitivity of 92% and specificity of 88% |
| [79] | Images of approximately 150 patients, either with or without breast cancer, totaling over 1000 (Kaggle available) | CNN SVM Random forest | The accuracy that CNN acquired was 99.67% SVM was 89.84% The accuracy that RF obtained was 90.55% |
| [80] | DMR_IR | CNN U_NET | Accuracy = 99.33% Sensitivity = 100% Specificity = 98.67% |
5. Discussion
5.1. Datasets
5.2. Results
- ▪
- It is clear from earlier studies that researchers put a lot of effort into applying various machine learning models, including Support Vector Machine Learning (SVM), Probability Neural Networks (PNN), and K-Nearest Neighbors (KNN). They ran the models on various medical images then compared the outcomes.
- ▪
- Researchers have proposed convolutional neural networks (CNNs) for early breast cancer detection. Various CNN architectures, including -Resnet18, Resnet34, -Resnet50, -Resnet152, -vgg16, and vgg19, have been used, along with each architecture’s median and interquartile range. The best outcomes were from the resnet34 and resnet50 convolutional neural network designs, with 100% predicted accuracy in blind validation.
- ▪
- ML and DL methods for breast cancer still have significant limits and challenges that need to be addressed despite the positive findings of the reviewed literature.
- ▪
- These approaches offer outstanding outcomes in early breast cancer diagnosis and categorization. And as a result of the review, several important issues were found.
5.3. Challenges
- ▪
- DL needs considerable training data because the data set’s size and quality significantly impact the classifier’s effectiveness. But a lack of data is one of the biggest obstacles to using DL in medical imaging. Generating significant amounts of medical imaging data is challenging because eliminating human error takes a great deal of work from experts and one person. Large medical imaging data sets are difficult to construct because annotating the data takes a great deal of time and effort from a single expert and many experts to eliminate human error. The absence of substantial training datasets has made it challenging to construct deep-learning models for medical imaging, which was the first problem we saw in our studies. Most reviewed studies evaluated and assessed these using various datasets that cancer research organizations or clinics privately collected. The main issue with this method is that it is impossible to compare how well such models function across several investigations.
- ▪
- The absence of benchmarks provided a hurdle and highlighted a lack of flexibility.
- ▪
- Another issue with specific papers is using data expansion techniques rather than transferring learning to minimize overfitting.
- ▪
- Techniques for breast cancer categorization using unsupervised grouping: The supervised learning method was used to classify breast cancer in most of the selected primary papers. These strategies have provided superior results when labeled images are used throughout the training. However, finding breast cancer images with precise, medically labeled criteria might be difficult. There are frequently many unidentified medical images available. Despite being useful knowledge sources, many blank labels cannot be used for supervised learning. Therefore, there is a pressing need for a breast cancer categorization model that may be created using several grouping techniques without supervision.
- ▪
- Methodology of reinforcement learning for breast cancer classification: The fundamental issue is a lack of sufficient breast cancer image examples to depict all types of breast cancer. Creating a machine learning model that simultaneously learns from its surroundings can be difficult. Therefore, systems for identifying breast cancer from medical photos can perform and be more effective when employing a learning-based reinforcement model.
- ▪
- Reliability of data collection techniques: The robustness issue of various clinical and technical circumstances must be addressed to integrate new datasets gradually. Different image acquisition scanners, lighting configurations, sizes, and views across many picture modalities, and varying presentation aspects of the coloring and enlargement factors, are a few examples of these modifications.
- ▪
- Despite its significance in medical picture segmentation, the segmentation’s influence still falls short of what is required for practical use.
