Abstract
While ground-glass opacity, consolidation, and fibrosis in the lungs are some of the hallmarks of acute SAR-CoV-2 infection, it remains unclear whether these pulmonary radiological findings would resolve after acute symptoms have subsided. We conducted a systematic review and meta-analysis to evaluate chest computed tomography (CT) abnormalities stratified by COVID-19 disease severity and multiple timepoints post-infection. PubMed/MEDLINE was searched for relevant articles until 23 May 2023. Studies with COVID-19-recovered patients and follow-up chest CT at least 12 months post-infection were included. CT findings were evaluated at short-term (1–6 months) and long-term (12–24 months) follow-ups and by disease severity (severe and non-severe). A generalized linear mixed-effects model with random effects was used to estimate event rates for CT findings. A total of 2517 studies were identified, of which 43 met the inclusion (N = 8858 patients). Fibrotic-like changes had the highest event rate at short-term (0.44 [0.3–0.59]) and long-term (0.38 [0.23–0.56]) follow-ups. A meta-regression showed that over time the event rates decreased for any abnormality (β = −0.137, p = 0.002), ground-glass opacities (β = −0.169, p < 0.001), increased for honeycombing (β = 0.075, p = 0.03), and did not change for fibrotic-like changes, bronchiectasis, reticulation, and interlobular septal thickening (p > 0.05 for all). The severe subgroup had significantly higher rates of any abnormalities (p < 0.001), bronchiectasis (p = 0.02), fibrotic-like changes (p = 0.03), and reticulation (p < 0.001) at long-term follow-ups when compared to the non-severe subgroup. In conclusion, significant CT abnormalities remained up to 2 years post-COVID-19, especially in patients with severe disease. Long-lasting pulmonary abnormalities post-SARS-CoV-2 infection signal a future public health concern, necessitating extended monitoring, rehabilitation, survivor support, vaccination, and ongoing research for targeted therapies.
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has been a watershed moment in global health, causing unprecedented strain on individual well-being and healthcare systems [1]. As the catastrophic waves of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection begin to subside, the long-term consequences of the virus are coming into focus. There is mounting evidence that COVID-19 has effects, such as chronic cough, dyspnea, increased susceptibility to pulmonary infections, and intolerance to exercise, that persist well beyond the acute phase, commonly referred to as “long COVID” [2,3,4,5]. A recent meta-analysis revealed that about one-third of non-hospitalized patients, and more than half of hospitalized patients, reported persistent symptoms up to a year post-COVID [6].
Although the exact etiology of long COVID is currently unknown, it has been hypothesized to occur as a result of potential long-term tissue damage due to pulmonary-cardiovascular compromise, sepsis, and pathological inflammation during the acute and subacute phases of COVID-19 [7,8]. Lung damage, in particular, may play a significant role in the development of long COVID, since respiratory symptoms, such as cough, chest pain, and dyspnea are common presenting symptoms in those infected with SARS-CoV-2 [8,9,10,11]. Residual pulmonary damage could also contribute to the development to new clinical disorders (such as cardiac disorders, hypertension, diabetes, and renal disorders) as well as the worsening of pre-existing clinical disorders among individuals with COVID-19 compared to those of matched controls [12,13,14,15,16,17,18,19,20], which has broad health implications. Thoracic imaging, such a computed tomography (CT), can be used to evaluate the residual pulmonary effects from COVID-19 and provide valuable insights into the long-term morphological changes in the respiratory system, with ground-glass opacities (GGO), consolidations, and fibrosis being characteristic features frequently identified in the chest imaging of individuals with acute COVID-19 [8,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35]. Emerging evidence suggests that the above-mentioned lingering respiratory symptoms are often accompanied by distinct CT findings, providing a visual narrative of the protracted aftermath of SARS-CoV-2 infection.
