Pediatric COVID-19 and Diabetes: An Investigation into the Intersection of Two Pandemics
Abstract
1. Introduction
1.1. T1DM
1.2. T2DM
2. Diabetes Mellitus and COVID-19 in Children
2.1. T1DM and COVID-19
2.2. T2DM and COVID-19
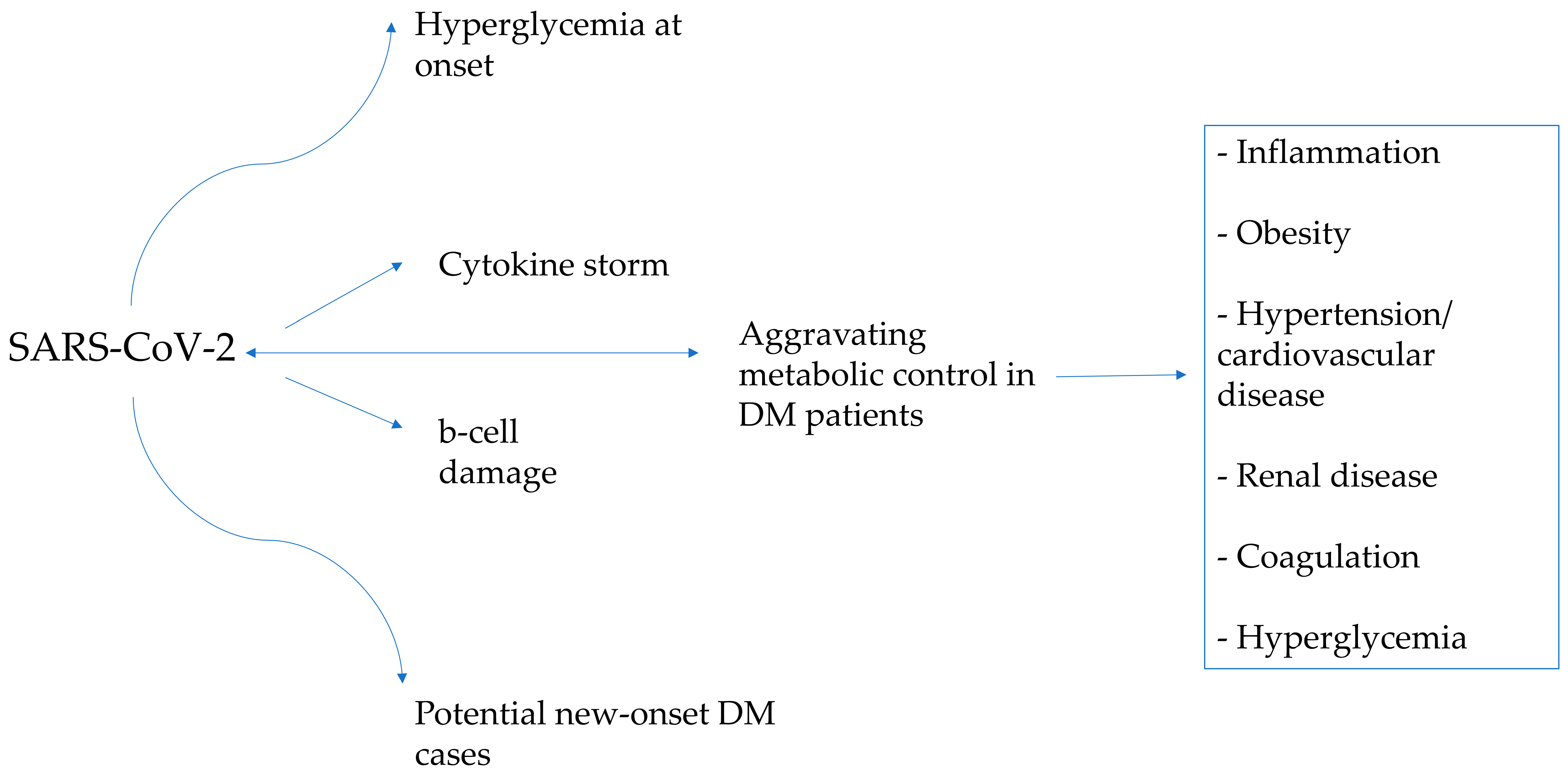
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cardona-Hernandez, R.; Cherubini, V.; Iafusco, D.; Schiaffini, R.; Luo, X.; Maahs, D.M. Children and youth with diabetes are not at increased risk for hospitalization due to COVID-19. Pediatr. Diabetes 2021, 22, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Filippi, C.M.; von Herrath, M.G. Viral trigger for type 1 diabetes: Pros and cons. Diabetes 2008, 57, 2863–2871. [Google Scholar] [CrossRef] [PubMed]
- Graff, K.; Smith, C.; Silveira, L.; Jung, S.; Curran-Hays, S.; Jarjour, J.; Carpenter, L.; Pickard, K.; Mattiucci, M.; Fresia, J.; et al. Risk Factors for Severe COVID-19 in Children. Pediatr. Infect. Dis. J. 2021, 40, e137–e145. [Google Scholar] [CrossRef] [PubMed]
- Nielsen-Saines, K.; Li, E.; Olivera, A.M.; Martin-Blais, R.; Bulut, Y. Case Report: Insulin-Dependent Diabetes Mellitus and Diabetic Keto-Acidosis in a Child with COVID-19. Front. Pediatr. 2021, 9, 628810. [Google Scholar] [CrossRef] [PubMed]
- CDC COVID-19 Response Team. Coronavirus Disease 2019 in Children-United States, February 12–April 2, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 422–426. [Google Scholar] [CrossRef]
- Weisberg, S.P.; Connors, T.J.; Zhu, Y.; Baldwin, M.R.; Lin, W.H.; Wontakal, S.; Szabo, P.A.; Wells, S.B.; Dogra, P.; Gray, J.; et al. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat. Immunol. 2021, 22, 25–31. [Google Scholar] [CrossRef]
- Vogel, T.P.; Top, K.A.; Karatzios, C.; Hilmers, D.C.; Tapia, L.I.; Moceri, P.; Giovannini-Chami, L.; Wood, N.; Chandler, R.E.; Klein, N.P.; et al. Multisystem inflammatory syndrome in children and adults (MIS-C/A): Case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2021, 39, 3037–3049. [Google Scholar]
- Matic, K.M. SARS-CoV-2 and Multisystem Inflammatory Syndrome in Children (MIS-C). Curr. Probl. Pediatr. Adolesc. Health Care 2021, 51, 101000. [Google Scholar] [CrossRef]
- Mihai, C.M.; Chisnoiu, T.; Cambrea, C.S.; Frecus, C.E.; Mihai, L.; Balasa, A.L.; Stroe, A.Z.; Gogu, A.E.; Docu Axelerad, A. Neurological manifestations found in children with multisystem inflammatory syndrome. Exp. Ther. Med. 2022, 23, 261. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, L.; Du, H.; Zhang, J.; Li, Y.Y.; Qu, J.; Zhang, W.; Wang, Y.; Bao, S.; Li, Y.; et al. SARS-CoV-2 Infection in Children. N. Engl. J. Med. 2020, 382, 1663–1665. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41 (Suppl. 1), S13–S27. [Google Scholar] [CrossRef] [PubMed]
- Roep, B.O.; Thomaidou, S.; van Tienhoven, R.; Zaldumbide, A. Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?). Nat. Rev. Endocrinol. 2021, 17, 150–161. [Google Scholar] [CrossRef]
- Crawford, J.M. Liver, biliary tract, and pancreas. In Pocket Companion to Robbins Pathologic Basis of Disease; Robbins, S.L., Cotran, R.S., Kumar, V., Eds.; WB Saunders: Philadelphia, PA, USA, 1991; pp. 313–317. [Google Scholar]
- Ziegler, R.; Neu, A. Diabetes in Childhood and Adolescence. Dtsch. Arztebl. Int. 2018, 115, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Wolfsdorf, J.I.; Allgrove, J.; Craig, M.E.; Edge, J.; Glaser, N.; Jain, V.; Lee, W.W.; Mungai, L.N.; Rosenbloom, A.L.; Sperling, M.A.; et al. ISPAD Clinical Practice Consensus Guidelines 2014. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr. Diabetes 2014, 15, 154–179. [Google Scholar] [CrossRef] [PubMed]
- Couper, J.J.; Haller, M.J.; Greenbaum, C.J.; Ziegler, A.G.; Wherrett, D.K.; Knip, M.; Craig, M.E. ISPAD Clinical Practice Consensus Guidelines 2018: Stages of type 1 diabetes in children and adolescents. Pediatr. Diabetes 2018, 19 (Suppl. 27), 20–27. [Google Scholar] [CrossRef] [PubMed]
