Early Optical Coherence Tomography Biomarkers for Selected Retinal Diseases—A Review
Abstract
1. Introduction
2. Materials and Methods
3. Age-Related Macular Degeneration
3.1. Drusen Height
3.2. Drusen Volume
3.3. RPE Changes
3.4. Geographic Atrophy
3.5. Choriocapillaris
3.6. Predicting the Type of MNV in the Fellow-Eye
4. Idiopathic Macular Telangiectasia
5. Drug-Induced Maculopathies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Cachulo, L.; Silva, R.; Fonseca, P.; Pires, I.; Carvajal-Gonzalez, S.; Bernardes, R.; Cunha-Vaz, J.G. Early markers of choroidal neovascularization in the fellow eye of patients with unilateral exudative age-related macular degeneration. Ophthalmologica 2011, 225, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Aumann, S.; Donner, S.; Fischer, J.; Müller, F. Optical Coherence Tomography (OCT): Principle and Technical Realization. In High Resolution Imaging in Microscopy and Ophthalmology New Frontiers in Biomedical Optics; Bille, J.F., Ed.; Springer: Cham, Switzerland, 2019; pp. 59–85. [Google Scholar]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef] [PubMed]
- Leitgeb, R.; Hitzenberger, C.; Fercher, A. Performance of Fourier domain vs. time domain optical coherence tomography. Opt. Express 2003, 11, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Sull, A.C.; Vuong, L.N.; Price, L.L.; Srinivasan, V.J.; Gorczynska, I.; Fujimoto, J.G.; Schuman, J.S.; Duker, J.S. Comparison of spectral/Fourier domain optical coherence tomography instruments for assessment of normal macular thickness. Retina 2010, 30, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.R.; Chhablani, J. Optical Coherence Tomography Imaging: Advances in Ophthalmology. J. Clin. Med. 2022, 11, 2858. [Google Scholar] [CrossRef]
- Yun, S.H.; Tearney, G.J.; Bouma, E.B.; Park, B.H.; de Boer, J.F. High-speed spectral-domain optical coherence tomography at 1.3 µm wavelength. Opt. Express 2003, 11, 3598–3604. [Google Scholar] [CrossRef]
- Emre, S.; Ulusoy, M.O. Optical coherence tomography angiography findings of the fellow eye of patients with unilateral neovascular age-related macular degeneration OCT-A Evaluation of Fellow Eyes of CNV. Rom. J. Ophthalmol. 2019, 63, 231–237. [Google Scholar] [CrossRef]
- Harada, N.; Nagai, N.; Mushiga, Y.; Ozawa, Y. Choriocapillaris Flow Imbalance in Fellow Eyes in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2022, 63, 13. [Google Scholar] [CrossRef]
- Rozing, M.P.; Durhuus, J.A.; Krogh Nielsen, M.; Subhi, Y.; Kirkwood, T.B.; Westendorp, R.G.; Sørensen, T.L. Age-related macular degeneration: A two-level model hypothesis. Prog. Retin. Eye Res. 2020, 76, 100825. [Google Scholar] [CrossRef]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Minami, S.; Suzuki, M.; Shinoda, H.; Kurihara, T.; Sonobe, H.; Watanabe, K.; Uchida, A.; Ban, N.; Tsubota, K.; et al. Macular Pigment Optical Density and Photoreceptor Outer Segment Length as Predisease Biomarkers for Age-Related Macular Degeneration. J. Clin. Med. 2020, 9, 1347. [Google Scholar] [CrossRef] [PubMed]
- Lamin, A.; El Nokrashy, A.; Chandra, S.; Sivaprasad, S. Association of Longitudinal Changes in Drusen Characteristics and Retinal Layer Volumes with Subsequent Subtype of Choroidal Neovascularisation. Ophthalmic Res. 2020, 63, 375–382. [Google Scholar] [CrossRef]
- Spaide, R.F.; Jaffe, G.J.; Sarraf, D.; Freund, K.B.; Sadda, S.R.; Staurenghi, G.; Waheed, N.K.; Chakravarthy, U.; Rosenfeld, P.J.; Holz, F.G.; et al. Consensus Nomenclature for Reporting Neovascular Age-Related Macular Degeneration Data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group. Ophthalmology 2020, 127, 616–636. [Google Scholar] [CrossRef] [PubMed]
- Sacconi, R.; Fragiotta, S.; Sarraf, D.; Sadda, S.R.; Freund, K.B.; Parravano, M.; Corradetti, G.; Cabral, D.; Capuano, V.; Miere, A.; et al. Towards a better understanding of non-exudative choroidal and macular neovascularization. Prog. Retin. Eye Res. 2023, 92, 101113. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Parachuri, N.; Kumar, N.; Bandello, F.; Kuppermann, B.D.; Loewenstein, A.; Regillo, C.D.; Chakravarthy, U. Terms non-exudative and non-neovascular: Awaiting entry at the doors of AMD reclassification. Graefe’s Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Fur Klin. Und Exp. Ophthalmol. 2021, 259, 1381–1383. [Google Scholar] [CrossRef]
