Deep Learning Convolutional Neural Network Reconstruction and Radial k-Space Acquisition MR Technique for Enhanced Detection of Retropatellar Cartilage Lesions of the Knee Joint
Abstract
1. Introduction
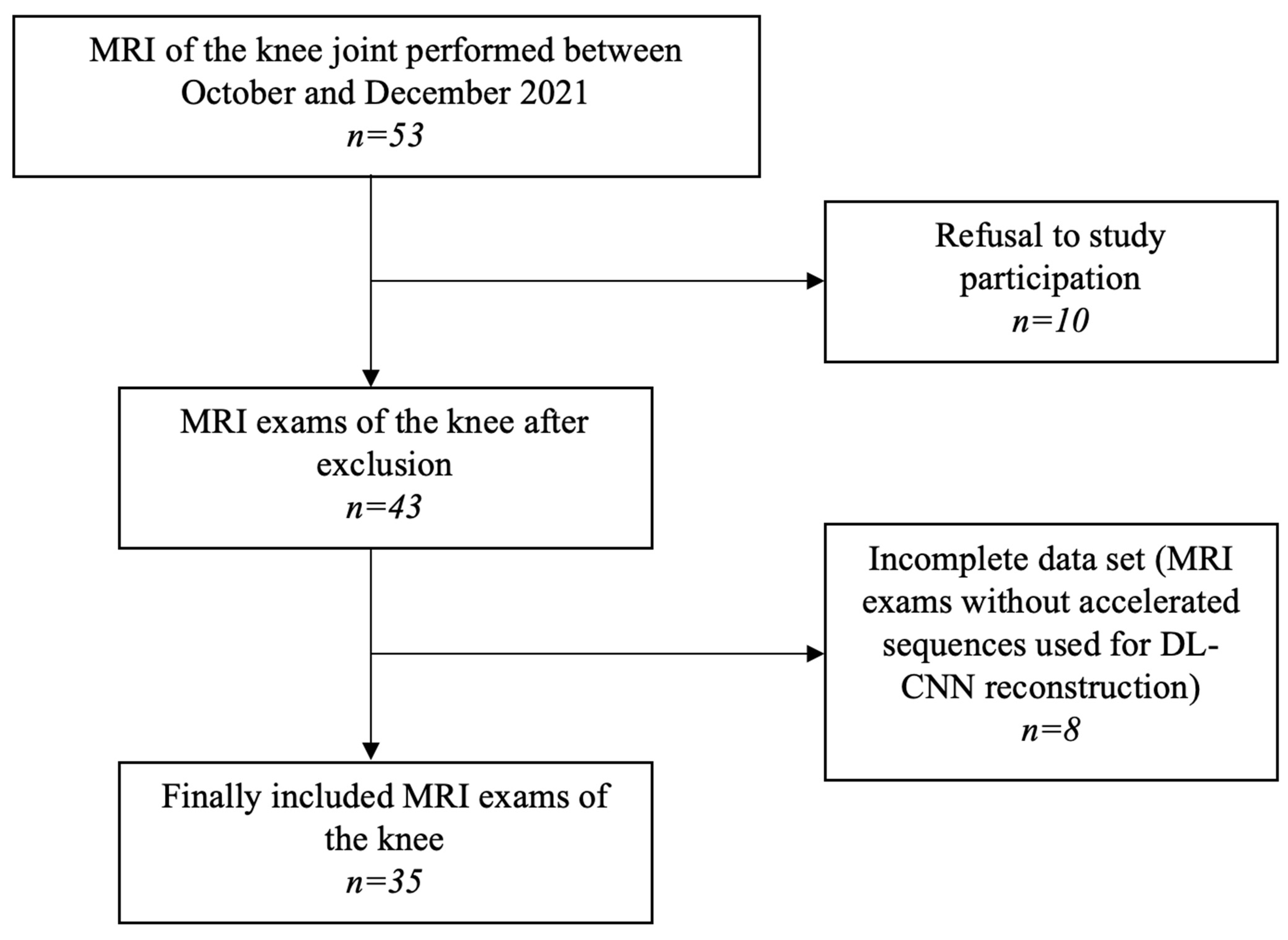
2. Materials and Methods
2.1. Participants
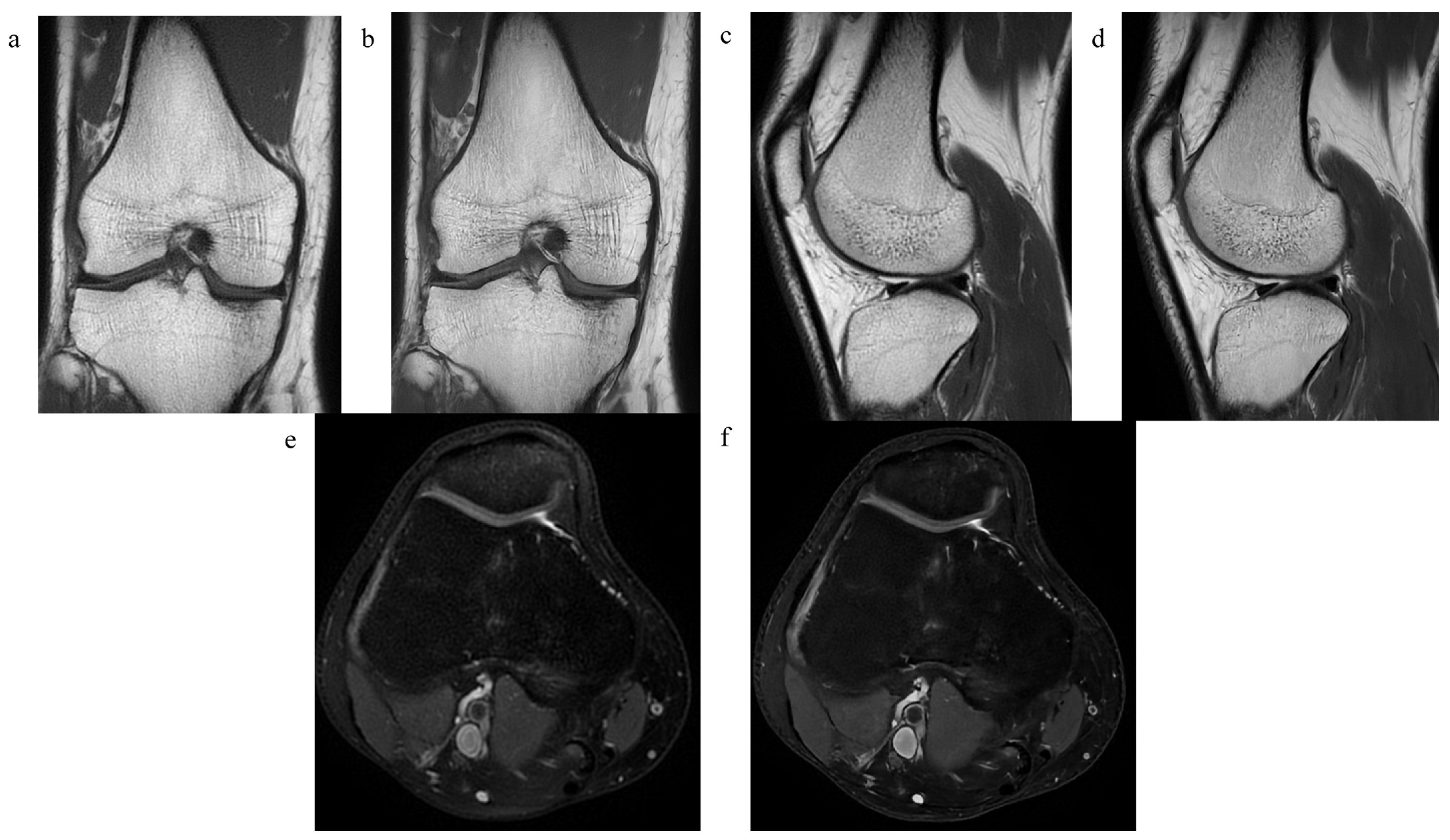
2.2. MRI Examination
2.3. Assessment of Image Quality and Diagnostic Confidence
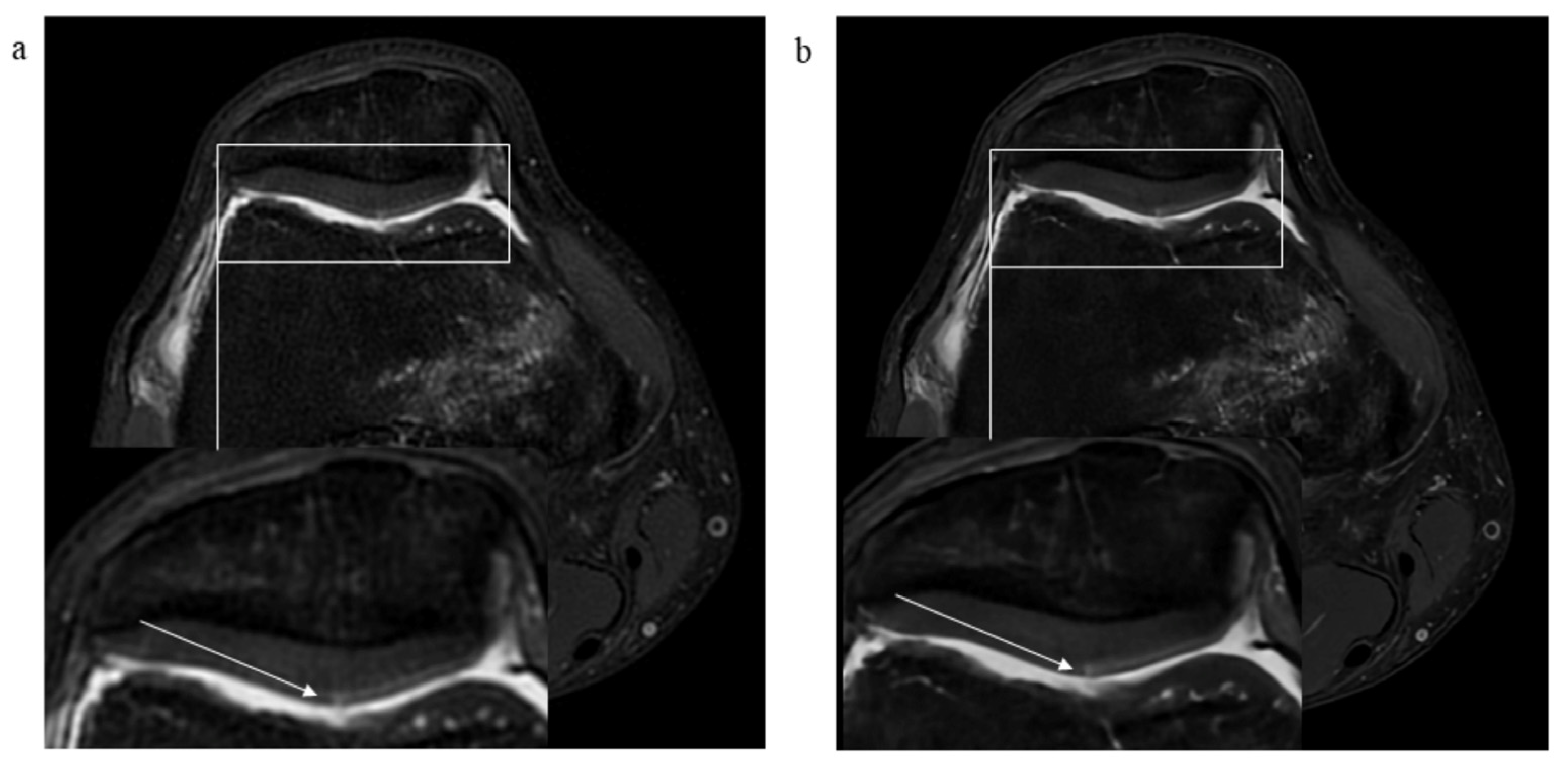
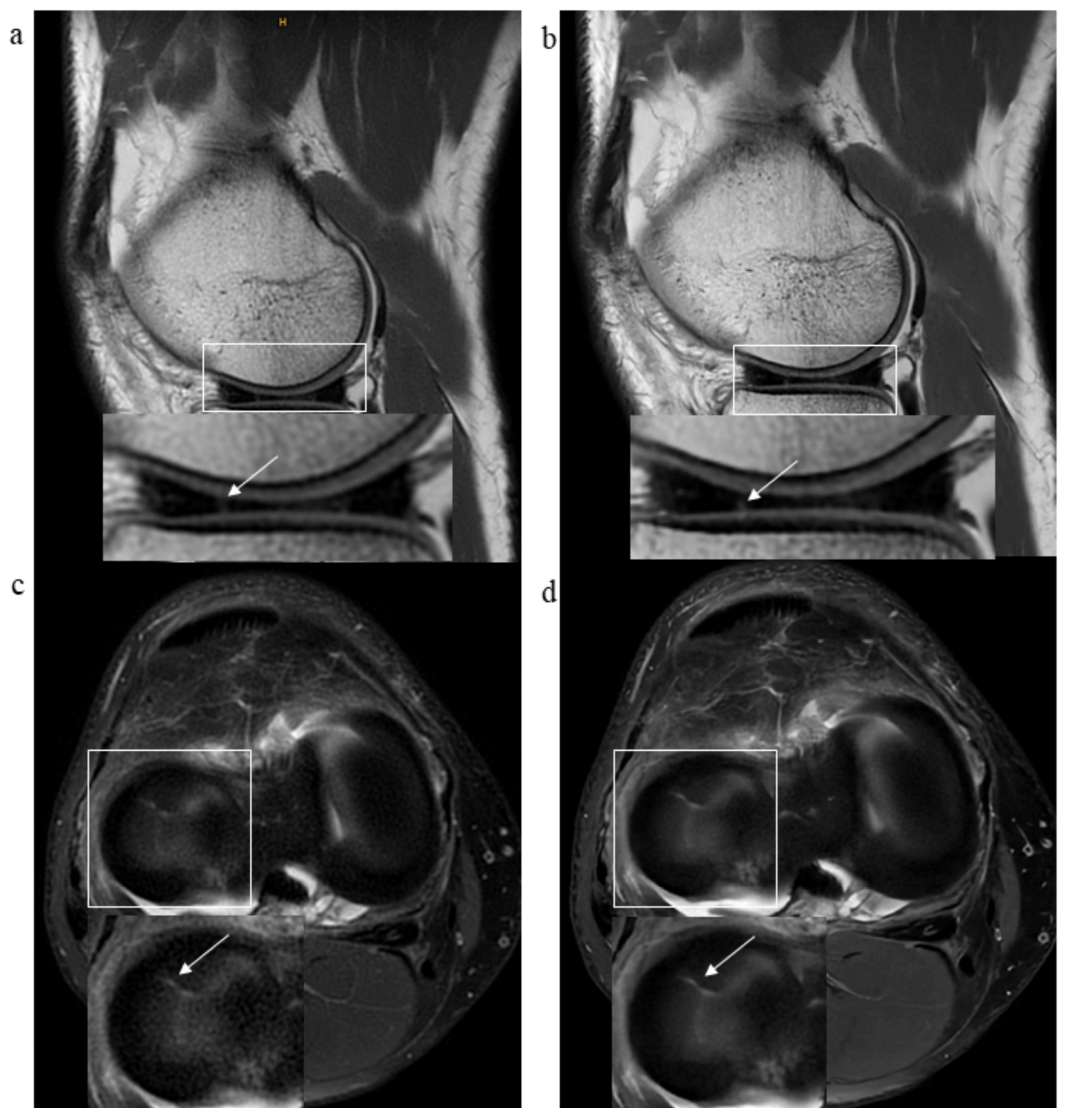
2.4. Assessment of Pathological Findings
2.5. Signal-to-Noise and Contrast-to-Noise Ratio
2.6. Statistical Analysis
3. Results
3.1. The Quality of the Images and the Level of Diagnostic Confidence
3.2. Pathological Findings
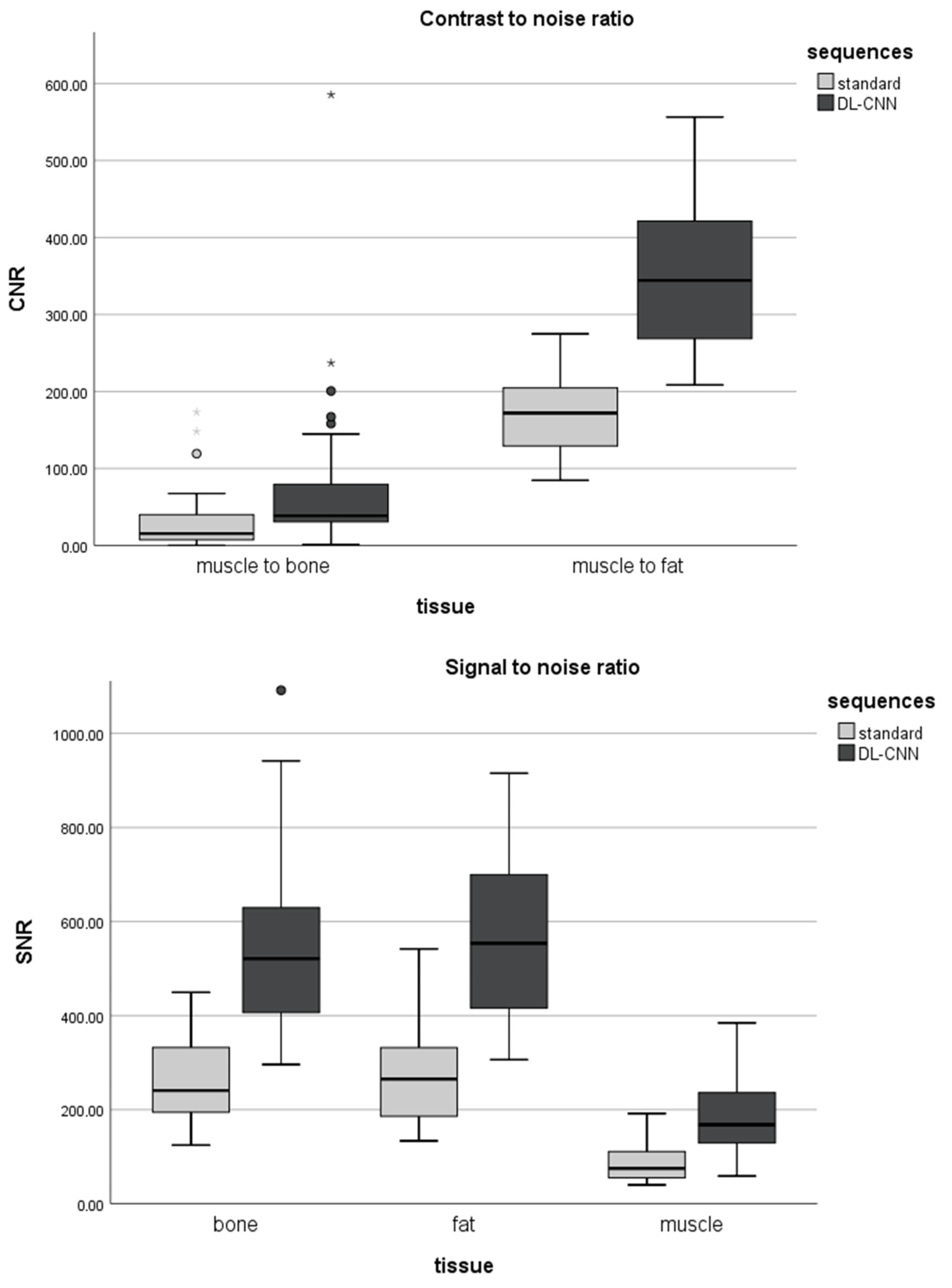
3.3. Signal-to-Noise and Contrast-to-Noise Ratios
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACL | anterior cruciate ligament |
| CNR | contrast-to-noise ratio |
| DL-CNN | deep learning-based convolutional neural networks |
| FOV | field of view |
| FSE | fast spin-echo sequences |
| ICC | intraclass correlation coefficient |
| IRA | inter-reader agreement |
| LCL | lateral collateral ligament |
| MCL | medial collateral ligament |
| PCL | posterior cruciate ligament |
| PROPELLER | periodically rotated overlapping parallel lines with enhanced reconstruction |
| ROI | region of interest |
| SD | standard deviation |
| SI | signal intensity |
| SNR | signal-to-noise ratio |
References
- Ben-Galim, P.; Steinberg, E.L.; Amir, H.; Ash, N.; Dekel, S.; Arbel, R. Accuracy of Magnetic Resonance Imaging of the Knee and Unjustified Surgery. Clin. Orthop. Relat. Res. 2006, 447, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Gold, G.E.; Han, E.; Stainsby, J.; Wright, G.; Brittain, J.; Beaulieu, C. Musculoskeletal MRI at 3.0 T: Relaxation Times and Image Contrast. Am. J. Roentgenol. 2004, 183, 343–351. [Google Scholar] [CrossRef]