5.4. Future
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arevalo, J.; Gonzalez, F.A.; Ramos-Pollan, R.; Oliveira, J.L.; Guevara Lopez, M.A. Representation learning for mammography mass lesion classification with convolutional neural networks. Comput. Methods Programs Biomed. 2016, 127, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Roslidar, R.; Rahman, A.; Muharar, R.; Syahputra, M.R.; Arnia, F.; Syukri, M.; Pradhan, B.; Munadi, K. A Review on Recent Progress in Thermal Imaging and Deep Learning Approaches for Breast Cancer Detection. IEEE Access 2020, 8, 116176–116194. [Google Scholar] [CrossRef]
- Lancet, T. Breastfeeding: Achieving the new normal. Lancet 2016, 387, 404. [Google Scholar] [CrossRef]
- Razzak, M.I.; Naz, S.; Zaib, A. Deep learning for medical image processing: Overview, challenges and the future. In Classification in BioApps; Springer: Cham, Switzerland, 2018; pp. 323–350. [Google Scholar]
- Erickson, B.J.; Korfiatis, P.; Akkus, Z.; Kline, T.L. Machine learning for medical imaging. Radiographics 2017, 37, 505–515. [Google Scholar] [CrossRef]
- Witten, H.; Frank, E.; Kaufmann, M.; Geller, J. Data Mining: Practical Machine Learning Tools and Techniques with Java Implementations. ACM Sigmod Rec. 2000, 31, 76–77. [Google Scholar] [CrossRef]
- Amin, R.K.; Sibaroni, Y. Implementation of Decision Tree Using C4.5 Algorithm in Decision Making of Loan Application by Debtor (Case Study:Bank Pasar of Yogyakarta Special Region). In Proceedings of the 2015 3rd International Conference on Information and Communication Technology (ICoICT), Bali, Indonesia, 27–29 May 2015. [Google Scholar]
- Lim, T.-S.; Loh, W.-Y.; Shih, Y.-S. A Comparison of Prediction Accuracy, Complexity, and Training Time of Thirty-Three Old and New Classification Algorithms. Mach. Learn. 2000, 40, 203–228. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Vatsa, M.; Singh, R.; Noore, A. Improving biometric recognition accuracy and robustness using DWT and SVM watermarking. IEICE Electron. Express 2005, 2, 362–367. [Google Scholar] [CrossRef][Green Version]
- Tharwat, A.; Hassanien, A.E.; Elnaghi, B.E. A BA-based algorithm for parameter optimization of Support Vector Machine. Pattern Recognit. Lett. 2017, 93, 13–22. [Google Scholar] [CrossRef]
- Deepak, P.A.; Chouhan, S. ICATE 2013 Paper Identification Number-102 SVM Kernel Functions for Classification. 2013. Available online: https://zero.sci-hub.se/2209/fd9e0a644e382bebb08d8cfa46399f2c/patle2013.pdf (accessed on 1 June 2023).
- Boser, B.; Denker, J.S.; Henderson, D.; Howard, R.E.; Hubbard, W.; Jackel, L.D.; Laboratories, H.Y.L.B.; Zhu, Z.; Cheng, J.; Zhao, Y.; et al. Backpropagation Applied to Handwritten Zip Code Recognition. Neural Comput. 1989, 1, 541–551. [Google Scholar]
- Shen, C. A Transdisciplinary Review of Deep Learning Research and Its Relevance for Water Resources Scientists. Water Resour. Res. 2018, 54, 8558–8593. [Google Scholar] [CrossRef]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. Imagenet classification with deep convolutional neural networks. Commun. ACM 2017, 60, 84–90. [Google Scholar] [CrossRef]
- Russakovsky, O.; Deng, J.; Su, H.; Krause, J.; Satheesh, S.; Ma, S.; Huang, Z.; Karpathy, A.; Khosla, A.; Bernstein, M.; et al. ImageNet Large Scale Visual Recognition Challenge. Int. J. Comput. Vis. 2015, 12, 211–252. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Y.; Oerlemans, A.; Lao, S.; Wu, S.; Lew, M.S. Deep learning for visual understanding: A review. Neurocomputing 2016, 187, 27–48. [Google Scholar] [CrossRef]