- Fasler, K.; Fu, D.J.; Moraes, G.; Wagner, S.; Gokhale, E.; Kortuem, K.; Chopra, R.; Faes, L.; Preston, G.; Pontikos, N.; et al. Moorfields AMD database report 2: Fellow eye involvement with neovascular age-related macular degeneration. Br. J. Ophthalmol. 2020, 104, 684–690. [Google Scholar] [CrossRef]
- Amissah-Arthur, K.N.; Panneerselvam, S.; Narendran, N.; Yang, Y.C. Optical coherence tomography changes before the development of choroidal neovascularization in second eyes of patients with bilateral wet macular degeneration. Eye 2012, 26, 394–399. [Google Scholar] [CrossRef]
- Bek, T.; Klug, S.E. Incidence and risk factors for neovascular age-related macular degeneration in the fellow eye. Graefe’s Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Fur Klin. Und Exp. Ophthalmol. 2018, 256, 2061–2068. [Google Scholar] [CrossRef]
- Schick, T.; Ersoy, L.; Hoyng, C.B.; Kirchhof, B.; Liakopoulos, S. Phenotype Characteristics of Fellow Eyes in Patients With Early Onset of Neovascular Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7269–7273. [Google Scholar] [CrossRef]
- Lövestam Adrian, M.; Schroeder, M.; Westborg, I. What about the fellow eye in treatment of neovascular age-related macular degeneration? Analysis of data from the Swedish macula register. Acta Ophthalmol. 2022, 100, 769–774. [Google Scholar] [CrossRef]
- Wong, T.Y.; Lanzetta, P.; Bandello, F.; Eldem, B.; Navarro, R.; Lövestam-Adrian, M.; Loewenstein, A. Current concepts and modalities for monitoring the fellow eye in neovascular age-related macular degeneration: An Expert Panel Consensus. Retina 2020, 40, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Hilely, A.; Au, A.; Freund, K.B.; Loewenstein, A.; Souied, E.H.; Zur, D.; Sacconi, R.; Borrelli, E.; Peiretti, E.; Iovino, C.; et al. Non-neovascular age-related macular degeneration with subretinal fluid. Br. J. Ophthalmol. 2021, 105, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Au, A.; Santina, A.; Abraham, N.; Levin, M.F.; Corradetti, G.; Sadda, S.; Sarraf, D. Relationship Between Drusen Height and OCT Biomarkers of Atrophy in Non-Neovascular AMD. Investig. Ophthalmol. Vis. Sci. 2022, 63, 24. [Google Scholar] [CrossRef]
- Abdelfattah, N.S.; Zhang, H.; Boyer, D.S.; Rosenfeld, P.J.; Feuer, W.J.; Gregori, G.; Sadda, S.R. Drusen Volume as a Predictor of Disease Progression in Patients With Late Age-Related Macular Degeneration in the Fellow Eye. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1839–1846. [Google Scholar] [CrossRef]
- Lamin, A.; Dubis, A.M.; Sivaprasad, S. Changes in macular drusen parameters preceding the development of neovascular age-related macular degeneration. Eye 2019, 33, 910–916. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Chakravarthy, U.; Freund, K.B.; Guymer, R.H.; Holz, F.G.; Liakopoulos, S.; Monés, J.M.; Rosenfeld, P.J.; Sadda, S.R.; Sarraf, D.; et al. Imaging Features Associated with Progression to Geographic Atrophy in Age-Related Macular Degeneration: Classification of Atrophy Meeting Report 5. Ophthalmology. Retina 2021, 5, 855–867. [Google Scholar] [PubMed]
- Trinh, M.; Kalloniatis, M.; Nivison-Smith, L. Vascular Changes in Intermediate Age-Related Macular Degeneration Quantified Using Optical Coherence Tomography Angiography. Transl. Vis. Sci. Technol. 2019, 8, 20. [Google Scholar] [CrossRef]
- Menteş, J.; Yıldırım, Ş. Optical Coherence Tomography Characteristics of Quiescent Type 1 Neovascularization in Eyes with Nonexudative Age-related Macular Degeneration. Turk. J. Ophthalmol. 2019, 49, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Motulsky, E.H.; Goldhardt, R.; Zohar, Y.; Thulliez, M.; Feuer, W.; Gregori, G.; Rosenfeld, P.J. Predictive Value of the OCT Double-Layer Sign for Identifying Subclinical Neovascularization in Age-Related Macular Degeneration. Ophthalmol. Retin. 2019, 3, 211–219. [Google Scholar] [CrossRef]