- Couch, R.; Jetha, M.; Dryden, D.M.; Hooton, N.; Liang, Y.; Durec, T.; Sumamo, E.; Spooner, C.; Milne, A.; O’Gorman, K.; et al. Diabetes Education for Children with Type 1 Diabetes Mellitus and Their Families; (Evidence Reports/Technology Assessments, No. 166.) 1, Introduction; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2008.
- UK Hypoglycaemia Study Group. Risk of hypoglycaemia in types 1 and 2 diabetes: Effects of treatment modalities and their duration. Diabetologia 2007, 50, 1140–1147. [Google Scholar] [CrossRef]
- Rundle, A.G.; Park, Y.; Herbstman, J.B.; Kinsey, E.W.; Wang, Y.C. COVID-19 related school closings and risk of weight gain among children. Obesity 2020, 28, 1008. [Google Scholar] [CrossRef]
- Urakami, T. Severe Hypoglycemia: Is It Still a Threat for Children and Adolescents with Type 1 Diabetes? Front. Endocrinol. 2020, 11, 609. [Google Scholar] [CrossRef]
- Mine, K.; Nagafuchi, S.; Mori, H.; Takahashi, H.; Anzai, K. SARS-CoV-2 Infection and Pancreatic β Cell Failure. Biology 2022, 11, 22. [Google Scholar] [CrossRef]
- Vlad, A.; Serban, V.; Timar, R.; Sima, A.; Botea, V.; Albai, O.; Timar, B.; Vlad, M. Increased Incidence of Type 1 Diabetes during the COVID-19 Pandemic in Romanian Children. Medicina 2021, 57, 973. [Google Scholar] [CrossRef]
- Hollstein, T.; Schulte, D.M.; Schulz, J.; Glück, A.; Ziegler, A.G.; Bonifacio, E.; Wendorff, M.; Franke, A.; Schreiber, S.; Bornstein, S.R.; et al. Autoantibody-negative insulin-dependent diabetes mellitus after SARS-CoV-2 infection: A case report. Nat. Metab. 2020, 2, 1021–1024. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, T. Type 2 diabetes mellitus in children and adolescents. World J. Diabetes 2013, 4, 270–281. [Google Scholar] [CrossRef]
- Mayer-Davis, E.J.; Kahkoska, A.R.; Jefferies, C.; Dabelea, D.; Balde, N.; Gong, C.X.; Aschner, P.; Craig, M.E. ISPAD Clinical Practice Consensus Guidelines 2018: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr. Diabetes 2018, 19 (Suppl. 27), 7–19. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.M.; Carpenter, J.R.; Morris, T.P.; Sharma, M.; Petersen, I. Ethnic Differences in the Prevalence of Type 2 Diabetes Diagnoses in the UK: Cross-Sectional Analysis of the Health Improvement Network Primary Care Database. Clin. Epidemiol. 2019, 11, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Tamborlane, W.V.; Haymond, M.W.; Dunger, D.; Shankar, R.; Gubitosi-Klug, R.; Bethin, K.; Karres, J.; Tomasi, P.; Libman, I.; Hale, P.H.; et al. Expanding treatment options for youth with type 2 diabetes: Current problems and proposed solutions: A white paper from the NICHD diabetes working group. Diabetes Care 2016, 393, 323–329. [Google Scholar] [CrossRef]
- Hutchins, J.; Barajas, R.A.; Hale, D.; Escaname, E.; Lynch, J. Type 2 diabetes in a 5-year-old and single center experience of type 2 diabetes in youth under 10. Pediatr. Diabetes 2017, 18, 674–677. [Google Scholar] [CrossRef]
- Nambam, B.; Silverstein, J.; Cheng, P.; Ruedy, K.J.; Beck, R.W.; Paul Wadwa, R.; Klingensmith, G.; Willi, S.M.; Wood, J.R.; Bacha, F.; et al. A cross-sectional view of the current state of treatment of youth with type 2 diabetes in the USA: Enrollment data from the Pediatric Diabetes Consortium Type 2 Diabetes Registry: PDC Type 2 Diabetes Registry. Pediatr. Diabetes 2017, 18, 222–229. [Google Scholar] [CrossRef]
- Scaramuzza, A.E.; Rabbone, I.; Maffeis, C.; Schiaffini, R.; Diabetes Study Group of the Italian Society for Pediatric Endocrinology, Diabetes. Seasonal flu and COVID-19 recommendations for children, adolescents and young adults with diabetes. Diabet. Med. 2021, 38, e14427. [Google Scholar] [CrossRef]
- Güemes, M.; Storch-de-Gracia, P.; Enriquez, S.V.; Martín-Rivada, Á.; Brabin, A.G.; Argente, J. Severity in pediatric type 1 diabetes mellitus debut during the COVID-19 pandemic. J. Pediatr. Endocrinol. Metab. 2020, 33, 1601–1603. [Google Scholar] [CrossRef]