- Simonyan, K.; Zisserman, A. Very Deep Convolutional Networks for Large-Scale Image Recognition. arXiv 2014, arXiv:1409.1556. [Google Scholar]
- Szegedy, C.; Liu, W.; Jia, Y.; Sermanet, P.; Reed, S.; Anguelov, D.; Erhan, D.; Vanhoucke, V.; Rabinovich, A.; Liu, W.; et al. Going deeper with convolutions. In Proceedings of the 2015 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Boston, MA, USA, 7–12 June 2015. [Google Scholar]
- Szegedy, C.; Vanhoucke, V.; Ioffe, S.; Shlens, J.; Wojna, Z. Rethinking the Inception Architecture for Computer Vision. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016; pp. 2818–2826. [Google Scholar]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the IEEE Computer Society Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar]
- Chollet, F. Xception: Deep learning with depthwise separable convolutions. In Proceedings of the 2017 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Honolulu, HI, USA, 21–26 July 2017. [Google Scholar]
- Szegedy, C.; Ioffe, S.; Vanhoucke, V.; Alemi, A. Inception-v4, Inception-ResNet and the Impact of Residual Connections on Learning. arXiv 2017, arXiv:160207261v2. [Google Scholar] [CrossRef]
- Xie, S.; Girshick, R.; Dollár, P.; Tu, Z.; He, K. Aggregated Residual Transformations for Deep Neural Networks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Honolulu, HI, USA, 21–26 July 2017; pp. 5987–5995. [Google Scholar]
- Wang, F.; Tax, D.M.J. Survey on the attention based RNN model and its applications in computer vision. arXiv 2016, arXiv:1601.06823. [Google Scholar]
- Mechria, H.; Gouider, M.; Hassine, S.K. Breast cancer detection using deep convolutional neural network. In Proceedings of the ICAART 2019—Proceedings of the 11th International Conference on Agents and Artificial Intelligence, Prague, Czech Republic, 19–21 February 2019; Volume 2, pp. 655–660. [Google Scholar]
- Shen, L.; Margolies, L.R.; Rothstein, J.H.; Fluder, E.; McBride, R.; Sieh, W. Deep Learning to Improve Breast Cancer Detection on Screening Mammography. Sci. Rep. 2019, 9, 12495. [Google Scholar] [CrossRef]
- Hossam, A.; Harb, H.M.; Abd, H.M.; Kader, E. Automatic Image Segmentation Method for Breast Cancer Analysis Using Thermography. JES J. Eng. Sci. 2018, 46, 12–32. [Google Scholar] [CrossRef]
- Robert, M.; Miller, J. About Cancer. Available online: http://www.aboutcancer.com/breast_pet_scans.htm (accessed on 17 July 2023).
- Vijayarajeswari, R.; Parthasarathy, P.; Vivekanandan, S.; Basha, A.A. Classification of mammogram for early detection of breast cancer using SVM classifier and Hough transform. Measurement 2019, 146, 800–805. [Google Scholar] [CrossRef]
- Zemmal, N.; Azizi, N.; Sellami, M. CAD system for classification of mammographic abnormalities using transductive semi supervised learning algorithm and heterogeneous features. In Proceedings of the 2015 12th International Symposium on Programming and Systems (ISPS), Algiers, Algeria, 28–30 April 2015; pp. 1–9. [Google Scholar]
- Anitha, J.; Dinesh, P.J. A wavelet based morphological mass detection and classification in mammograms. In Proceedings of the 2012 International Conference on Machine Vision and Image Processing (MVIP), Tamil Nadu, India, 14–15 December 2012; pp. 25–28. [Google Scholar]