- Nacey, N.C.; Geeslin, M.G.; Miller, G.W.; Pierce, J.L. Magnetic resonance imaging of the knee: An overview and update of conventional and state of the art imaging. J. Magn. Reson. Imaging 2017, 45, 1257–1275. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.; Harish, M.; Hargreaves, B.; Staroswiecki, E.; Gold, G. Advances in musculoskeletal MRI: Technical considerations. J. Magn. Reson. Imaging 2012, 36, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Friemert, B.; Schwarz, W.; Danz, B.; Oberländer, Y.; Häberle, H.J.; Bähren, W.; Gerngroß, H. Diagnosis of chondral lesions of the knee joint: Can MRI replace arthroscopy? Knee Surg. Sports Traumatol. Arthrosc. 2004, 12, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.; Jennings, L.; Maywood, R.; Berger, P. Magnetic resonance imaging of the knee. Am. J. Sports Med. 1988, 16, 29–38. [Google Scholar] [CrossRef]
- Fritz, J.; Guggenberger, R.; del Grande, F. Rapid Musculoskeletal MRI in 2021: Clinical Application of Advanced Accelerated Techniques. Am. J. Roentgenol. 2021, 216, 718–733. [Google Scholar] [CrossRef]
- Del Grande, F.; Rashidi, A.; Luna, R.; Delcogliano, M.; Stern, S.E.; Dalili, D.; Fritz, J. Five-Minute Five-Sequence Knee MRI Using Combined Simultaneous Multislice and Parallel Imaging Acceleration: Comparison with 10-Minute Parallel Imaging Knee MRI. Radiology 2021, 299, 635–646. [Google Scholar] [CrossRef]
- Dietrich, T.J.; Ulbrich, E.J.; Zanetti, M.; Fucentese, S.F.; Pfirrmann, C.W.A. PROPELLER Technique to Improve Image Quality of MRI of the Shoulder. Am. J. Roentgenol. 2011, 197, W1093–W1100. [Google Scholar] [CrossRef]
- Jia-gao, F.; Yang, F.; Lian-jin, N. Clinical Application of 3.0 T MRI BLADE Technique in Correcting Joint Motion Artifacts. Prog. Mod. Biomed. 2010, 23, 4547–4549. [Google Scholar]
- Lavdas, E.; Vlychou, M.; Zaloni, E.; Vassiou, K.; Tsagkalis, A.; Dailiana, Z.H.; Fezoulidis, I.V. Elimination of motion and pulsation artifacts using BLADE sequences in shoulder MR imaging. Skelet. Radiol. 2015, 44, 1619–1626. [Google Scholar] [CrossRef]