Numerous studies have reported CT lung abnormalities post-COVID [22,36,37], including a limited number of reviews and meta-analyses [8,38,39,40,41]. However, only a few studies covered multiple timepoints post-COVID [39,40] or were stratified by COVID-19 disease severity [40], and none covered beyond 12 months post-COVID. This meta-analysis of CT lung abnormalities post-COVID aims to build on prior meta-analyses by including more recent chest CT studies, studies at longer durations (up to 2 years post-COVID), as well as stratifying findings at multiple follow-ups and by COVID-19 disease severity. Our findings provide further insights into persistent lung abnormalities that could help inform clinical decision making and guide future research.
2. Materials and Methods
2.1. Protocol and Registration
The systematic review and meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [42]. The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO: CRD42023447766).
2.2. Eligibility Criteria
The inclusion criteria were as follows: (1) studies that included adult patients who recovered from acute COVID-19, confirmed by a SARS-CoV-2–positive reverse-transcription polymerase chain reaction test via nasopharyngeal swabs; (2) prospective or retrospective cohort studies, or cross-sectional studies; and (3) studies that included follow-up chest CT at least 12 months post-infection. Case reports, small case series (N < 10 patients), conference abstracts, and studies not in English were excluded.
2.3. Search Strategy
PubMed/MEDLINE was used to systematically search for relevant articles from 1 January 2020 to 23 May 2023. The search strategy included the following terms: ((“COVID” OR “COVID-19” OR “Coronavirus” OR “Coronavirus disease” OR “Coronavirus disease 2019” OR “SARS-CoV-2” OR “CoV-2” OR “SARS-CoV” OR “SARS” OR “Severe acute respiratory syndrome” OR “2019-nCoV” OR “nCoV” OR “Novel coronavirus”) AND (“Long-COVID” OR “Post-COVID” OR “Follow-up” OR “Long-term” OR “Chronic” OR “sequelae”) AND (“Computed tomography” OR “CT” OR “Chest CT”)) NOT (Review [Publication Type])).
After removing duplicates, two authors independently reviewed the search results using Covidence [43] and selected studies based on the inclusion criteria. Relevant studies were further identified through a manual search of secondary sources, including references of initially identified articles and reviews. After a full-text review, studies that met our eligibility criteria were included. Disagreements were resolved through consensus.
2.4. Data Extraction
Two authors independently extracted the data for study characteristics (author, year of publication, country, study design, percentage of patients with chest CT at long-term follow-up, longest follow-up time), patient characteristics (total sample size, age, sex, smoking habits, comorbidities), and chest-CT findings (any abnormalities, GGO, reticulation, consolidation, interlobular septal thickening, bronchiectasis, honeycombing, and fibrotic-like changes (combination of GGO, reticulation, bronchiectasis, and/or honeycombing)). Disagreements were resolved through consensus.
2.5. Meta-Analysis
2.5.1. Data Processing
Observational time intervals for CT findings were harmonized into monthly units. Time expressed in days was converted by a factor of 30, and when provided as a range, the midpoint was used for standardization. These intervals were aggregated into two broad temporal categories: short-term (≤6 months) and long-term (≥12 months).
The severe group was reported for patients with “severe” or “critical” COVID-19 disease severity, and the non-severe group was reported for patients with “mild” or “moderate” COVID-19 disease severity. Individuals who had any of the various signs and symptoms of COVID-19 but did not have shortness of breath, dyspnea, or abnormal chest imaging were classified as having “mild” disease. Individuals who showed lower respiratory disease during clinical assessment or imaging and who had an oxygen saturation ≥ 94% on room air at sea level were classified as having “moderate” disease. Individuals who had an oxygen saturation < 94% on room air at sea level, a ratio of an arterial partial pressure of oxygen to fraction of inspired oxygen < 300 mm Hg, a respiratory rate > 30 breaths/min, or lung infiltrates > 50% were classified as having “severe” disease. Finally, individuals who had respiratory failure, septic shock, and/or multiple organ dysfunctions were classified as having “critical” disease [44]. Cases that were not clearly “non-severe” or “severe” were categorized as “mixed”.