- Zheng, B.; Yoon, S.W.; Lam, S.S. Breast cancer diagnosis based on feature extraction using a hybrid of K-means and support vector machine algorithms. Expert Syst. Appl. 2014, 41, 1476–1482. [Google Scholar] [CrossRef]
- Wichakan, I.; Vateekul, P. Combining Deep Convolutional Networks SVMs for Mass Detection on Digital Mammograms. In Proceedings of the 2016 8th International Conference on Knowledge and Smart Technology (KST), Chiang Mai, Thailand, 3–6 February 2016; pp. 239–244. [Google Scholar]
- Li, H.; Zhuang, S.; Li, D.-A.; Zhao, J.; Ma, Y. Benign and malignant classification of mammogram images based on deep learning. Biomed. Signal Process. Control. 2019, 51, 347–354. [Google Scholar] [CrossRef]
- Abdel-Nasser, M.; Rashwan, H.A.; Puig, D.; Moreno, A. Analysis of Tissue Abnormality and Breast Density in Mammographic Images Using a Uniform Local Directional Pattern; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Spanhol, F.A.; Oliveira, L.S.; Petitjean, C.; Heutte, L. A dataset for breast cancer histopathological image classification. IEEE Trans. Biomed. Eng. 2016, 63, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Wajid, S.K.; Hussain, A. Local energy-based shape histogram feature extraction technique for breast cancer diagnosis. Expert Syst. Appl. 2015, 42, 6990–6999. [Google Scholar] [CrossRef]
- Phadke, A.C.; Rege, P.P. Fusion of local and global features for classification of abnormality in mammograms. Sādhanā 2016, 41, 385–395. [Google Scholar] [CrossRef]
- Khan, S.; Hussain, M.; Aboalsamh, H.; Bebis, G. A comparison of different Gabor feature extraction approaches for mass classification in mammography. Multimed. Tools Appl. 2017, 76, 33–57. [Google Scholar] [CrossRef]
- Pratiwi, M.; Alexander; Harefa, J.; Nanda, S. Mammograms Classification Using Gray-level Co-occurrence Matrix and Radial Basis Function Neural Network. Proc. Comput. Sci. 2015, 59, 83–91. [Google Scholar] [CrossRef]
- Hamoud, M.; Merouani, H.F.; Laimeche, L. The power laws: Zipf and inverse Zipf for automated segmentation and classification of masses within mammograms. Evol. Syst. 2015, 6, 209–227. [Google Scholar] [CrossRef]
- Abubacker, N.F.; Azman, A.; Doraisamy, S.; Murad, M.A.A. An integrated method of associative classification and neuro-fuzzy approach for effective mammographic classification. Neural Comput. Appl. 2017, 28, 3967–3980. [Google Scholar] [CrossRef]
- Gardezi, S.J.S.; Faye, I.; Bornot, J.M.S.; Kamel, N.; Hussain, M. Mammogram classification using dynamic time warping. Multimed. Tools Appl. 2018, 77, 3941–3962. [Google Scholar] [CrossRef]
- Rouhi, R.; Jafari, M. Classification of benign and malignant breast tumors based on hybrid level set segmentation. Expert Syst. Appl. 2016, 46, 45–59. [Google Scholar] [CrossRef]
- Peng, W.; Mayorga, R.; Hussein, E. An automated confirmatory system for analysis of mammograms. Comput. Methods Programs Biomed. 2016, 125, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Hai, J.; Tan, H.; Chen, J.; Wu, M.; Qiao, K.; Xu, J.; Zeng, L.; Gao, F.; Shi, D.; Yan, B. Multi-level features combined end-to-end learning for automated pathological grading of breast cancer on digital mammograms. Comput. Med. Imaging Graph. 2019, 71, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Saber, A.; Sakr, M.; Abo-Seida, O.M.; Keshk, A.; Chen, H. A Novel Deep-Learning Model for Automatic Detection and Classification of Breast Cancer Using the Transfer-Learning Technique. IEEE Access 2021, 9, 71194–71209. [Google Scholar] [CrossRef]