- de Oliveira Dias, J.R.; Zhang, Q.; Garcia, J.M.B.; Zheng, F.; Motulsky, E.H.; Roisman, L.; Miller, A.; Chen, C.L.; Kubach, S.; de Sisternes, L.; et al. Natural History of Subclinical Neovascularization in Nonexudative Age-Related Macular Degeneration Using Swept-Source OCT Angiography. Ophthalmology 2018, 125, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Nassisi, M.; Lei, J.; Abdelfattah, N.S.; Karamat, A.; Balasubramanian, S.; Fan, W.; Uji, A.; Marion, K.M.; Baker, K.; Huang, X.; et al. OCT Risk Factors for Development of Late Age-Related Macular Degeneration in the Fellow Eyes of Patients Enrolled in the HARBOR Study. Ophthalmology 2019, 126, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Yoon, E.G.; Nam, K.T.; Yun, C. Chorioretinal thickness and retinal pigment epithelial degeneration of fellow eyes in patients with unilateral neovascular age-related macular degeneration with subretinal drusenoid deposits. BMC Ophthalmol. 2022, 22, 304. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.H.; Park, W.K.; Kim, R.Y.; Kim, M.; Park, Y.G.; Park, Y.H. Unaffected fellow eye neovascularization in patients with type 3 neovascularization: Incidence and risk factors. PLoS ONE 2021, 16, e0254186. [Google Scholar] [CrossRef] [PubMed]
- Toprak, I.; Yaylalı, V.; Yildirim, C. Early deterioration in ellipsoid zone in eyes with non-neovascular age-related macular degeneration. Int. Ophthalmol. 2017, 37, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Sadda, S.R.; Guymer, R.; Holz, F.G.; Schmitz-Valckenberg, S.; Curcio, C.A.; Bird, A.C.; Blodi, B.A.; Bottoni, F.; Chakravarthy, U.; Chew, E.Y.; et al. Consensus Definition for Atrophy Associated with Age-Related Macular Degeneration on OCT: Classification of Atrophy Report 3. Ophthalmology 2018, 125, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Waldstein, S.M.; Klimscha, S.; Sadeghipour, A.; Hu, X.; Gerendas, B.S.; Osborne, A.; Bogunovic, H. Prediction of Individual Disease Conversion in Early AMD Using Artificial Intelligence. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3199–3208. [Google Scholar] [CrossRef]
- Obana, A.; Hiramitsu, T.; Gohto, Y.; Ohira, A.; Mizuno, S.; Hirano, T.; Bernstein, P.S.; Fujii, H.; Iseki, K.; Tanito, M.; et al. Macular carotenoid levels of normal subjects and age-related maculopathy patients in a Japanese population. Ophthalmology 2008, 115, 147–157. [Google Scholar] [CrossRef]
- Nag, T.C.; Wadhwa, S. Ultrastructure of the human retina in aging and various pathological states. Micron 2012, 43, 759–781. [Google Scholar] [CrossRef]
- Ozawa, Y.; Shigeno, Y.; Nagai, N.; Suzuki, M.; Kurihara, T.; Minami, S.; Hirano, E.; Shinoda, H.; Kobayashi, S.; Tsubota, K. Absolute and estimated values of macular pigment optical density in young and aged Asian participants with or without age-related macular degeneration. BMC Ophthalmol. 2017, 17, 161. [Google Scholar] [CrossRef]
- Guymer, R.H.; Rosenfeld, P.J.; Curcio, C.A.; Holz, F.G.; Staurenghi, G.; Freund, K.B.; Schmitz-Valckenberg, S.; Sparrow, J.; Spaide, R.F.; Tufail, A.; et al. Incomplete Retinal Pigment Epithelial and Outer Retinal Atrophy in Age-Related Macular Degeneration: Classification of Atrophy Meeting Report 4. Ophthalmology 2020, 127, 394–409. [Google Scholar] [CrossRef]
- Curcio, C.A.; Zanzottera, E.C.; Ach, T.; Balaratnasingam, C.; Freund, K.B. Activated Retinal Pigment Epithelium, an Optical Coherence Tomography Biomarker for Progression in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO211–BIO226. [Google Scholar]
- Lutty, G.A.; McLeod, D.S.; Bhutto, I.A.; Edwards, M.M.; Seddon, J.M. Choriocapillaris dropout in early age-related macular degeneration. Exp. Eye Res. 2020, 192, 107939. [Google Scholar] [CrossRef] [PubMed]
- Chatziralli, I.; Theodossiadis, G.; Panagiotidis, D.; Pousoulidi, P.; Theodossiadis, P. Choriocapillaris Vascular Density Changes in Patients with Drusen: Cross-Sectional Study Based on Optical Coherence Tomography Angiography Findings. Ophthalmol. Ther. 2018, 7, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Ahn, J.; Yun, C. Variation of retinal and choroidal vasculatures in patients with age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5246–5255. [Google Scholar] [CrossRef]
- Toto, L.; Borrelli, E.; Di Antonio, L.; Carpineto, P.; Mastropasqua, R. Retinal vascular plexuses’ changes in dry age-related macular degeneration, evaluated by means of optical coherence tomography angiography. Retina 2016, 36, 1566–1572. [Google Scholar] [CrossRef]
- Chang, B.; Yannuzzi, L.A.; Ladas, I.D.; Guyer, D.R.; Slakter, J.S.; Sorenson, J.A. Choroidal neovascularization in second eyes of patients with unilateral exudative age-related macular degeneration. Ophthalmology 1995, 102, 1380–1386. [Google Scholar] [CrossRef]
- Sheth, J.; Anantharaman, G.; Chandra, S.; Sivaprasad, S. “Double-layer sign” on spectral domain optical coherence tomography in pachychoroid spectrum disease. Indian J. Ophthalmol. 2018, 66, 1796–1801. [Google Scholar] [CrossRef]