2.5.2. Statistical Analysis
A generalized linear mixed-effects model (GLMM) with a random-effects component was utilized to estimate pooled event rates for lung abnormalities. Logit transformation with a continuity correction of 0.5 for the zero event effect sizes was applied to individual study proportions to stabilize variances. Confidence intervals for individual studies were calculated using the Clopper–Pearson method. These estimations were made separately for the short- and long-term categories to avoid dependency between effect sizes. If a study reported multiple event rates in a time interval, only the last one was included in the GLMM model. Due to limited data points in some instances, the I2 statistic for heterogeneity was not always calculable. The data was represented in forest plots and figures after returning them to the original scale.
Meta-regression was utilized to inspect the impact of the time on the prevalence of lung abnormalities. For subgroup analysis, data classified as “mixed” for severity was excluded to focus on the “non-severe” and “severe” classifications. The p-value of Cochren’s Q was reported to indicate if there is a significant difference between the subgroups at the same time period. Statistical significance between CT findings at the 12- and 24-month follow-ups was calculated using a chi-square test. Meta-regression was also used in each severity strata to inspect relationships between the prevalence of lung abnormalities and time since a diagnosis of COVID-19. Statistical analysis was performed using the R statistical programming environment, version 4.3.1. Package meta, version 6.5, was used for all the meta-analysis elaborations.
2.6. Quality Assessment
The quality of each included study was critically appraised by two authors using the validated risk of bias tool by Hoy et al. [45], which comprises 10 items and a summary assessment. Items 1 to 4 assess the external validity of the study (selection and nonresponse bias), and items 5 to 10 assess the internal validity (items 5 to 9 assess measurement bias, and item 10 assesses bias related to the analysis). The final score for each study was categorized into three classes: 0–3, 4–6, and 7–9, indicating low, moderate, and high risk of bias, respectively.
To evaluate the presence of publication bias, funnel plots were generated for each pooled event rate of lung abnormalities. Publication bias was visually assessed through funnel plots.
3. Results
3.1. Study Selection and Characteristics
A total of 2517 studies were identified, of which 43 met the inclusion (N = 8858 patients) (Figure 1) [46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88]. The majority of studies were from China (14 studies [51,58,59,60,61,64,65,66,74,83,85,86,87,88], 32.6%) or Italy (13 studies [46,47,48,49,50,52,54,55,56,70,75,77,84], 30.2%), and were prospective in nature (41 studies [46,47,48,49,50,51,53,54,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88], 95.3%).
Figure 1.
Flowchart of study selection. A total of 2513 records were screened, 108 were assessed for eligibility, and 43 were included in the analysis.
Patients were infected with SARS-CoV-2 between December 2019 and December 2021. The median age of the patients was 60.3 years (57–63), with 61.5% being males and 37.8% being current/former smokers. Thirty-nine studies [47,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,65,66,67,68,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88] reported outcomes stratified by disease severity; 17 for non-severe disease, 31 for severe disease, and 7 for mixed disease severity (Table 1). The most common comorbidities were hypertension (35.8%), cardiovascular disease, (28%), and obesity (24.9%) (Supplemental Table S1).

Table 1.
Characteristics of included studies (n = 43). R: retrospective, P: prospective, H: hospitalized, NH: non-hospitalized, NR: not reported.
Of the 8858 patients included, 4223 (48%) had a chest CT at a short-term follow-up (median 3 months [3,4,5,6]) and 4872 patients (55%) had chest CT at long-term follow-up (median 12 months [12–12]). An overview of the lung abnormalities at long-term follow-ups is shown Table 2.

Table 2.
Chest-CT evaluation of residual lung abnormalities at long-term follow-up after COVID-19. NR: not reported.
3.2. Pooled Event Rates of Follow-Up Chest-CT Lung Abnormalities over Time for Entire Population
The interval between 6 and 12 months was not included in the pooled effect-sizes’ synthesis analysis because of an inadequate number of effect sizes but was utilized in the meta-regression. Figure 2 illustrates the pooled event rates of each CT lung abnormality over time. The forest plots are reported in Supplemental Figure S1 and summarized in Supplemental Table S2. Meta-regression analysis using the number of months since the diagnosis of COVID-19 as the predictor variable are reported in Supplemental Figure S2 and summarized in Supplemental Table S3. Subgroup analysis comparing chest-CT findings between the 12- and 24-month follow-ups is reported in Supplemental Table S4.