- Dhahbi, S.; Barhoumi, W.; Zagrouba, E. Breast cancer diagnosis in digitized mammograms using curvelet moments. Comput. Biol. Med. 2015, 64, 79–90. [Google Scholar] [CrossRef]
- Aminikhanghahi, S.; Shin, S.; Wang, W.; Jeon, S.I.; Son, S.H. A new fuzzy Gaussian mixture model (FGMM) based algorithm for mammography tumor image classification. Multimed. Tools Appl. 2017, 76, 10191–10205. [Google Scholar] [CrossRef]
- Khan, S.U.; Islam, N.; Jan, Z.; Ud Din, I.; Rodrigues, J.J. A novel deep learning based framework for the detection and classification of breast cancer using transfer learning. Pattern Recognit. Lett. 2019, 125, 1–6. [Google Scholar] [CrossRef]
- Liu, X.; Mei, M.; Liu, J.; Hu, W. Microcalcification detection in full-field digital mammograms with PFCM clustering and weighted SVM-based method. EURASIP J. Adv. Signal Process. 2015, 2015, 73. [Google Scholar] [CrossRef]
- Tan, M.; Qian, W.; Pu, J.; Liu, H.; Zheng, B. A new approach to develop computer-aided detection schemes of digital mammograms. Phys. Med. Biol. 2015, 60, 4413–4427. [Google Scholar] [CrossRef]
- Suzuki, S.; Zhang, X.; Homma, N.; Ichiji, K.; Sugita, N.; Kawasumi, Y.; Ishibashi, T.; Yoshizawa, M. Mass detection using deep convolutional neural networks for mammoghraphic computer-aided diagnosis. In Proceedings of the 55th Annual Conference of the Society of Intruments and Control Engineers of Japan (SICE), Tsukuba, Japan, 20–23 September 2016; pp. 1382–1386. [Google Scholar]
- Jasti, V.D.P.; Zamani, A.S.; Arumugam, K.; Naved, M.; Pallathadka, H.; Sammy, F.; Raghuvanshi, A.; Kaliyaperumal, K. Computational Technique Based on Machine Learning and Image Processing for Medical Image Analysis of Breast Cancer Diagnosis. Secur. Commun. Netw. 2022, 2022, 1918379. [Google Scholar] [CrossRef]
- Ranjbarzadeh, R.; Tataei Sarshar, N.; Jafarzadeh Ghoushchi, S.; Saleh Esfahani, M.; Parhizkar, M.; Pourasad, Y.; Anari, S.; Bendechache, M. MRFE-CNN: Multi-route feature extraction model for breast tumor segmentation in Mammograms using a convolutional neural network. Ann. Oper. Res. 2022, 11, 1–22. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, Y.; Yan, S.; Tan, M.; Cheng, S.; Liu, H.; Zheng, B. An initial investigation on developing a new method to predict short-term breast cancer risk based on deep learning technology. In Medical Imaging 2016: Computer-Aided Diagnosis; SPIE: Cergy, France, 2016; Volume 9785, p. 978521. [Google Scholar]
- Cai, L.; Wang, X.; Wang, Y.; Guo, Y.; Yu, J.; Wang, Y. Robust phase-based texture descriptor for classification of breast ultrasound images. Biomed. Eng. Online 2015, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Alam, S.K.; Garra, B.; Zhang, Y.; Ahmed, T. Computer-Aided Diagnosis for Breast Ultrasound Using Computerized BI-RADS Features and Machine Learning Methods. Ultrasound Med. Biol. 2016, 42, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhang, L.; Chen, Y.; Pi, Y.; Chen, Y.; Lv, Q.; Yi, Z. Automated diagnosis of breast ultrasonography images using deep neural networks. Med. Image Anal. 2019, 52, 185–198. [Google Scholar] [CrossRef]