- Pipe, J.G. Motion correction with PROPELLER MRI: Application to head motion and free-breathing cardiac imaging. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 1999, 42, 963–969. [Google Scholar] [CrossRef]
- Lebel, R.M. Performance characterization of a novel deep learning-based MR image reconstruction pipeline. arXiv 2020, arXiv:200806559. [Google Scholar]
- Recht, M.P.; Zbontar, J.; Sodickson, D.K.; Knoll, F.; Yakubova, N.; Sriram, A.; Murrell, T.; Defazio, A.; Rabbat, M.; Rybak, L.; et al. Using Deep Learning to Accelerate Knee MRI at 3 T: Results of an Interchangeability Study. Am. J. Roentgenol. 2020, 215, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Yaman, B.; Hosseini, S.A.H.; Moeller, S.; Ellermann, J.; Uğurbil, K.; Akçakaya, M. Self-Supervised Physics-Based Deep Learning MRI Reconstruction Without Fully-Sampled Data. In Proceedings of the 2020 IEEE 17th International Symposium on Biomedical Imaging (ISBI), Iowa City, IA, USA, 3–7 April 2020. [Google Scholar]
- Hahn, S.; Yi, J.; Lee, H.-J.; Lee, Y.; Lim, Y.-J.; Bang, J.-Y.; Kim, H.; Lee, J. Image Quality and Diagnostic Performance of Accelerated Shoulder MRI with Deep Learning–Based Reconstruction. Am. J. Roentgenol. 2021, 218, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.D.; Heide, H.R. The Clinical Benefits of AIR™ Recon DL for MR Image Reconstruction; GE Healthcare: Singapore, 2020. [Google Scholar]
- Ng, A.W.H.; Griffith, J.F.; Hung, E.H.Y.; Law, K.Y.; Yung, P.S.H. MRI diagnosis of ACL bundle tears: Value of oblique axial imaging. Skelet. Radiol. 2013, 42, 209–217. [Google Scholar] [CrossRef]
- Ghasemi, A.; Zahediasl, S. Normality Tests for Statistical Analysis: A Guide for Non-Statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef]
- Razali, N.M.; Wah, Y.B. Power comparisons of shapiro-wilk, kolmogorov-smirnov, lilliefors and anderson-darling tests. J. Stat. Model. Anal. 2011, 2, 21–33. [Google Scholar]