- Kamrath, C.; Mönkemöller, K.; Biester, T.; Rohrer, T.R.; Warncke, K.; Hammersen, J.; Holl, R.W. Ketoacidosis in Children and Adolescents with Newly Diagnosed Type 1 Diabetes During the COVID-19 Pandemic in Germany. JAMA 2020, 324, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Unsworth, R.; Wallace, S.; Oliver, N.S.; Yeung, S.; Kshirsagar, A.; Naidu, H.; Kwong, R.M.W.; Kumar, P.; Logan, K.M. New-onset type 1 diabetes in children during COVID-19: Multicenter regional findings in the U.K. Diabetes Care 2020, 43, e170–e171. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Uhl, S.; Zhang, T.; Xue, D.; Li, B.; Vandana, J.J.; Acklin, J.A.; Bonnycastle, L.L.; Narisu, N.; Erdos, M.R.; et al. SARS-CoV-2 infection induces beta cell transdifferentiation. Cell Metab. 2021, 33, 1577–1591.e7. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.V.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Wu, C.T.; Lidsky, P.V.; Xiao, Y.; Lee, I.T.; Cheng, R.; Nakayama, T.; Jiang, S.; Demeter, J.; Bevacqua, R.J.; Chang, C.A.; et al. SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment. Cell Metab. 2021, 33, 1565–1576.e5. [Google Scholar] [CrossRef]
- Shirakawa, J. Pancreatic β-cell fate in subjects with COVID-19. J. Diabetes Investig. 2021, 12, 2126–2128. [Google Scholar] [CrossRef]
- Tittel, S.R.; Rosenbauer, J.; Kamrath, C.; Ziegler, J.; Reschke, F.; Hammersen, J.; Mönkemöller, K.; Pappa, A.; Kapellen, T.; Holl, R.W. Did the COVID-19 Lockdown Affect the Incidence of Pediatric Type 1 Diabetes in Germany? Diabetes Care 2020, 43, e172–e173. [Google Scholar] [CrossRef]
- Vasconez, W.A.; Bustamante Escobar, C.L.; Agarwal, N.; Solano, J.P.; Sanchez, J.E. Severe Diabetic Ketoacidosis in a Child with Type-1 Diabetes, Asthma, and COVID-19. J. Pediatr. Intensive Care 2021, 10, 232–234. [Google Scholar] [CrossRef]
- Ordooei, M.; Behniafard, N.; Soheilipour, F.; Akbarian, E. New onset of diabetes in a child infected with COVID-19: A case report. J. Diabetes Metab. Disord. 2021, 20, 2129–2132. [Google Scholar] [CrossRef]
- Brothers, E.M.; Lidsky, K.; Simmons, J.; Nakagawa, T. A Child With COVID-19, Type 1 Diabetes, and Candida glabrata: A Case Report and Literature Review. Clin. Pediatr. 2021, 60, 554–558. [Google Scholar] [CrossRef]
- Naguib, M.N.; Raymond, J.K.; Vidmar, A.P. New onset diabetes with diabetic ketoacidosis in a child with multisystem inflammatory syndrome due to COVID-19. J. Pediatr. Endocrinol. Metab. 2020, 34, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Grigore, I.; Miron, I.; Gavrilovici, C.; Lupu, V.V.; Antal, D.C.; Schreiner, T.G.; Prazaru, C.; Lupu, A.; Dragan, F.; Grigore, E. SARS-CoV-2 Possible Etiology of Cerebral Venous Thrombosis in a Teenager: Case Report and Review of Literature. Viruses 2023, 15, 405. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, F.; O’Halloran, A.; Alghamdi, A.; Chen, C.; Trissal, M.; Traum, A.; DeCourcey, D. Toddler with New Onset Diabetes and Atypical Hemolytic-Uremic Syndrome in the Setting of COVID-19. Pediatrics 2021, 147, e2020016774. [Google Scholar] [CrossRef] [PubMed]
- Rabbone, I.; Schiaffini, R.; Cherubini, V.; Maffeis, C.; Scaramuzza, A.; Diabetes Study Group of the Italian Society for Pediatric Endocrinology and Diabetes. Has COVID-19 Delayed the Diagnosis and Worsened the Presentation of Type 1 Diabetes in Children? Diabetes Care 2020, 43, 2870–2872. [Google Scholar] [CrossRef]
- Dayal, D.; Gupta, S.; Raithatha, D.; Jayashree, M. Missing during COVID-19 lockdown: Children with onset of type 1 diabetes. Acta Paediatr. 2020, 109, 2144–2146. [Google Scholar] [CrossRef]
- Williams, G.; McLean, R.; Liu, J.F.; Ritzmann, T.A.; Dandapani, M.; Shanmugavadivel, D.; Sachdev, P.; Brougham, M.; Mitchell, R.T.; Conway, N.T.; et al. Multicentre service evaluation of presentation of newly diagnosed cancers and type 1 diabetes in children in the UK during the COVID-19 pandemic. BMJ Paediatr. Open 2021, 5, e001078. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Gottesman, B.L.; Yu, J.; Tanaka, C.; Longhurst, C.A.; Kim, J.J. Incidence of New-Onset Type 1 Diabetes among US Children during the COVID-19 Global Pandemic. JAMA Pediatr. 2022, 176, 414–415. [Google Scholar] [CrossRef]