- Byra, M.; Galperin, M.; Ojeda-Fournier, H.; Olson, L.; O’Boyle, M.; Comstock, C.; Andre, M. Breast mass classification in sonography with transfer learning using a deep convolutional neural network and color conversion. Med. Phys. 2019, 46, 746–755. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Yuan, J.; Cheng, Q.; Wang, X.; Carson, P.L. Medical breast ultrasound image segmentation by machine learning. Ultrasonics 2019, 91, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yap, M.H.; Pons, G.; Marti, J.; Ganau, S.; Sentis, M.; Zwiggelaar, R.; Davison, A.K.; Marti, R. Automated Breast Ultrasound Lesions Detection Using Convolutional Neural Networks. IEEE J. Biomed. Health Inform. 2018, 22, 1218–1226. [Google Scholar] [CrossRef]
- Huang, Q.; Yang, F.; Liu, L.; Li, X. Automatic segmentation of breast lesions for interaction in ultrasonic computer-aided diagnosis. Inf. Sci. 2015, 314, 293–310. [Google Scholar] [CrossRef]
- Moon, W.K.; Huang, Y.-S.; Lo, C.-M.; Huang, C.-S.; Bae, M.S.; Kim, W.H.; Chen, J.-H.; Chang, R.-F. Computer-aided diagnosis for distinguishing between triple-negative breast cancer and fibroadenomas based on ultrasound texture features. Med. Phys. 2015, 42, 3024–3035. [Google Scholar] [CrossRef]
- Prabusankarlal, K.M.; Thirumoorthy, P.; Manavalan, R. Assessment of combined textural and morphological features for diagnosis of breast masses in ultrasound. Hum.-Centric Comput. Inf. Sci. 2015, 5, 12. [Google Scholar] [CrossRef]
- Han, S.; Kang, H.-K.; Jeong, J.-Y.; Park, M.-H.; Kim, W.; Bang, W.-C.; Seong, Y.-K. A deep learning framework for supporting the classification of breast lesions in ultrasound images. Phys. Med. Biol. 2017, 62, 7714–7728. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.K.; Saxena, S.; Lakshmanan, K.; Sangaiah, A.K.; Chauhan, H.; Shrivastava, S.; Singh, R.K. Deep feature learning for histopathological image classification of canine mammary tumors and human breast cancer. Inf. Sci. 2020, 508, 405–421. [Google Scholar] [CrossRef]
- Chmielewski, A.; Dufort, P.; Scaranelo, A.M. A Computerized System to Assess Axillary Lymph Node Malignancy from Sonographic Images. Ultrasound Med. Biol. 2015, 41, 2690–2699. [Google Scholar] [CrossRef]
- Shibusawa, M.; Nakayama, R.; Okanami, Y.; Kashikura, Y.; Imai, N.; Nakamura, T.; Kimura, H.; Yamashita, M.; Hanamura, N.; Ogawa, T. The usefulness of a computer-aided diagnosis scheme for improving the performance of clinicians to diagnose non-mass lesions on breast ultrasonographic images. J. Med. Ultrason. 2016, 43, 387–394. [Google Scholar] [CrossRef]
- Lo, C.-M.; Chan, S.-W.; Yang, Y.-W.; Chang, Y.-C.; Huang, C.-S.; Jou, Y.-S.; Chang, R.-F. Feasibility Testing: Three-dimensional Tumor Mapping in Different Orientations of Automated Breast Ultrasound. Ultrasound Med. Biol. 2016, 42, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Nasser, M.; Melendez, J.; Moreno, A.; Omer, O.A.; Puig, D. Breast tumor classification in ultrasound images using texture analysis and super-resolution methods. Eng. Appl. Artif. Intell. 2017, 59, 84–92. [Google Scholar] [CrossRef]
- Moon, W.K.; Chen, I.-L.; Chang, J.M.; Shin, S.U.; Lo, C.-M.; Chang, R.-F. The adaptive computer-aided diagnosis system based on tumor sizes for the classification of breast tumors detected at screening ultrasound. Ultrasonics 2017, 76, 70–77. [Google Scholar] [CrossRef]
- Ali, M.A.; Sayed, G.I.; Gaber, T.; Hassanien, A.E.; Snasel, V.; Silva, L.F. Detection of breast abnormalities of thermograms based on a new segmentation method. In Proceedings of the Federated Conference on Computer Science and Information Systems (FedCSIS), Lodz, Poland, 13–16 September 2015; pp. 255–261. [Google Scholar]