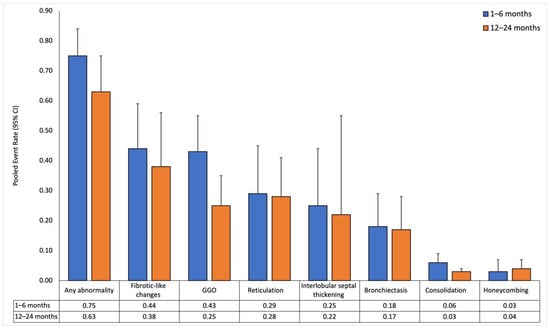
Figure 2.
Pooled event rates of chest-CT findings over time for all patients. Data is reported at short-term (1–6 months) and long-term (12–24 months) follow-ups.
3.2.1. Short-Term Follow-Up (1 to 6 Months)
Thirty-two studies reported sizes in the short-term follow-up (median 3 months [3,4,5,6], range 1–6 months). CT findings at this follow-up revealed a high prevalence of lung abnormalities. The pooled event rate of any abnormality was 0.75 (0.63–0.84) with high heterogeneity (I2: 0.89). Fibrotic-like changes were the most common abnormality, with a pooled event rate of 0.44 (0.3–0.59) and high heterogeneity (I2: 0.9). GGO followed closely, with a pooled event rate of 0.43 (0.32–0.55) and also with high heterogeneity (I2: 0.94). Honeycombing had the lowest pooled event rate of 0.03 (0.02–0.07), and I2 could not be estimated due to an insufficient number of effect sizes.
3.2.2. Long-Term Follow-Up (12 to 24 Months)
In 43 studies, long-term follow-up (median 12 months [12–12], range 12–24 months) showed a decrease in the pooled event rate of any abnormality to 0.63 (0.49–0.75) with high heterogeneity (I2: 0.95). GGO decreased to 0.25 (0.17–0.35) with high heterogeneity (I2: 0.93). Other abnormalities showed similar but smaller declining trends, except for honeycombing, which slightly increased to 0.04 (0.02–0.07) with low heterogeneity (I2: 0.4).
3.2.3. Temporal Trends in Chest-CT Lung Abnormalities
In the meta-regression analysis, any abnormality and GGO significantly decreased over time (β = −0.137, p = 0.002 and β = −0.169, p < 0.001, respectively). In contrast, honeycombing was associated with an upward trend (β = 0.075, p = 0.03). The other lung abnormalities did not show significant associations (p > 0.05 for all).
A total of 37 studies [47,48,49,50,51,52,53,54,56,57,60,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,78,79,80,81,82,83,84,85,86,87,88] reported chest-CT findings at 12 months, while only 3 studies [55,59,61] reported findings at 24 months. When comparing CT abnormalities between the 12- and 24-month follow-ups, consolidation (12 months: 3.6% vs. 24 months: 0.9%, p = 0.036) and interlobular septal thickening (12 months: 17.3% vs. 24 months: 7%, p = 0.043) significantly decreased over time. The other lung abnormalities showed no significant change between the 12- and 24-month follow-ups.
3.3. Pooled Event Rates of Follow-Up Chest-CT Lung Abnormalities over Time with COVID-19 Severity as the Mediator
The severity subgroup estimates for each chest-CT finding for the short- and long-term follow-ups are illustrated in Figure 3, and the forest plots for the conducted meta-analysis are shown in Supplemental Figure S3 and summarized in Supplemental Table S5. Meta-regression analysis for each severity strata using the number of months since the diagnosis of COVID-19 as the predictor variable is reported in Supplemental Figure S4 and summarized in Supplemental Table S6.