- Menteş, J.; Barış, M.E. Multimodal Imaging Characteristics of Quiescent Type 1 Neovascularization in Best Vitelliform Macular Dystrophy. Turk. J. Ophthalmol. 2021, 51, 188–191. [Google Scholar] [CrossRef]
- Iovino, C.; Pellegrini, M.; Bernabei, F.; Borrelli, E.; Sacconi, R.; Govetto, A.; Vagge, A.; Di Zazzo, A.; Forlini, M.; Finocchio, L.; et al. Choroidal Vascularity Index: An In-Depth Analysis of This Novel Optical Coherence Tomography Parameter. J. Clin. Med. 2020, 9, 595. [Google Scholar] [CrossRef]
- Lei, J.; Balasubramanian, S.; Abdelfattah, N.S.; Nittala, M.G.; Sadda, S.R. Proposal of a simple optical coherence tomography-based scoring system for progression of age-related macular degeneration. Graefe’s Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Fur Klin. Und Exp. Ophthalmol. 2017, 255, 1551–1558. [Google Scholar] [CrossRef]
- Nagiel, A.; Sarraf, D.; Sadda, S.R.; Spaide, R.F.; Jung, J.J.; Bhavsar, K.V.; Ameri, H.; Querques, G.; Freund, K.B. Type 3 neovascularization: Evolution, association with pigment epithelial detachment, and treatment response as revealed by spectral domain optical coherence tomography. Retina 2015, 35, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Ooto, S.; Curcio, C.A. Subretinal drusenoid deposits AKA pseudodrusen. Surv. Ophthalmol. 2018, 63, 782–815. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Daniel, E.; Maguire, M.G.; Grunwald, J.E.; Martin, E.R.; Martin, D.F.; Ying, G.S. Comparison of Age-Related Macular Degeneration Treatments Trials Research Group. Pseudodrusen and Incidence of Late Age-Related Macular Degeneration in Fellow Eyes in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2016, 123, 1530–1540. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F. Improving the age-related macular degeneration construct: A New Classification System. Retina 2018, 38, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F. Disease expression in nonexudative age-related macular degeneration varies with choroidal thickness. Retina 2018, 38, 708–716. [Google Scholar] [CrossRef]
- Jang, S.; Park, S.Y.; Ahn, S.M.; Hwang, S.Y.; Kim, S.W.; Oh, J.; Yun, C. Morphologic features of the retinal pigment epithelium and associated chorioretinal characteristics in eyes with early age-related macular degeneration and subretinal drusenoid deposits. Retina 2020, 40, 686–694. [Google Scholar] [CrossRef]
- Freund, K.B.; Ho, I.V.; Barbazetto, I.A.; Koizumi, H.; Laud, K.; Ferrara, D.; Matsumoto, Y.; Sorenson, J.A.; Yannuzzi, L. Type 3 neovascularization: The expanded spectrum of retinal angiomatous proliferation. Retina 2008, 28, 201–211. [Google Scholar] [CrossRef]
- Yannuzzi, L.A.; Negrão, S.; Iida, T.; Carvalho, C.; Rodriguez-Coleman, H.; Slakter, J.; Freund, K.B.; Sorenson, J.; Orlock, D.; Borodoker, N. Retinal angiomatous proliferation in age-related macular degeneration. Retina 2001, 21, 416–434. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.S.; Kim, J.H.; Yoo, S.J.; Lew, Y.J.; Kim, J. Fellow-eye neovascularization in unilateral retinal angiomatous proliferation in a Korean population. Acta Ophthalmol. 2016, 94, e49–e53. [Google Scholar] [CrossRef] [PubMed]
- Gass, J.D.; Oyakawa, R.T. Idiopathic juxtafoveolar retinal telangiectasis. Arch. Ophthalmol. 1982, 100, 769780. [Google Scholar] [CrossRef] [PubMed]
- Kedarisetti, K.C.; Narayanan, R.; Stewart, M.W.; Reddy Gurram, N.; Khanani, A.M. Macular Telangiectasia Type 2: A Comprehensive Review. Clin. Ophthalmol. 2022, 16, 3297–3309. [Google Scholar] [CrossRef] [PubMed]
- Marsonia, K.; Kiran Chandra, K.; Ali, M.H.; Chhablani, J.; Narayanan, R. Long term follow-up of visual acuity and incidence of subretinal neovascularization in Mactel Type 2 in 82 Eyes. Semin. Ophthalmol. 2022, 37, 136–141. [Google Scholar] [CrossRef]
- Samadi, M.; Azarkish, A.; Riazi-Esfahani, H.; Mahmoudi, A.; Ebrahimiadib, N. Macular telangiectasia with bilateral obliterated capillaries: A case report. J. Med. Case Rep. 2022, 16, 372. [Google Scholar] [CrossRef] [PubMed]
- Gass, J.D.; Blodi, B.A. Idiopathic juxtafoveolar retinal telangiectasis. Update of classification and follow-up study. Ophthalmology 1993, 100, 1536–1546. [Google Scholar] [CrossRef] [PubMed]
- Yannuzzi, L.A.; Bardal, A.M.; Freund, K.B.; Chen, K.J.; Eandi, C.M.; Blodi, B. Idiopathic macular telangiectasia. Arch. Ophthalmol. 2006, 124, 450–460. [Google Scholar] [CrossRef]