- Pramanik, S.; Bhattacharjee, D.; Nasipuri, M. Wavelet based thermogram analysis for breast cancer detection. In Proceedings of the 2015 International Symposium on Advanced Computing and Communication (ISACC), Silchar, India, 14–15 September 2015; pp. 205–212. [Google Scholar]
- Santana, M.; Pereira, J.; Silva, F.; Lima, N.; Sousa, F.; Arruda, G.; Lima, R.; Silva, W.; Santos, W. Breast cancer diagnosis based on mammary thermography and extreme learning machines. Int. J. Artif. Intell. Mach. Learn. 2018, 34, 45–53. [Google Scholar] [CrossRef]
- Sánchez-Ruiz, D.; Olmos-Pineda, I.; Olvera-López, J.A. Automatic region of interest segmentation for breast thermogram image classification. Pattern Recognit. Lett. 2020, 135, 72–81. [Google Scholar] [CrossRef]
- Ekici, S.; Jawzal, H. Breast cancer diagnosis using thermography and convolutional neural networks. Med. Hypotheses 2020, 137, 109542. [Google Scholar] [CrossRef]
- Reddy, A.V. Breast cancer detection based on thermographic images using machine learning and deep learning algorithms Healthcare View project Breast cancer detection based on thermographic images using machine learning and deep learning algorithms. Comput. Sci. 2022, 4, 49–56. [Google Scholar]
- Mohamed, E.A.; Rashed, E.A.; Gaber, T.; Karam, O. Deep learning model for fully automated breast cancer detection system from thermograms. PLoS ONE 2022, 17, e0262349. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ovies, F.; Alférez, S.; DeAndrés-Galiana, E.J.; Cernea, A.; Fernández-Muñiz, Z.; Fernández-Martínez, J.L. Detection of Breast Cancer Using Infrared Thermography and Deep Neural Networks. In Bioinformatics and Biomedical Engineering; Rojas, I., Valenzuela, O., Rojas, F., Ortuño, F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 514–523. [Google Scholar]
- Mookiaha, M.R.K.; Acharyaa, U.R.; Ng, E.Y. Data mining technique for breast cancer detection in thermograms using hybrid feature extraction strategy. Quant. InfraRed Thermogr. J. 2012, 9, 151–165. [Google Scholar] [CrossRef]
- Milosevic, M.; Jankovic, D.; Peulic, A. Thermography based breast cancer detection using texture features and minimum variance quantization. EXCLI J. 2014, 13, 1204. [Google Scholar] [PubMed]
- Gaber, T.; Ismail, G.; Anter, A.; Soliman, M.; Ali, M.; Semary, N.; Hassanien, A.E.; Snasel, V. Thermogram breast cancer prediction approach based on Neutrosophic sets and fuzzy c-means algorithm. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 4254–4257. [Google Scholar]
- Sayed, G.I.; Soliman, M.; Hassanien, A.E. Bio-Inspired Swarm Techniques for Thermogram Breast Cancer Detection; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- De Freitas Barbosa, V.A.; de Santana, M.A.; Andrade, M.K.S.; de Lima, R.d.C.F.; dos Santos, W.P. Deep-wavelet neural networks for breast cancer early diagnosis using mammary termographies. In Deep Learning for Data Analytics; Elsevier: Hoboken, NJ, USA, 2020; pp. 99–124. [Google Scholar]
- Tello-Mijares, S.; Woo, F.; Flores, F. Breast Cancer Identification via Thermography Image Segmentation with a Gradient Vector Flow and a Convolutional Neural Network. J. Health Eng. 2019, 2019, 9807619. [Google Scholar] [CrossRef]
- Al Husaini, M.A.S.; Habaebi, M.H.; Gunawan, T.S.; Islam, M.R.; Elsheikh, E.A.A.; Suliman, F.M. Thermal-based early breast cancer detection using inception V3, inception V4 and modified inception MV4. Neural Comput. Appl. 2022, 34, 333–348. [Google Scholar] [CrossRef]