- Zar, J.H. Spearman rank correlation. In Encyclopedia of Biostatistics; Wiley: Hoboken, NJ, USA, 2005; Volume 7. [Google Scholar]
- Zimmerman, D.W.; Zumbo, B.D. Relative power of the Wilcoxon test, the Friedman test, and repeated-measures ANOVA on ranks. J. Exp. Educ. 1993, 62, 75–86. [Google Scholar] [CrossRef]
- Abdi, H. Holm’s sequential Bonferroni procedure. Encycl. Res. Des. 2010, 1, 1–8. [Google Scholar]
- McGraw, K.O.; Wong, S.P. Forming inferences about some intraclass correlation coefficients. Psychol. Methods 1996, 1, 30. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Fleiss, J.L.; Levin, B.; Paik, M.C. The measurement of interrater agreement. Stat. Methods Rates Proportions 1981, 2, 22–23. [Google Scholar]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Medica 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Cohen, J. Statistical power analysis. Curr. Dir. Psychol. Sci. 1992, 1, 98–101. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Afat, S.; Wessling, D.; Afat, C.; Nickel, D.; Arberet, S.; Herrmann, J.; Othman, A.E.; Gassenmaier, S. Analysis of a Deep Learning-Based Superresolution Algorithm Tailored to Partial Fourier Gradient Echo Sequences of the Abdomen at 1.5 T: Reduction of Breath-Hold Time and Improvement of Image Quality. Investig. Radiol. 2022, 57, 157–162. [Google Scholar] [CrossRef]
- Chaudhari, A.S.; Fang, Z.; Kogan, F.; Wood, J.; Stevens, K.J.; Gibbons, E.K.; Lee, J.H.; Gold, G.E.; Hargreaves, B.A. Super-resolution musculoskeletalMRIusing deep learning. Magn. Reson. Med. 2018, 80, 2139–2154. [Google Scholar] [CrossRef]
- Kelkar, V.; Zhang, X.; Granstedt, J.; Li, H.; Anastasio, M. Task-based evaluation of deep image super-resolution in medical imaging. In Medical Imaging 2021: Image Perception, Observer Performance, and Technology Assessment; SPIE: Bellingham, WA, USA, 2021. [Google Scholar]