- Kim, Y.H.; Chung, Y.R.; Oh, J.; Kim, S.W.; Lee, C.S.; Yun, C.; Lee, B.; Ahn, S.M.; Choi, E.Y.; Jang, S.; et al. Optical coherence tomographic features of macular telangiectasia type 2: Korean Macular Telangiectasia Type 2 Study-Report No. 1. Sci. Rep. 2020, 10, 16594. [Google Scholar] [CrossRef]
- Gaudric, A.; Ducos de Lahitte, G.; Cohen, S.Y.; Massin, P.; Haouchine, B. Optical coherence tomography in group 2A idiopathic juxtafoveolar retinal telangiectasis. Arch. Ophthalmol. 2006, 124, 1410–1419. [Google Scholar] [CrossRef]
- Zeimer, M.; Gutfleisch, M.; Heimes, B.; Spital, G.; Lommatzsch, A.; Pauleikhoff, D. Association between changes in macular vasculature in optical coherence tomography- and fluorescein- angiography and distribution of macular pigment in type 2 idiopathic macular telangiectasia. Retina 2015, 35, 2307–2316. [Google Scholar] [CrossRef]
- Venkatesh, R.; Reddy, N.G.; Mishra, P.; Agrawal, S.; Mutalik, D.; Yadav, N.K.; Chhablani, J. Spectral domain OCT features in type 2 macular telangiectasia (type 2 MacTel): Its relevance with clinical staging and visual acuity. Int. J. Retin. Vitr. 2022, 8, 26. [Google Scholar] [CrossRef]
- Powner, M.B.; Gillies, M.C.; Zhu, M.; Vevis, K.; Hunyor, A.P.; Fruttiger, M. Loss of Müller’s cells and photoreceptors in macular telangiectasia type 2. Ophthalmology 2013, 120, 2344–2352. [Google Scholar] [CrossRef]
- Baumüller, S.; Charbel Issa, P.; Scholl, H.P.; Schmitz-Valckenberg, S.; Holz, F.G. Outer retinal hyperreflective spots on spectral-domain optical coherence tomography in macular telangiectasia type 2. Ophthalmology 2010, 117, 2162–2168. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, A.; Unterlauft, J.D.; Wiedemann, R.; Barth, T.; Rehak, M.; Wiedemann, P. Two different populations of Müller cells stabilize the structure of the fovea: An optical coherence tomography study. Int. Ophthalmol. 2020, 40, 2931–2948. [Google Scholar] [CrossRef] [PubMed]
- Sallo, F.B.; Leung, I.; Chung, M.; Wolf-Schnurrbusch, U.E.; Dubra, A.; Williams, D.R.; Clemons, T.; Pauleikhoff, D.; Bird, A.C.; Peto, T. & MacTel Study Group. Retinal crystals in type 2 idiopathic macular telangiectasia. Ophthalmology 2011, 118, 2461–2467. [Google Scholar] [PubMed]
- Charbel Issa, P.; Gillies, M.C.; Chew, E.Y.; Bird, A.C.; Heeren, T.F.; Peto, T.; Holz, F.G.; Scholl, H.P. Macular telangiectasia type 2. Prog. Retin. Eye Res. 2013, 34, 49–77. [Google Scholar] [CrossRef]
- Nagasaka, Y.; Ito, Y.; Ueno, S.; Terasaki, H. Number of Hyperreflective Foci in the Outer Retina Correlates with Inflammation and Photoreceptor Degeneration in Retinitis Pigmentosa. Ophthalmol. Retin. 2018, 2, 726–734. [Google Scholar] [CrossRef]
- Lee, H.; Jang, H.; Choi, Y.A.; Kim, H.C.; Chung, H. Association between soluble CD14 in the aqueous humor and hyperreflective foci on optical coherence tomography in patients with diabetic macular edema. Investig. Ophthalmol. Vis. Sci. 2018, 59, 715–721. [Google Scholar] [CrossRef]
- Coscas, G.; De Benedetto, U.; Coscas, F.; Li Calzi, C.I.; Vismara, S.; Roudot-Thoraval, F.; Bandello, F.; Souied, E. Hyperreflective dots: A new spectral-domain optical coherence tomography entity for follow-up and prognosis in exudative age-related macular degeneration. Ophthalmologica J. Int. D’ophtalmologie. Int. J. Ophthalmol. 2013, 229, 32–37. [Google Scholar] [CrossRef]
- Alex, D.; Giridhar, A.; Gopalakrishnan, M.; Manayath, G.; Amar, S.; Raman, R.; Sreenivasan, R.; Ayachit, A.; Sivaprasad, S. Early spectral-domain optical coherence tomography biomarkers to confirm fellow eye changes in asymmetric type-2 macular telangiectasia: A Case-Control Study (India Macular Telangiectasia Report 1). Retina 2021, 41, 471–479. [Google Scholar] [CrossRef]
- Corradetti, G.; Violanti, S.; Au, A.; Sarraf, D. Wide field retinal imaging and the detection of drug associated retinal toxicity. Int. J. Retin. Vitr. 2019, 5 (Suppl. S1), 26. [Google Scholar] [CrossRef]
- Trenkic Božinovic, M.S.; Stankovic Babic, G.; Petrovic, M.; Karadžic, J.; Šarenac Vulovic, T.; Trenkic, M. Role of optical coherence tomography in the early detection of macular thinning in rheumatoid arthritis patients with chloroquine retinopathy. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2019, 24, 55. [Google Scholar] [CrossRef] [PubMed]