- Chambers, M.A.; Mecham, C.; Arreola, E.V.; Sinha, M. Increase in the number of pediatric new-onset diabetes and diabetic ketoacidosis cases during the COVID-19 pandemic. Endocr. Pract. 2022, 28, 479–485. [Google Scholar] [CrossRef]
- Dżygało, K.; Nowaczyk, J.; Szwilling, A.; Kowalska, A. Increased frequency of severe diabetic ketoacidosis at type 1 diabetes onset among children during COVID-19 pandemic lockdown: An observational cohort study. Pediatr. Endocrinol. Diabetes Metab. 2020, 26, 167–175. [Google Scholar] [CrossRef]
- Ng, S.M.; Woodger, K.; Regan, F.; Soni, A.; Wright, N.; Agwu, J.C.; Williams, E.; Timmis, A.; Kershaw, M.; Moudiotis, C.; et al. Presentation of newly diagnosed type 1 diabetes in children and young people during COVID-19: A national UK survey. BMJ Paediatr. Open 2020, 4, e000884. [Google Scholar] [CrossRef] [PubMed]
- Mameli, C.; Scaramuzza, A.; Macedoni, M.; Marano, G.; Frontino, G.; Luconi, E.; Pelliccia, C.; Felappi, B.; Guerraggio, L.P.; Spiri, D.; et al. Type 1 diabetes onset in Lombardy region, Italy, during the COVID-19 pandemic: The doublewave occurrence. EClinicalMedicine 2021, 39, 101067. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Rosolowsky, E.; Pacaud, D.; Huang, C.; Lemay, J.A.; Brockman, N.; Rath, M.; Doulla, M. Diabetic ketoacidosis at type 1 diabetes diagnosis in children during the COVID-19 pandemic. Pediatr. Diabetes 2021, 22, 552–557. [Google Scholar] [CrossRef] [PubMed]
- McGlacken-Byrne, S.M.; Drew, S.E.V.; Turner, K.; Peters, C.; Amin, R. The SARS-CoV-2 pandemic is associated with increased severity of presentation of childhood onset type 1 diabetes mellitus: A multi-centre study of the first COVID-19 wave. Diabet. Med. 2021, 38, e14640. [Google Scholar] [CrossRef]
- Alonso, G.T.; Ebekozien, O.; Gallagher, M.P.; Rompicherla, S.; Lyons, S.K.; Choudhary, A.; Majidi, S.; Pinnaro, C.T.; Balachandar, S.; Gangat, M.; et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J. Diabetes 2021, 13, 681–687. [Google Scholar] [CrossRef]
- Trieu, C.; Sunil, B.; Ashraf, A.P.; Cooper, J.; Yarbrough, A.; Pinninti, S.; Boppana, S. SARS-CoV-2 infection in hospitalized children with type 1 and type 2 diabetes. J. Clin. Transl. Endocrinol. 2021, 26, 100271. [Google Scholar] [CrossRef]
- Kostopoulou, E.; Eliopoulou, M.I.; Rojas Gil, A.P.; Chrysis, D. Impact of COVID-19 on new-onset type 1 diabetes mellitus—A one-year prospective study. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5928–5935. [Google Scholar] [CrossRef]
- Pulungan, A.B.; Afifa, I.T.; Annisa, D. Type 2 diabetes mellitus in children and adolescent: An Indonesian perspective. Ann. Pediatr. Endocrinol. Metab. 2018, 23, 119. [Google Scholar] [CrossRef]
- Prosperi, S.; Chiarelli, F. COVID-19 and diabetes in children. Ann. Pediatr. Endocrinol. Metab. 2022, 27, 157–168. [Google Scholar] [CrossRef]
- Boutari, C.; Mantzoros, C.S. A 2022 update on the epidemiology of obesity and a call to action: As its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 2022, 133, 155217. [Google Scholar] [CrossRef]
- Adams, E.L.; Caccavale, L.J.; Smith, D.; Bean, M.K. Food Insecurity, the Home Food Environment, and Parent Feeding Practices in the Era of COVID-19. Obesity 2020, 28, 2056–2063. [Google Scholar] [CrossRef] [PubMed]
- Dutta, M. COVID-19 and Impact of School Closures on the Children of the United States; a Point of View with an Empirical Analysis. SSRN J. 2020, 3596096. [Google Scholar] [CrossRef]
- Fernandez-Rio, J.; Cecchini, J.A.; Mendez-Gimenez, A.; Carriedo, A. Weight changes during the COVID-19 home confinement. Effects on psychosocial variables. Obes. Res. Clin. Pract. 2020, 14, 383–385. [Google Scholar] [CrossRef]
- Sidor, A.; Rzymski, P. Dietary Choices and Habits during COVID-19 Lockdown: Experience from Poland. Nutrients 2020, 12, 1657. [Google Scholar] [CrossRef] [PubMed]