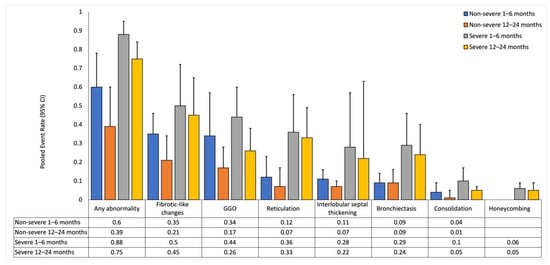
Figure 3.
Pooled event rates for chest-CT findings with COVID-19 severity as the mediator. Data is reported for non-severe and severe subgroups at short-term (1–6 months) and long-term (12–24 months) follow-ups. Event rates for each severity subgroup is compared with that in the other severity subgroup at the respective follow-up. UE: Unable to estimate.
3.3.1. Non-Severe Subgroup
In the non-severe subgroup, event rates for various abnormalities (any abnormality, GGO, consolidation, and interlobular septal thickening) decreased from the short-term to the long-term follow-up and was supported by negative trends in meta-regression (p < 0.05 for all). Additionally, the event rates for fibrotic-like changes, bronchiectasis, and reticulation remained stable or slightly decreased over time, but meta-regression did not show a significant trend (p > 0.05 for all). Honeycombing was negligible at both timepoints, and meta-regression could not be calculated due to insufficient data. At the short-term follow-up, I2 ranged between 0.53 and 0.90, with interlobular septal thickening having the lowest I2 and GGO having the highest I2. At the long-term follow-up, I2 ranged between 0.64 and 0.93, with consolidation having the lowest I2 and fibrotic-like changes having the highest I2.
3.3.2. Severe Subgroup
In the severe subgroup, event rates for any abnormality, GGO, and consolidation decreased from the short-term to the long-term follow-up and was supported by negative trends in meta-regression (p < 0.05 for all). Additionally, the event rates for fibrotic-like changes, bronchiectasis, reticulation, interlobular septal thickening, and honeycombing remained stable or slightly decreased over time, but meta-regression did not show a significant trend (p > 0.05 for all). At the short-term follow-up, I2 ranged between 0.83 and 0.96, with fibrotic-like changes having the lowest I2 and bronchiectasis having the highest I2. At the long-term follow-up, I2 ranged between 0.60 and 0.96, with honeycombing having the lowest I2 and bronchiectasis having the highest I2.
3.3.3. Comparison between Severity Subgroups
The severe subgroup had significantly higher event rates for any abnormalities (p = 0.01), bronchiectasis (p < 0.001), and reticulation (p = 0.01) at the short-term follow-up, and any abnormalities (p < 0.001), bronchiectasis (p = 0.02), fibrotic-like changes (p = 0.03), and reticulation (p < 0.001) at the long-term follow-up when compared to the non-severe subgroup.
3.4. Quality Assessment
The risk-of-bias assessment is shown in Supplemental Figure S5. Thirteen studies (30.2%) had a moderate risk of bias while the remaining 30 studies (69.8%) had a low risk of bias. The items that had the highest scores were those that were tested for external validity (items 1 to 5), with item 2 and item 3 having the highest summative scores of 39/43 and 42/43, respectively. A visual inspection of the funnel plots revealed publication bias in some CT findings (Supplemental Figures S6 and S7).
4. Discussion
Our meta-analysis of 43 studies, stratified by COVID-19 severity, revealed significant CT abnormalities up to 2 years after SARS-CoV-2 infection. While some abnormalities like GGO and consolidation decreased over time, others including fibrotic-like changes, bronchiectasis, reticulation, and interlobular septal thickening remained unchanged. Notably, honeycombing increased over time. Patients with severe COVID-19 exhibited higher incidences of any abnormality, bronchiectasis, fibrotic-like changes, and reticulation up to 2 years post-COVID compared to those with non-severe COVID-19.