- Garrity, S.T.; Jung, J.Y.; Zambrowski, O.; Pichi, F.; Su, D.; Arya, M.; Waheed, N.K.; Duker, J.S.; Chetrit, Y.; Miserocchi, E.; et al. Early hydroxychloroquine retinopathy: Optical coherence tomography abnormalities preceding Humphrey visual field defects. Br. J. Ophthalmol. 2019, 103, 1600–1604. [Google Scholar] [CrossRef] [PubMed]
- Stepien, K.E.; Han, D.P.; Schell, J.; Godara, P.; Rha, J.; Carroll, J. Spectral-domain optical coherence tomography and adaptive optics may detect hydroxychloroquine retinal toxicity before symptomatic vision loss. Trans. Am. Ophthalmol. Soc. 2009, 107, 28–33. [Google Scholar]
- Korah, S.; Kuriakose, T. Optical coherence tomography in a patient with chloroquine-induced maculopathy. Indian J. Ophthalmol. 2008, 56, 511–513. [Google Scholar] [CrossRef]
- Souza Monteiro de Araújo, D.; Brito, R.; Pereira-Figueiredo, D.; Dos Santos-Rodrigues, A.; De Logu, F.; Nassini, R.; Zin, A.; Calaza, K.C. Retinal Toxicity Induced by Chemical Agents. Int. J. Mol. Sci. 2022, 23, 8182. [Google Scholar] [CrossRef] [PubMed]
- Browning, D.J. Hydroxychloroquine and chloroquine retinopathy: Screening for drug toxicity. Am. J. Ophthalmol. 2002, 133, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Marmor, M.F.; Kellner, U.; Lai, T.Y.; Melles, R.B.; Mieler, W.F.; American Academy of Ophthalmology. Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy (2016 Revision). Ophthalmology 2016, 123, 1386–1394. [Google Scholar] [CrossRef]
- Melles, R.B.; Marmor, M.F. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014, 132, 1453–1460. [Google Scholar] [CrossRef]
- Sánchez Brea, L.; Andrade De Jesus, D.; Shirazi, M.F.; Pircher, M.; van Walsum, T.; Klein, S. Review on Retrospective Procedures to Correct Retinal Motion Artefacts in OCT Imaging. Appl. Sci. 2019, 9, 2700. [Google Scholar] [CrossRef]
- Shin, Y.U.; Lee, B.R.; Lim, H.W. A comparison of image quality between swept source optical coherence tomography and spectral domain optical coherence tomography according to ocular media opacity. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3359. [Google Scholar]
- Tappeiner, C.; Barthelmes, D.; Abegg, M.H.; Wolf, S.; Fleischhauer, J.C. Impact of Optic Media Opacities and Image Compression on Quantitative Analysis of Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1609–1614. [Google Scholar] [CrossRef] [PubMed]

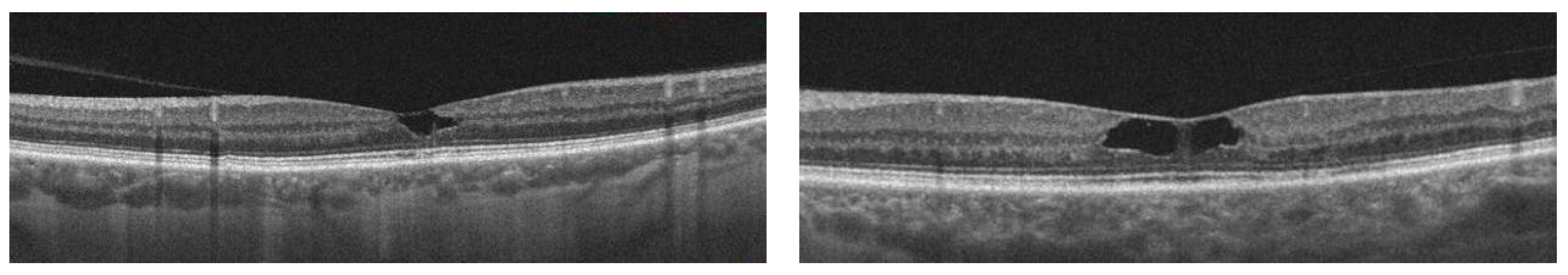
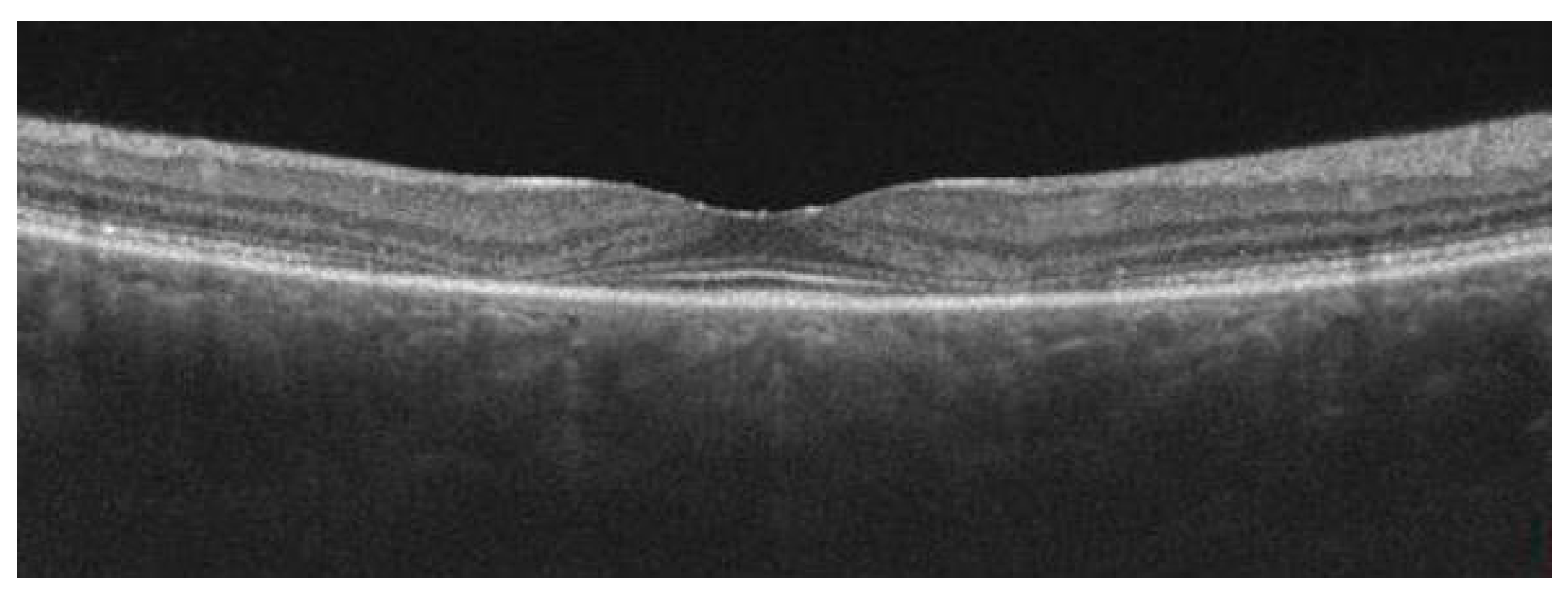
| Author | Year | Number of Patients/Eyes Included | Biomarker | Characteristics of Biomarker | Description | Correlation with AMD | Method | The Role of Biomarker in Clinical Practice |
|---|---|---|---|---|---|---|---|---|
| Hilely et al. [24] | 2021 | 45 eyes | Drusen height | Quantitative biomarker | The height of the drusen causing reduced choroidal perfusion might lead to RPE 1 pump failure | An early sign of atrophy on OCT 2 imaging | OCT 2 | Predictor |
| Au et al. [25] | 2022 | 155 eyes | Drusen height | Quantitative biomarker | Drusen height, more so than drusen GHD 3, is correlated with the presence of OCT 1 predictors of atrophy | Early biomarker for targeting early intervention to prevent atrophy and vision loss | OCT 2 | Predictor |
| Abdelfattah et al. [26] | 2016 | 89 patients | Drusen volume | Quantitative biomarker | Quantifying drusen volume through OCT 2 could be a promising biomarker for identifying patients at higher risk of developing MNV 4 | Important predictor for the development of advanced AMD 5 at 12 and 24 months of follow-up in the fellow eye | OCT 2 | Predictor |
| Lamin et al. [27] | 2019 | 248 patients | Drusen volume | Quantitative biomarker | Increase in the overall amount of drusen measured through optical coherence tomography before neovascular AMD 5 development | A useful method for detecting early signs of neovascular AMD 5 in those at risk for the disease | OCT 2 | Predictor |
| Amissah-Arthur et al. [19] | 2012 | 749 patients | RPE 1 changes | Qualitative biomarker | Abnormalities in the RPE 1 without any visible alterations in the outer neural retina | Possible to diagnose conversion to a wet form of AMD 5 in the fellow eye by looking for small changes in RPE 1 contours/elevation | OCT 2 | Predictor |
| Nagai et al. [13] | 2020 | 30 eyes | PROS 6 | Quantitative biomarker | The length of the PROS 6 where the visual pigment is concentrated | Potential sensitive biomarker for the degeneration of photoreceptors | OCT 2 | Risk factor |