- Vandoni, M.; Codella, R.; Pippi, R.; Carnevale Pellino, V.; Lovecchio, N.; Marin, L.; Silvestri, D.; Gatti, A.; Magenes, V.C.; Regalbuto, C.; et al. Combatting Sedentary Behaviors by Delivering Remote Physical Exercise in Children and Adolescents with Obesity in the COVID-19 Era: A Narrative Review. Nutrients 2021, 13, 4459. [Google Scholar] [CrossRef]
- Al Heialy, S.; Hachim, M.Y.; Senok, A.; Gaudet, M.; Abou Tayoun, A.; Hamoudi, R.; Alsheikh-Ali, A.; Hamid, Q. Regulation of Angiotensin- Converting Enzyme 2 in Obesity: Implications for COVID-19. Front. Physiol. 2020, 11, 555039. [Google Scholar] [CrossRef]
- Dicker, D.; Bettini, S.; Farpour-Lambert, N.; Frühbeck, G.; Golan, R.; Goossens, G.; Halford, J.; O’Malley, G.; Mullerova, D.; Ramos Salas, X.; et al. Obesity and COVID-19: The Two Sides of the Coin. Obes. Facts 2020, 13, 430–438. [Google Scholar] [CrossRef]
- Nogueira-de-Almeida, C.A.; Del Ciampo, L.A.; Ferraz, I.S.; Del Ciampo, I.R.L.; Contini, A.A.; Ued, F.D.V. COVID-19 and obesity in childhood and adolescence: A clinical review. J. Pediatr. 2020, 96, 546–558. [Google Scholar] [CrossRef]
- Tsankov, B.K.; Allaire, J.M.; Irvine, M.A.; Lopez, A.A.; Sauvé, L.J.; Vallance, B.A.; Jacobson, K. Severe COVID-19 Infection and Pediatric Comorbidities: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2021, 103, 246–256. [Google Scholar] [CrossRef]
- Neshteruk, C.D.; Zizzi, A.; Suarez, L.; Erickson, E.; Kraus, W.E.; Li, J.S.; Skinner, A.C.; Story, M.; Zucker, N.; Armstrong, S.C. Weight-Related Behaviors of Children with Obesity during the COVID-19 Pandemic. Child. Obes. 2021, 17, 371–378. [Google Scholar] [CrossRef]
- Storz, M.A. The COVID-19 pandemic: An unprecedented tragedy in the battle against childhood obesity. Clin. Exp. Pediatr. 2020, 63, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.J.; Kompaniyets, L.; Freedman, D.S.; Kraus, E.M.; Porter, R.; Blanck, H.M.; Goodman, A.B. Longitudinal trends in body mass index before and during the COVID-19 pandemic among persons aged 2–19 years—United States, 2018–2020. Morb. Mortal. Wkly. Rep. 2021, 70, 1278. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Luo, S.; Zheng, X.; Ding, Y.; Wang, S.; Ling, P.; Yue, T.; Xu, W.; Yan, J.; Weng, J. Glycemic control in children and teenagers with type 1 diabetes around lockdown for COVID-19: A continuous glucose monitoring-based observational study. J Diabetes Investig. 2021, 12, 1708–1717. [Google Scholar] [CrossRef]
- Anderson, L.N.; Yoshida-Montezuma, Y.; Dewart, N.; Jalil, E.; Khattar, J.; De Rubeis, V.; Carsley, S.; Griffith, L.E.; Mbuagbaw, L. Obesity and weight change during the COVID-19 pandemic in children and adults: A systematic review and meta-analysis. Obes. Rev. 2023, 24, e13550. [Google Scholar] [CrossRef]
- Sasidharan Pillai, S.; Has, P.; Quintos, J.B.; Serrano Gonzalez, M.; Kasper, V.L.; Topor, L.S.; Fredette, M.E. Incidence, Severity, and Presentation of Type 2 Diabetes in Youth During the First and Second Year of the COVID-19 Pandemic. Diabetes Care 2023, 46, dc221702. [Google Scholar] [CrossRef]
- Bond, D.M.; Seimon, R.; Schneuer, F.J.; Baur, L.A.; Craig, M.; Alexander, S.; Garnett, S.P.; Henderson, J.; Nassar, N. Impact and recovery of the COVID-19 pandemic on weight status of children and adolescents. Clin. Obes. 2023, 13, e12579. [Google Scholar] [CrossRef]
- Rubino, F.; Amiel, S.A.; Zimmet, P.; Alberti, G.; Bornstein, S.; Eckel, R.H.; Mingrone, G.; Boehm, B.; Cooper, M.E.; Chai, Z.; et al. New-onset diabetes in COVID-19. N. Engl. J. Med. 2020, 383, 789–790. [Google Scholar] [CrossRef]
- Hsia, D.S.; Lim, M.; Beyl, R.A.; Hasan, H.A.; Gardner, J. Initial Presentation of Children with Type 2 Diabetes during the COVID-19 Pandemic. Diabetes 2021, 70 (Suppl. 1), 153-LB. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, M.; Oh, K.; Kang, E.; Rhie, Y.J.; Lee, J.; Hong, Y.H.; Shin, Y.L.; Kim, J.H. Comparison of Initial Presentation of Pediatric Diabetes Before and During the Coronavirus Disease 2019 Pandemic Era. J. Korean Med. Sci. 2022, 37, e176. [Google Scholar] [CrossRef]