At the 12- and 24-month follow-ups, the only chest-CT abnormalities that showed significant improvement were consolidation and interlobular septal thickening. This underscores the persistent nature of lung abnormalities over years following the initial infection. However, the results should be interpreted with caution because of the small number of studies.
Although there have been a few prior meta-analyses of CT abnormalities in post-COVID-19 patients [38,39,40,41], our meta-analysis is the largest to date (43 studies) and is stratified by COVID-19 disease severity and multiple timepoints up to 2 years post-COVID. There is only one other meta-analysis that investigated chest CT up to 1 year post-COVID-19. Watanabe et al. [40] included 15 studies and found that residual CT abnormalities were common in hospitalized COVID-19 patients 1 year after recovery, especially for fibrotic-like changes in those with severe/critical severity. We also observed fibrotic-like changes, along with bronchiectasis and reticulation, to be greater in those with severe/critical severity. Watanabe et al. [40] reported a 21% prevalence of any abnormality at long-term follow-up for mild/moderate disease and 38% for severe/critical disease. However, in our study, we found approximately double these rates: 39% for mild/moderate disease and 75% severe/critical cases. These discrepancies may be due to differences in patient demographics (age, sex, race, comorbidities) when infection occurred, the duration of the follow-up, and the CT-in-utilization rate. In particular, our cohort had a higher median age (60.3 vs. 56 years old), percentage of males (61.5% vs. 51.3%), proportion of cardiovascular disease (28% vs. 7.5%), and longer duration of patient follow-up (up to 2 years vs. up to 1 year).
Post-COVID pulmonary fibrosis, with an incidence ranging between 5 and 75%, contributes to the burden of chronic respiratory issues among survivors [89]. Its pathophysiology likely stems from the local proinflammatory environment caused by macrophage and immune cell infiltration in the lungs, which disrupts the natural homeostatic tissue-repair functions [90]. Given that pulmonary fibrosis may represent permanent lung damage, identifying its risk factors is crucial for potential prophylactic interventions. Our study identified severe COVID-19 as a risk factor for residual pulmonary fibrosis, which is consistent with other studies [91,92]. Although there is no consensus on treatment, antifibrotic agents may benefit these patients [89].
The functional consequences of lingering CT abnormalities from long COVID remain uncertain [59]. Since these abnormalities suggest lung damage, they can potentially lead to chronic fatigue, post-exertional malaise, and a reduced quality of life [93,94]. Furthermore, the high prevalence of residual CT abnormalities in our study, raises concerns about the increased risk of new-onset pulmonary diseases, such as chronic obstructive pulmonary disease (COPD), asthma, pneumonia, and bronchitis, and compromised pulmonary–cardiovascular health, resulting in decreased exercise tolerance and increased fatigue. In addition, since there is a known association between immunology and cancer, pathological inflammation and immunological responses may increase the rate of lung cancer. Additionally, these radiological abnormalities may decrease the sensitivity of detecting lung cancer by obscuring certain details in imaging. These persistent lung abnormalities can also exacerbate pre-existing pulmonary diseases in the years to come [95]. Large population-based studies involving pulmonary-function testing and long-term follow-ups of at-risk patients with abnormal CT abnormalities are warranted [21,96].
An identification of the potential risk factors of long COVID is necessary to better understand who is at risk and to allow for early clinical support. A recent meta-analysis found certain demographics (female sex, older age, higher BMI, and smoking) and comorbidities (anxiety and/or depression, asthma, COPD, diabetes, ischemic heart disease, and immunosuppression) to be associated with an increased risk of long COVID, whereas vaccination had a protective role [97]. Besides taking preventative measures of receiving vaccination, individuals with risk factors and previous COVID-19 infection may require follow-up outpatient services to manage long COVID and explore the possible association between their symptoms and residual lung damage.