| Jaffe et al. (CAM Report 5) [28] | 2021 | Not available | Intraretinal hyperreflective foci, focal RPE 1 thickening, and choroidal hypertransmission | Qualitative biomarker | Early signs of RPE 1 damage and disturbance that may lead to geographic atrophy | Predictors of atrophy | OCT 2 | Predictor |
| Harada et al. [10] | 2022 | 24 eyes | CCFA 7 ratio | Quantitative biomarker | Percentage of choriocapillaris slab area to analyzed area from OCTA 8 images | Smaller CCFA 7 ratio in AMD high-risk fellow eyes than in control eyes, indicating decline in choriocapillaris flow | OCTA 8 | Risk factor |
| Trinh et al. [29] | 2019 | 63 eyes | GCL 9 thickness | Quantitative biomarker | Reduction in GCL 9 thickness in AMD eyes | Structural loss in the early stages of AMD may be linked to retinal vascular alterations | OCT 2/OCTA 8 | Predictor |
| Trinh et al. [29] | 2019 | 63 eyes | Vascular density in the superficial capillary plexus (SCP) 10 | Quantitative biomarker | As different OCTA 8 modalities utilize different scanning techniques and definitions to automatically separate the retinal vasculature from the scan, differences between OCTA 8 studies may be device-related | Substantial reduction in the SCP’s 10 vascular density in AMD patients; however, vascular density in DCP 11 was not significantly decreased | OCTA 8 | Risk factor |
| Lamin et al. [14] | 2020 | 51 eyes | Drusen load | Quantitative biomarker | Comparison of drusen load between the two types of CNV 12 | Significant rise in the area and volume of drusen during the 12 months preceding the onset of occult CNV 12 | OCT 2 | Risk factor |
| Lamin et al. [14] | 2020 | 51 eyes | Retinal layer volumes | Quantitative biomarker | Comparison of retinal layer volumes between CNV 12 types | Decrease in ONL 13 volume and increase in outer plexiform layer volume in eyes with occult CNV 12 | OCT 2 | Risk factor |
| Menteş et al. [30] | 2019 | 27 eyes | Drusen load | Quantitative biomarker | Comparison of drusen load between the two types of CNV 12 | Significant rise in the area and volume of drusen during the 12 months preceding the onset of occult CNV 12 | OCT 2 | Risk factor |
| Shi et al. [31] | 2019 | 100 eyes | DLS 14 | Qualitative biomarker | Identification of type 1 MNV in eyes with nonexudative AMD | DLS 14 has good predictive values for identifying type 1 MNV 2 | OCT 2 | Predictor |
| Oliveira Dias et al. [32] | 2018 | 160 patients | Subclinical neovascularization | Qualitative biomarker | Neovascularization was not visible on clinical exam, but detected on OCT 2 imaging | Increased risk of exudation in eyes with subclinical MNV 4 compared to eyes without detectable MNV 4 | OCTA 8 | Risk factor |
| Nassisi et al. [33] | 2019 | 501 eyes | IHRF 15, hRF 16, DLs 14, SDD 17, and Drusen volume | Qualitative biomarkers (except drusen volume—quantitative biomarker) | Various features visible on OCT imaging | IHRF 15, hRF 16 within a drusen-like lesion, and SDD 17 were significantly associated with an increased risk of progression to late AMD 5. SDD 17 is strongly associated with the development of GA 18 and type 3 MNV 4. Drusen volume was not significantly associated with the development of MNV 4 | OCT 2 | Risk factors |
| Kang et al. [34] | 2022 | 70 eyes | Chorioretinal thickness, RPE 1 degenerative features, and SDDs 17 | Chorioretinal thickness—quantitative biomarker; RPE 1 degenerative features and SDDs 17—qualitative biomarkers | The thickness of the retina and choroid in eyes with RPE 1 degeneration is comparatively reduced to those without this degeneration | Early AMD 5 eyes with SDDs 17 are prone to overall chorioretinal degeneration. Fellow eyes with neovascular AMD 5 showed greater proportions of RPE 1 degeneration and a thicker retina and choroid | OCT 2 | Risk factor |
| Kwak et al. [35] | 2021 | 93 eyes | CVI 19 | Quantitative biomarker | Lower CVI 19 values in unaffected fellow eyes may be associated with possible subclinical disease | Significant correlation between the incidence of fellow eye type 3 neovascularization and the presence of large soft drusen, reticular pseudodrusen, and lower choroidal vascularity index CVI 19 values | OCT 2 | Risk factor |
| Toprak et al. [36] | 2017 | 47 eyes | RPE 1 and EZ 20 reflectivity | Qualitative biomarker | Lower reflectivities suggest early photoreceptor damage | Non-neovascular AMD patients have significantly lower reflectivities compared to healthy controls. | OCT 2 | Predictor |