- Apicella, M.; Campopiano, M.C.; Mantuano, M.; Mazoni, L.; Coppelli, A.; Del Prato, S. COVID-19 in people with diabetes: Understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020, 8, 782–792, Erratum in Lancet Diabetes Endocrinol. 2020, 8, e5. [Google Scholar] [CrossRef]
- Ebekozien, O.A.; Noor, N.; Gallagher, M.P.; Alonso, G.T. Type 1 diabetes and COVID-19: Preliminary findings from a multicenter surveillance study in the US. Diabetes Care 2020, 43, e83–e85. [Google Scholar] [CrossRef]
- Gayoso, M.; Lim, W.Y.; Mulekar, M.S.; Kaulfers, A.D. Effect of COVID-19 quarantine on diabetes Care in Children. Clin. Diabetes Endocrinol. 2021, 7, 9. [Google Scholar] [CrossRef]
- Shawar, R.S.; Cymbaluk, A.L.; Bell, J.J.; Patel, T.; Treybig, C.W.; Poland, T.R.; DeSalvo, D.J.; Sonabend, R.Y.; Lyons, S.K.; Lin, Y. Isolation and Education During a Pandemic: Novel Telehealth Approach to Family Education for a Child with New-Onset Type 1 Diabetes and Concomitant COVID-19. Clin Diabetes 2021, 39, 124–127. [Google Scholar] [CrossRef]
- Zabeen, B.; Ahmed, B.; Nahar, J. Young people with type 1 diabetes on insulin pump therapy could fast safely during COVID-19 pandemic Ramadan: A telemonitoring experience in Bangladesh. J. Diabetes Investig. 2021, 12, 1060–1063. [Google Scholar] [CrossRef]
- Alharthi, S.K.; Alyusuf, E.Y.; Alguwaihes, A.M.; Alfadda, A.; Al-Sofiani, M.E. The impact of a prolonged lockdown and use of telemedicine on glycemic control in people with type 1 diabetes during the COVID-19 outbreak in Saudi Arabia. Diabetes Res. Clin. Pract. 2021, 173, 108682. [Google Scholar] [CrossRef]
- Sarteau, A.C.; Souris, K.J.; Wang, J.; Ramadan, A.A.; Addala, A.; Bowlby, D.; Corathers, S.; Forsander, G.; King, B.; Law, J.R.; et al. Changes to care delivery at nine international pediatric diabetes clinics in response to the COVID-19 global pandemic. Pediatr. Diabetes 2021, 22, 463–468. [Google Scholar] [CrossRef]
- Rachmiel, M.; Lebenthal, Y.; Mazor-Aronovitch, K.; Brener, A.; Levek, N.; Levran, N.; Chorna, E.; Dekel, M.; Barash, G.; Landau, Z.; et al. Glycaemic control in the paediatric and young adult population with type 1 diabetes following a single telehealth visit-What have we learned from the COVID-19 lockdown? Acta Diabetol. 2021, 58, 697–705. [Google Scholar] [CrossRef]
- Salmi, H.; Heinonen, S.; Hästbacka, J.; Lääperi, M.; Rautiainen, P.; Miettinen, P.J.; Vapalahti, O.; Hepojoki, J.; Knip, M. New-onset type 1 diabetes in Finnish children during the COVID-19 pandemic. Arch. Dis. Child. 2021, 107, 180–185. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; Pivari, F.; Soldati, L.; Attinà, A.; Cinelli, G.; Leggeri, C.; Caparello, G.; Barrea, L.; Scerbo, F.; et al. Eating habits and lifestyle changes during COVID-19 lockdown: An Italian survey. J. Transl. Med. 2020, 18, 229. [Google Scholar] [CrossRef]
- Predieri, B.; Leo, F.; Candia, F.; Lucaccioni, L.; Madeo, S.F.; Pugliese, M.; Vivaccia, V.; Bruzzi, P.; Iughetti, L. Glycemic Control Improvement in Italian Children and Adolescents with Type 1 Diabetes Followed Through Telemedicine during Lockdown due to the COVID-19 Pandemic. Front. Endocrinol. 2020, 11, 595735. [Google Scholar] [CrossRef]
- Lazzeroni, P.; Motta, M.; Monaco, S.; Laudisio, S.R.; Furoncoli, D.; Maffini, V.; Rubini, M.; Tchana, B.; Ruberto, C.; Dodi, I.; et al. Improvement in glycaemic control in paediatric and young adult type 1 diabetes patients during COVID-19 pandemic: Role of telemedicine and lifestyle changes. Acta Biomed. 2021, 92, e2021399. [Google Scholar] [CrossRef] [PubMed]
- Nwosu, B.U.; Al-Halbouni, L.; Parajuli, S.; Jasmin, G.; Zitek-Morrison, E.; Barton, B.A. COVID-19 Pandemic and Pediatric Type 1 Diabetes: No Significant Change in Glycemic Control during the Pandemic Lockdown of 2020. Front. Endocrinol. 2021, 12, 703905. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J. Effect of COVID-19 pandemic on treatment of Type 1 diabetes in children. Acta Paediatr. 2021, 110, 933–934. [Google Scholar] [CrossRef] [PubMed]