Similar to SARS-CoV-2, patients with SARS-CoV-1, Middle East respiratory syndrome (MERS), and influenza A exhibited residual pulmonary abnormalities at long-term follow-ups, especially fibrotic-like changes (SARS-CoV-2: 38% vs. SARS-CoV-1: 62% vs. MERS: 33% vs. influenza A: 42%) [98,99,100,101,102,103]. Genetic homology between SARS-CoV-2, SARS-CoV-1, and MERS suggests a genetically influenced fibroproliferative process contributing to increased risks of post-COVID pulmonary complications [104,105,106,107]. However, unlike other viruses, SARS-CoV-2 leads to a higher burden of extrapulmonary organ involvement, resulting in a higher level of health impairment during both the acute and post-acute phases [108]. Regarding symptoms, shortness of breath was less common in SARS-CoV-2 patients (17%) in comparison with SARS-CoV-1 (32%), MERS (51%), and influenza A (34%) [109,110]. Although the prevalence of pulmonary abnormalities (e.g., fibrotic-like changes) and symptoms may seem contradictory between the different viruses, these findings may be biased by reporting only confirmed cases and should therefore be considered when interpreting the data. However, unlike SARS-CoV-1 and MERS, which had a total of <11,000 combined confirmed cases, SARS-CoV-2 has an alarming of >700,000,000 confirmed cases to date [111,112]. The magnitude of cases with a persistence of pulmonary abnormalities is a matter of concern. Despite its high prevalence, SARS-CoV-2 has a significantly lower mortality rate (SARS-CoV-2: 2.1% vs. SARS-CoV-1: 9.5% vs. MERS: 34.4%) [113].
This study has several limitations. High heterogeneity in most of our pooled event-rate estimates suggest the presence of unaccounted mediating factors. Additionally, some studies did not report effect sizes for specific lung abnormalities, limiting our comprehensive analysis. Furthermore, limited observations in some severity groups at various time intervals hampered our precise assessment of initial infection severity on the evolution of specific abnormalities. Additionally, the CT abnormalities could not be graded according to their severity because there were only a few studies that graded the severity of the abnormalities, and the scoring system was neither standardized nor validated across studies. Therefore, it remains unclear how the abnormalities can be compared to those observed in a healthy cohort. Another limitation is the use of broad time brackets (1–6 months, 12–24 months) due to the limited number of suitable studies. Moreover, we did not collect pulmonary-function tests as this was not the focus of the study. Consequently, we were unable to correlate pulmonary function with imaging findings. Furthermore, a small percentage of patients had pre-existing pulmonary diseases, which may be a confounder. Finally, it is possible that some of the patients might have had pre-existing CT abnormalities prior to COVID-19.
5. Conclusions
Significant pulmonary CT abnormalities remained for up to 2 years post-COVID, especially in patients with severe disease. The sheer number of individuals infected with SARS-CoV-2 world-wide suggests that pulmonary sequela and related complications could be a major public-health issue in years to come. Our findings underscore the need for extended monitoring, rehabilitation, and support for COVID-19 survivors, vaccination for severe disease prevention, and ongoing research into targeted therapies to mitigate the enduring pulmonary consequences of SARS-CoV-2.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics14060621/s1, Supplemental Table S1. Breakdown of comorbidities. Supplemental Table S2. Pooled event rates of chest-CT findings over time for all patients. Supplemental Table S3. Summary of meta-regression models for the pooled event rate of each lung abnormality using months since primary infection as the independent predictor. Supplemental Table S4. Subgroup analysis comparing chest CT-findings between the 12- and 24-month follow-ups. Supplemental Table S5. Pooled event rates for chest-CT findings with COVID-19 severity as the mediator. Supplemental Table S6. Summary of meta-regression models for the pooled event rate for each lung abnormality stratified by primary COVID-19 infection severity using months since primary infection as the independent predictor. Supplemental Figure S1. Meta analysis of the follow-up chest-CT lung abnormalities over time for the entire population. Supplemental Figure S2. Meta-regression plots for the pooled event rate for each lung abnormality using months since primary infection as the independent predictor. Supplemental Figure S3. Meta-analysis of the follow-up chest-CT lung abnormalities over time for the entire population stratified by primary COVID-19 infection severity. Supplemental Figure S4. Meta-regression plots for the pooled event rate for each lung abnormality stratified by primary COVID-19 infection severity using months since primary infection as the independent predictor. Supplemental Figure S5. Risk-of-bias assessment summary plot using Hoy et al. tool. The tool consists of 10 items addressing four domains of bias. Items 1 to 4 assess the external validity of the study (domains are selection and nonresponse biases), and items 5 to 10 assess the internal validity (items 5 to 9 assess the domain of measurement bias, and item 10 assesses bias related to the analysis). Supplemental Figure S6. Funnel plots for the pooled meta-analysis. Supplemental Figure S7. Funnel plots for the stratified meta-analysis by severity.