- Wang, Z.; Chen, J.; Hoi, S.C.H. Deep Learning for Image Super-Resolution: A Survey. IEEE Trans. Pattern Anal. Mach. Intell. 2021, 43, 3365–3387. [Google Scholar] [CrossRef]
- McLeavy, C.; Chunara, M.; Gravell, R.; Rauf, A.; Cushnie, A.; Talbot, C.S.; Hawkins, R. The future of CT: Deep learning reconstruction. Clin. Radiol. 2021, 76, 407–415. [Google Scholar] [CrossRef]
- Salaha, Z.F.M.; Ammarullah, M.I.; Abdullah, N.N.A.A.; Aziz, A.U.A.; Gan, H.-S.; Abdullah, A.H.; Kadir, M.R.A.; Ramlee, M.H. Biomechanical Effects of the Porous Structure of Gyroid and Voronoi Hip Implants: A Finite Element Analysis Using an Experimentally Validated Model. Materials 2023, 16, 3298. [Google Scholar] [CrossRef] [PubMed]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Permana, M.S.; Winarni, T.I.; van der Heide, E. Adopted walking condition for computational simulation approach on bearing of hip joint prosthesis: Review over the past 30 years. Heliyon 2022, 8, e12050. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Hartono, R.; Supriyono, T.; Santoso, G.; Sugiharto, S.; Permana, M.S. Polycrystalline Diamond as a Potential Material for the Hard-on-Hard Bearing of Total Hip Prosthesis: Von Mises Stress Analysis. Biomedicines 2023, 11, 951. [Google Scholar] [CrossRef] [PubMed]
- Shanbhogue, K.; Tong, A.; Smereka, P.; Nickel, D.; Arberet, S.; Anthopolos, R.; Chandarana, H. Accelerated single-shot T2-weighted fat-suppressed (FS) MRI of the liver with deep learning–based image reconstruction: Qualitative and quantitative comparison of image quality with conventional T2-weighted FS sequence. Eur. Radiol. 2021, 31, 8447–8457. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, Y.; Isoda, H.; Maetani, Y.S.; Arizono, S.; Shimada, K.; Togashi, K. MRI Artifact Reduction and Quality Improvement in the Upper Abdomen with PROPELLER and Prospective Acquisition Correction (PACE) Technique. Am. J. Roentgenol. 2008, 191, 1154–1158. [Google Scholar] [CrossRef]