- Mianowska, B.; Fedorczak, A.; Michalak, A.; Pokora, W.; Barańska-Nowicka, I.; Wilczyńska, M.; Szadkowska, A. Diabetes Related Distress in Children with Type 1 Diabetes before and during the COVID-19 Lockdown in Spring 2020. Int. J. Environ. Res. Public Health 2021, 18, 8527. [Google Scholar] [CrossRef]
- Elbarbary, N.S.; Dos Santos, T.J.; de Beaufort, C.; Wiltshire, E.; Pulungan, A.; Scaramuzza, A.E. The Challenges of Managing Pediatric Diabetes and Other Endocrine Disorders during the COVID-19 Pandemic: Results from an International Cross-Sectional Electronic Survey. Front. Endocrinol. 2021, 12, 735554. [Google Scholar] [CrossRef] [PubMed]
- Nicodemo, M.; Spreghini, M.R.; Manco, M.; Wietrzykowska Sforza, R.; Morino, G. Childhood Obesity and COVID-19 Lockdown: Remarks on Eating Habits of Patients Enrolled in a Food-Education Program. Nutrients 2021, 13, 383. [Google Scholar] [CrossRef]
- Schmidt, S.C.E.; Anedda, B.; Burchartz, A.; Eichsteller, A.; Kolb, S.; Nigg, C.; Niessner, C.; Oriwol, D.; Worth, A.; Woll, A. Physical activity and screen time of children and adolescents before and during the COVID-19 lockdown in Germany: A natural experiment. Sci. Rep. 2020, 10, 21780. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.K.; Gupta, J.; Chowdhury, S.R.; Kumar, R.; Meena, A.K.; Madaan, P.; Sharawat, I.K.; Gulati, S. Psychological and Behavioral Impact of Lock-down and Quarantine Measures for COVID-19 Pandemic on Children, Adolescents and Caregivers: A Systematic Review and Meta-Analysis. J. Trop. Pediatr. 2021, 67, fmaa122. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Keshvari, M.; Mirnasuri, S.; Yon, D.K.; Lee, S.W.; Il Shin, J.; Smith, L. The global impact of COVID-19 pandemic on the incidence of pediatric new-onset type 1 diabetes and ketoacidosis: A systematic review and meta-analysis. J. Med. Virol. 2022, 94, 5112–5127. [Google Scholar] [CrossRef]
- de Sá-Ferreira, C.O.; da Costa, C.H.M.; Guimarães, J.C.W.; Sampaio, N.S.; Silva, L.M.L.; de Mascarenhas, L.P.; Rodrigues, N.G.; Dos Santos, T.L.; Campos, S.; Young, E.C. Diabetic ketoacidosis and COVID-19: What have we learned so far? Am. J. Physiol. Endocrinol. Metab. 2022, 322, E44–E53. [Google Scholar] [CrossRef]
- Alamuri, T.T.; Mahesh, S.; Dell’Aquila, K.; Leong, T.J.; Jennings, R.; Duong, T.Q. COVID-19 associated ketosis and diabetic ketoacidosis: A rapid review. Diabetes Obes. Metab. 2023, 25, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Barrett, C.E.; Koyama, A.K.; Alvarez, P.; Chow, W.; Lundeen, E.A.; Perrine, C.G.; Pavkov, M.E.; Rolka, D.B.; Wiltz, J.L.; Bull-Otterson, L.; et al. Risk for Newly Diagnosed Diabetes >30 Days after SARS-CoV-2 Infection among Persons Aged <18 Years-United States, March 1, 2020–June 28, 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.Y.; Wang, S.H.; Duong, T.Q. Patients with prediabetes are at greater risk of developing diabetes 5 months postacute SARS-CoV-2 infection: A retrospective cohort study. BMJ Open Diabetes Res. Care 2023, 11, e003257. [Google Scholar] [CrossRef] [PubMed]
- Wander, P.L.; Lowy, E.; Beste, L.A.; Tulloch-Palomino, L.; Korpak, A.; Peterson, A.C.; Kahn, S.E.; Boyko, E.J. The Incidence of Diabetes Among 2,777,768 Veterans with and without Recent SARS-CoV-2 Infection. Diabetes Care 2022, 45, 782–788, Erratum in Diabetes Care 2023. [Google Scholar] [CrossRef] [PubMed]
- Ilic, I.; Ilic, M. Diabetes Mellitus after SARS-CoV-2 Infection: An Epidemiological Review. Life 2023, 13, 1233. [Google Scholar] [CrossRef]
- Ng, S.M. COVID-19 and children with diabetes: Emerging knowledge. Pract. Diabetes 2020, 37, 147–148a. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Pal, R.; Bhadada, S.K.; Misra, A. COVID-19 vaccination in patients with diabetes mellitus: Current concepts, uncertainties and challenges. Diabetes Metab. Syndr. 2021, 15, 505–508. [Google Scholar] [CrossRef]
- Gregory, J.M.; Slaughter, J.C.; Duffus, S.H.; Smith, T.J.; LeStourgeon, L.M.; Jaser, S.S.; McCoy, A.B.; Luther, J.M.; Giovannetti, E.R.; Boeder, S.; et al. COVID-19 Severity Is Tripled in the Diabetes Community: A Prospective Analysis of the Pandemic’s Im-pact in Type 1 and Type 2 Diabetes. Diabetes Care 2021, 44, 526–532. [Google Scholar] [CrossRef]