| Schick et al. [21] | 2015 | 104 patients | Macular drusen | Quantitative biomarker | More than 20 drusen in non-affected eyes indicate potential biomarkers for more severe progression of the disease | Prevalence of more than 20 macular drusen is more prevalent in non-affected eyes of individuals with early onset of unilateral neovascular AMD 5 | OCT 2 | Risk factor |
| RPD 21 | Qualitative biomarker | Prevalence increases with age and is more common in the late-onset CNV 12 group; link with neovascular AMD 5 | RPDs 21 were more commonly observed in the late-onset CNV 12 group than in the no-CNV 12 group. | Predictor |
| OCT Features | Description |
|---|---|
| Enlarged foveal pit | A thin temporal juxtafoveal retina leads to enlargement of the foveal pit in the temporal region (thinning takes place in the outer nuclear/Henle’s fiber layer) [63]. |
| Hypo-reflective cavities | Located in both inner and outer neurosensory retina [68]. |
| Disruptions of retinal layers | Disruption of the external limiting membrane (ELM), photoreceptor inner segment–outer segment border, and interdigitation zone—one of the most frequently observed OCT features in patients with idiopathic macular telangiectasia type 2 [68]. |
| Thicker temporal retina | Early subretinal neovascularization may be indicated by a thicker temporal retina compared to nasal fovea without retinal fluid [69]. |
| Hyper-reflective lesions | Thick, hyper-reflective lesions in the outer retina, with highly reflective dots in the inner and the outer nuclear layers [69]. |
| Decrease in vascular density | In type 2 MacTel, the earliest vascular changes are observed in the deep vascular plexus, which are characterized by a decrease in vascular density and the presence of telangiectatic vessels (changes can be visualized using OCT angiography) [70]. |
| Hyperreflective middle retinal layer (MRL) | Loss of Müller cells in the perifoveal region may contribute to increased hyperreflectivity of MRL [70,71,72]. In type 2 MacTel, hyperreflective MRL in the perfoveal region was recognized as the most frequent early OCT finding [71]. |
| Superficial intraretinal crystals | Lesions present in all stages of disease provide evidence of Müller cell involvement. Useful for early disease diagnosis [71]. |
| Retinal pigment clumps (RPC) | The presence of retinal pigment clumps can potentially serve as an early indicator or biomarker for predicting the onset of the proliferative stage of the disease [71]. |
| Clustered hyperreflective foci (HF) | Clustered hyperreflective foci are the early biomarker of the neurodegenerative process; additional research is needed [73]. |
| Biomarker | Description |
|---|---|
| Parafoveal thinning | The localized thinning of retinal layers in the parafoveal region detected through OCT imaging confirms the early stage of toxicity before significant visual field loss was detected [82]. |
| Inner segment ellipsoid attenuation | The focal area of parafoveal inner segment ellipsoid attenuation with subsequent loss, particularly in the inferotemporal quadrant [83]. |
| “Flying saucer” sign | This distinct OCT biomarker visible in the spectral domain OCT can progress from the initial stage of retinal toxicity, which is characterized by parafoveal inner segment ellipsoid attenuation [83]. |
| “Moth eaten” photoreceptor inner/outer segments junction | This photoreceptor appearance is due to the preferential loss of cone photoreceptors, which describes the preclinical stage of hydroxychloroquine toxicity [84]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goździewska, E.; Wichrowska, M.; Kocięcki, J. Early Optical Coherence Tomography Biomarkers for Selected Retinal Diseases—A Review. Diagnostics 2023, 13, 2444. https://doi.org/10.3390/diagnostics13142444
Goździewska E, Wichrowska M, Kocięcki J. Early Optical Coherence Tomography Biomarkers for Selected Retinal Diseases—A Review. Diagnostics. 2023; 13(14):2444. https://doi.org/10.3390/diagnostics13142444
Chicago/Turabian StyleGoździewska, Ewa, Małgorzata Wichrowska, and Jarosław Kocięcki. 2023. "Early Optical Coherence Tomography Biomarkers for Selected Retinal Diseases—A Review" Diagnostics 13, no. 14: 2444. https://doi.org/10.3390/diagnostics13142444
APA StyleGoździewska, E., Wichrowska, M., & Kocięcki, J. (2023). Early Optical Coherence Tomography Biomarkers for Selected Retinal Diseases—A Review. Diagnostics, 13(14), 2444. https://doi.org/10.3390/diagnostics13142444