- Ebrahimkhani, S.; Dharmaratne, A.; Jaward, M.H.; Wang, Y.; Cicuttini, F.M. Automated segmentation of knee articular cartilage: Joint deep and hand-crafted learning-based framework using diffeomorphic mapping. Neurocomputing 2022, 467, 36–55. [Google Scholar] [CrossRef]
- Shen, S.; Tian, H.; Lu, H.; Chen, H.-L.; Nie, C.; Zhang, J.; Zhang, C.-C.; Wang, H. T1ρ magnetic resonance imaging for quantitative assessment of patella cartilage in healthy subjects and athletes. Measurement 2022, 191, 110785. [Google Scholar] [CrossRef]
- Wu, M.; Ma, Y.-J.; Liu, M.; Xue, Y.; Gong, L.; Wei, Z.; Jerban, S.; Jang, H.; Chang, D.G.; Chang, E.Y.; et al. Quantitative assessment of articular cartilage degeneration using 3D ultrashort echo time cones adiabatic T1ρ (3D UTE-Cones-AdiabT1ρ) imaging. Eur. Radiol. 2022, 32, 6178–6186. [Google Scholar] [CrossRef]
- Kidoh, M.; Shinoda, K.; Kitajima, M.; Isogawa, K.; Nambu, M.; Uetani, H.; Morita, K.; Nakaura, T.; Tateishi, M.; Yamashita, Y.; et al. Deep Learning Based Noise Reduction for Brain MR Imaging: Tests on Phantoms and Healthy Volunteers. Magn. Reson. Med. Sci. 2020, 19, 195–206. [Google Scholar] [CrossRef]
- Kladny, B.; Hofmann, G.; Glückert, K.; Blank-Schäl, A. MRI of the knee joint with a 3-D gradient echo sequence. Arch. Orthop. Trauma Surg. 1992, 112, 5–14. [Google Scholar] [CrossRef]

| Standard PROPELLER | DL-CNN Sequences | |||||
|---|---|---|---|---|---|---|
| ax PD FS | sag PD | cor T1w | ax PD FS | sag PD | cor T1w FS | |
| TE (ms) | 49.9 | 44.2 | 12.8 | 46.7 | 42.9 | 42.2 |
| TR (ms) | 4649 | 2517 | 511 | 4421 | 2500 | 541 |
| SL (mm) | 3 | 3 | 3 | 3 | 3 | 3 |
| Spacing between slices | 4 | 4 | 4 | 4 | 4 | 4 |
| Echo train length | 15 | 14 | 7 | 15 | 14 | 7 |
| Echo numbers | 1 | 1 | 1 | 1 | 1 | 1 |
| Matrix | 300 × 300 | 320 × 320 | 340 × 340 | 320 × 320 | 360 × 360 | 360 × 360 |
| Flip angle (°) | 160 | 160 | 160 | 160 | 160 | 160 |
| Receiver bandwidth (kHz) | 162.7 | 195.3 | 195.3 | 195.3 | 244.1 | 244.1 |
| Number of averages | 3.0 | 2.8 | 2.6 | 1.6 | 1.5 | 1.6 |