- Mohseni Afshar, Z.; Babazadeh, A.; Janbakhsh, A.; Mansouri, F.; Sio, T.T.; Sullman, M.J.M.; Carson-Chahhoud, K.; Hosseinzadeh, R.; Barary, M.; Ebrahimpour, S. Coronavirus disease 2019 (COVID-19) vaccination recommendations in special populations and pa-tients with existing comorbidities. Rev. Med. Virol. 2022, 32, e2309. [Google Scholar]
- Muñoz, C.E.; Chao, L.C. Impact of COVID-19 on Youth with Type 2 Diabetes: Lessons Learned from a Pediatric Endocrinologist and a Psychologist. Front. Endocrinol. 2021, 12, 650492. [Google Scholar] [CrossRef] [PubMed]

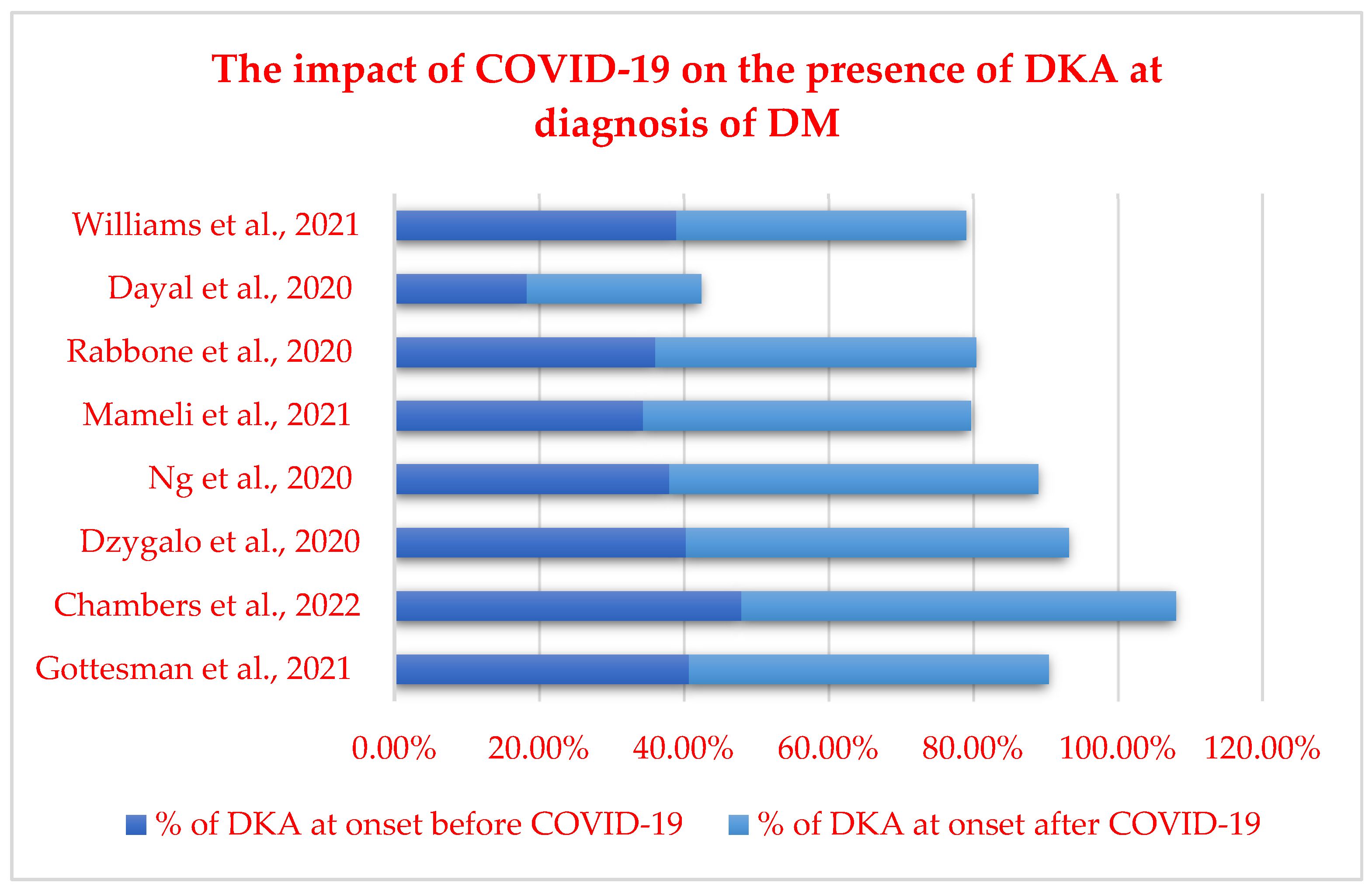
| Author | Year | Regions | Age (Years) | Study Period | Findings |
|---|---|---|---|---|---|
| Rabbone et al. [46] | 2020 | Italy | NA | 2019–2020 | Decrease in diabetes presentation and in severity of DKA |
| Dayal et al. [47] | 2020 | India | Pediatric population | April 2019–March 2020 | Major reduction in hospitalization of children with onset of T1DM in April 2020 |
| Brothers et al. [42] | 2021 | NA | 12 | NA | Sepsis due to Candida glabrata in a teenager with COVID-19 and T1DM |
| Williams et al. [48] | 2021 | United Kingdom | Pediatric population | 1 January–31 July 2020 | No evidence of diagnostic delay or increased illness severity for childhood cancer or T1DM |
| Ng et al. [108] | 2020 | United Kingdom | Pediatric population | 1 March 2020–30 June 2020 | An increased percentage of DKA was observed during the pandemic |
| Mianowska et al. [96] | 2021 | Poland | T1DM aged 8–18 years and their parents | November 2019–February 2020 | COVID-19 lockdown did not seem to aggravate diabetes manifestations |
| Rahmati et al. [101] | 2022 | NA | T1DM pediatric patients | Up to March 2022 | Notable increase in the worldwide incidence of pediatric new-onset T1DM, DKA, and severe DKA during the initial year of the pandemic |
| Lee et al. [81] | 2022 | South Korea | T1DM or T2DM patients <18 years | 2018–2020 | During the pandemic, the proportion of DKA cases increased compared to the pre-pandemic period |
| Wu et al. [109] | 2021 | China | T1DM pediatric patients | 1 November 2019–31 July 2020 | Glycemic control did not decrease in T1DM pediatric patients during the COVID-19 pandemic |
| Sadisharan Pillai et al. [77] | 2023 | United States | New-onset T2DM pediatric patients | 1 January 2017–31 December 2021 | The yearly occurrence of T2DM demonstrated an almost threefold rise during the pandemic compared to the preceding period |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotea, S.; Ghiciuc, C.M.; Stefanescu, G.; Cianga, A.L.; Mihai, C.M.; Lupu, A.; Butnariu, L.I.; Starcea, I.M.; Salaru, D.L.; Mocanu, A.; et al. Pediatric COVID-19 and Diabetes: An Investigation into the Intersection of Two Pandemics. Diagnostics 2023, 13, 2436. https://doi.org/10.3390/diagnostics13142436
Fotea S, Ghiciuc CM, Stefanescu G, Cianga AL, Mihai CM, Lupu A, Butnariu LI, Starcea IM, Salaru DL, Mocanu A, et al. Pediatric COVID-19 and Diabetes: An Investigation into the Intersection of Two Pandemics. Diagnostics. 2023; 13(14):2436. https://doi.org/10.3390/diagnostics13142436
Chicago/Turabian StyleFotea, Silvia, Cristina Mihaela Ghiciuc, Gabriela Stefanescu, Anca Lavinia Cianga, Cristina Maria Mihai, Ancuta Lupu, Lacramioara Ionela Butnariu, Iuliana Magdalena Starcea, Delia Lidia Salaru, Adriana Mocanu, and et al. 2023. "Pediatric COVID-19 and Diabetes: An Investigation into the Intersection of Two Pandemics" Diagnostics 13, no. 14: 2436. https://doi.org/10.3390/diagnostics13142436
APA StyleFotea, S., Ghiciuc, C. M., Stefanescu, G., Cianga, A. L., Mihai, C. M., Lupu, A., Butnariu, L. I., Starcea, I. M., Salaru, D. L., Mocanu, A., Chisnoiu, T., Thet, A. A., Miron, L., & Lupu, V. V. (2023). Pediatric COVID-19 and Diabetes: An Investigation into the Intersection of Two Pandemics. Diagnostics, 13(14), 2436. https://doi.org/10.3390/diagnostics13142436