| Case | Number of Images |
|---|---|
| Benign | 487 |
| Malignant | 210 |
| Normal | 133 |
| Total | 780 |
| References | Dataset | ML Method | Performance Evaluation |
|---|---|---|---|
| [58] | 138 private cases | SVM classifier | Accuracy, Sn value, and Sp value all 86.9% AUC value is 0.89 |
| [66] | 120 private images -(benign 70) -(malignant 50) | SVM classifier | Accuracy is 95.85% Sn value is 96.0% Sp value is 91.5% The AUC value is 0.94 |
| [69] | 105 private images | SVM classifier | Sn value is 95% Sp value is 90% AUC value is 95% |
| [65] | 169 private cases | SVM classifier | Accuracy is 94.8% Sn value is 94.1% Sp value is 96.7% |
| [64] | 46 private Images | SVM classifier | Accuracy is 0.98 ± 0.013 Sn value is 0.97 ± 0.035 Sp value is 0.98 ± 0.019 AUC value is 0.997 ± 0.003 |
| [70] | 97 private images | K-NN | Sn value is 87.8% Sp value is 89.5% AUC value is 0.93 |
| [71] | 18 private cases | Binary-LR | Accuracy is 80.4% |
| [72] | 59 private images | RF | AUC value is 99% |
| [73] | 156 owned cases | LR ANN | Accuracy is 81.8% Sn Value is 85.4% Sp Value is 77.8% AUC value is 0.855 |
| [59] | 283 owned cases | DT KNN RF SVM | SVM accuracy is 77.7% AUC Value is 0.84 RF accuracy is 78.5% AUC value is 0.83 |
| [67] | 7408 | CNN based on VGG19 | Accuracy value is 91.2% TP value is 84.3% TN value is 96.1% AUC value is 96.0% |
| [61] | 882 | CNN based on VGG19 | Acc value is 88.7% TP value is 84.8% TN value is 89.7% AUC value is 93.6% |
| [63] | 306 | FCN–AlexNet | TP value is 98% |
| [68] | 433 | U-Net | TP value is 84% |
| Dataset | Image Type | URL |
|---|---|---|
| MIAS | mammogram | https://www.repository.cam.ac.uk/handle/1810/250394 (accessed on 1 June 2023) |
| DDSM | mammogram | http://marathon.csee.usf.edu/Mammography/Database.html (accessed on 1 June 2023) |
| mini-MIAS | mammogram | http://peipa.essex.ac.uk/info/mias.html (accessed on 1 June 2023) |
| Break-His | -histological | https://web.inf.ufpr.br/vri/databases/breastcancer-histopathological-databasebreakhis/ (accessed on 1 June 2023) |
| DMR-IR | Thermography | http://visual.ic.uff.br/dmi (accessed on 1 June 2023) |
| BI-RADS | mammogram | https://radiopaedia.org/articles/breast-imaging-reporting-and-data-system-bi-rads (accessed on 1 June 2023) |
| INbreast | mammogram | http://dx.doi.org/10.17632/x7bvzv6cvr.1 (accessed on 1 June 2023) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalloul, R.; Chethan, H.K.; Alkhatib, R. A Review of Machine Learning Techniques for the Classification and Detection of Breast Cancer from Medical Images. Diagnostics 2023, 13, 2460. https://doi.org/10.3390/diagnostics13142460
Jalloul R, Chethan HK, Alkhatib R. A Review of Machine Learning Techniques for the Classification and Detection of Breast Cancer from Medical Images. Diagnostics. 2023; 13(14):2460. https://doi.org/10.3390/diagnostics13142460
Chicago/Turabian StyleJalloul, Reem, H. K. Chethan, and Ramez Alkhatib. 2023. "A Review of Machine Learning Techniques for the Classification and Detection of Breast Cancer from Medical Images" Diagnostics 13, no. 14: 2460. https://doi.org/10.3390/diagnostics13142460
APA StyleJalloul, R., Chethan, H. K., & Alkhatib, R. (2023). A Review of Machine Learning Techniques for the Classification and Detection of Breast Cancer from Medical Images. Diagnostics, 13(14), 2460. https://doi.org/10.3390/diagnostics13142460