| Imaging frequency | 63.8 | 63.8 | 63.8 | 63.8 | 63.8 | 63.8 |
| Acquisition time (min:s) | 04:09 | 02:46 | 03:08 | 02:00 | 01:09 | 01:36 |
| Overall acquisition time (min:s) | 10:03 | 04:45 | ||||
| Standard PROPELLER (Mean ± SD) | DL-CNN (Mean ± SD) | Wilcoxon-Signed Rank Test (p-Value) | |
|---|---|---|---|
| Bone | 2.05 ± 0.57 | 2.90 ± 0.08 | |
| Cartilage | 1.95 ± 0.29 | 2.85 ± 0.22 | |
| Retropatellar cartilage | 1.94 ± 0.05 | 2.95 ± 0.50 | |
| Muscle | 2.22 ± 0.51 | 2.42 ± 0.16 | |
| Anterior cruciate ligament | 1.80 ± 0.05 | 2.60 ± 0.51 | |
| Posterior cruciate ligament | 2.24 ± 0.31 | 2.97 ± 0.20 | |
| Medial collateral ligament | 1.98 ± 0.02 | 2.91 ± 0.11 | |
| Lateral collateral ligament | 1.95 ± 0.02 | 2.88 ± 0.05 | |
| Medial meniscus | 1.91 ± 0.11 | 2.82 ± 0.30 | |
| Lateral meniscus | 2.04 ± 0.20 | 2.92 ± 0.86 | |
| Contour sharpness in central FOV | 1.95 ± 0.42 | 2.92 ± 0.02 | |
| Contour sharpness in peripheral FOV | 2.05 ± 0.40 | 2.85 ± 0.32 | |
| Homogeneity of fat saturation in central FOV | 2.32 ± 1.05 | 2.40 ± 0.65 | |
| Homogeneity of fat saturation in peripheral FOV | 1.84 ± 1.34 | 2.30 ± 0.71 | all < 0.05 |
| Standard PROPELLER (Mean ± SD) | DL-CNN (Mean ± SD) | Wilcoxon Signed-Rank Test (p-Value) | |
|---|---|---|---|
| Bone | 2.07 ± 0.47 | 2.94 ± 0.23 | |
| Cartilage | 1.91 ± 0.40 | 2.85 ± 0.35 | |
| Muscle | 2.41 ± 0.55 | 2.92 ± 0.25 | |
| Ligaments | 2.14 ± 0.48 | 2.87 ± 0.33 | |
| Medial and lateral meniscus | 2.12 ± 0.52 | 2.89 ± 0.31 | |
| Subcutaneous fat tissue | 2.49 ± 0.55 | 2.90 ± 0.33 | |
| Overall | 2.04 ± 0.31 | 2.95 ± 0.20 | all < 0.05 |
| Assessment of Pathologies | Inter-Reader Agreement | |||
|---|---|---|---|---|
| Standard PROPELLER (Mean ± SD) | DL-CNN (Mean ± SD) | Standard PROPELLER (κ-Value) | DL-CNN (κ-Value) | |
| Bone | 1.87 ± 0.89 | 1.80 ± 0.89 | 0.479 | 0.508 |
| Cartilage | 1.31 ± 0.46 | 1.34 ± 0.47 | 0.739 | 0.832 |
| Patellar cartilage | 1.37 ± 0.48 | 1.45 ± 0.50 | 0.756 | 0.770 |
| Anterior cruciate ligament | 1.12 ± 0.33 | 1.14 ± 0.35 | 0.624 | 0.717 |
| Posterior cruciate ligament | 1.02 ± 0.16 | 1.04 ± 0.20 | 0.653 | 0.729 |
| Medial collateral ligament | 1.12 ± 0.33 | 1.12 ± 0.33 | 0.873 | 0.873 |
| Lateral collateral ligament | 1.01 ± 0.11 | 1.01 ± 0.11 | 0.810 | 0.814 |
| Medial meniscus | 1.27 ± 0.44 | 1.24 ± 0.43 | 0.784 | 0.767 |
| Lateral meniscus | 1.14 ± 0.35 | 1.11 ± 0.32 | 0.710 | 0.720 |
| Joint effusion | 1.32 ± 0.47 | 1.31 ± 0.46 | 0.807 | 0.736 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaniewska, M.; Deininger-Czermak, E.; Lohezic, M.; Ensle, F.; Guggenberger, R. Deep Learning Convolutional Neural Network Reconstruction and Radial k-Space Acquisition MR Technique for Enhanced Detection of Retropatellar Cartilage Lesions of the Knee Joint. Diagnostics 2023, 13, 2438. https://doi.org/10.3390/diagnostics13142438
Kaniewska M, Deininger-Czermak E, Lohezic M, Ensle F, Guggenberger R. Deep Learning Convolutional Neural Network Reconstruction and Radial k-Space Acquisition MR Technique for Enhanced Detection of Retropatellar Cartilage Lesions of the Knee Joint. Diagnostics. 2023; 13(14):2438. https://doi.org/10.3390/diagnostics13142438
Chicago/Turabian StyleKaniewska, Malwina, Eva Deininger-Czermak, Maelene Lohezic, Falko Ensle, and Roman Guggenberger. 2023. "Deep Learning Convolutional Neural Network Reconstruction and Radial k-Space Acquisition MR Technique for Enhanced Detection of Retropatellar Cartilage Lesions of the Knee Joint" Diagnostics 13, no. 14: 2438. https://doi.org/10.3390/diagnostics13142438
APA StyleKaniewska, M., Deininger-Czermak, E., Lohezic, M., Ensle, F., & Guggenberger, R. (2023). Deep Learning Convolutional Neural Network Reconstruction and Radial k-Space Acquisition MR Technique for Enhanced Detection of Retropatellar Cartilage Lesions of the Knee Joint. Diagnostics, 13(14), 2438. https://doi.org/10.3390/diagnostics13142438