Author Contributions
Conceptualization, M.B., H.J., N.M. and T.Q.D.; Methodology, M.B., H.J., N.M. and T.Q.D.; Software, H.J.; Formal Analysis, M.B. and H.J.; Investigation, T.Q.D.; Data Curation, M.B., N.M. and A.M.; Writing—Original Draft Preparation, M.B., H.J., N.M., A.M. and T.Q.D.; Writing—Review and Editing, M.B., H.J., N.M., A.M. and T.Q.D.; Supervision, T.Q.D.; Project Administration, T.Q.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Filip, R.; Gheorghita Puscaselu, R.; Anchidin-Norocel, L.; Dimian, M.; Savage, W.K. Global Challenges to Public Health Care Systems during the COVID-19 Pandemic: A Review of Pandemic Measures and Problems. J. Pers. Med. 2022, 12, 1295. [Google Scholar] [CrossRef]
- Bowe, B.; Xie, Y.; Al-Aly, Z. Postacute sequelae of COVID-19 at 2 years. Nat. Med. 2023, 29, 2347–2357. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Lippi, G.; Sanchis-Gomar, F.; Henry, B.M. COVID-19 and its long-term sequelae: What do we know in 2023? Pol. Arch. Intern. Med. 2023, 133, 16402. [Google Scholar] [CrossRef]
- Lai, C.C.; Hsu, C.K.; Yen, M.Y.; Lee, P.I.; Ko, W.C.; Hsueh, P.R. Long COVID: An inevitable sequela of SARS-CoV-2 infection. J. Microbiol. Immunol. Infect. 2023, 56, 1–9. [Google Scholar] [CrossRef]
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global Prevalence of Post-Coronavirus Disease 2019 (COVID-19) Condition or Long COVID: A Meta-Analysis and Systematic Review. J. Infect. Dis. 2022, 226, 1593–1607. [Google Scholar] [CrossRef]
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef]
- Bazdar, S.; Kwee, A.; Houweling, L.; de Wit-van Wijck, Y.; Mohamed Hoesein, F.A.A.; Downward, G.S.; Nossent, E.J.; Maitland-van der Zee, A.H. A Systematic Review of Chest Imaging Findings in Long COVID Patients. J. Pers. Med. 2023, 13, 282. [Google Scholar] [CrossRef] [PubMed]
- Michelen, M.; Manoharan, L.; Elkheir, N.; Cheng, V.; Dagens, A.; Hastie, C.; O’Hara, M.; Suett, J.; Dahmash, D.; Bugaeva, P.; et al. Characterising long COVID: A living systematic review. BMJ Glob. Health 2021, 6, e005427. [Google Scholar] [CrossRef] [PubMed]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long COVID-mechanisms, risk factors, and management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef] [PubMed]
- George, P.M.; Barratt, S.L.; Condliffe, R.; Desai, S.R.; Devaraj, A.; Forrest, I.; Gibbons, M.A.; Hart, N.; Jenkins, R.G.; McAuley, D.F.; et al. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax 2020, 75, 1009–1016. [Google Scholar] [CrossRef]
- Lu, J.Q.; Lu, J.Y.; Wang, W.; Liu, Y.; Buczek, A.; Fleysher, R.; Hoogenboom, W.S.; Zhu, W.; Hou, W.; Rodriguez, C.J.; et al. Clinical predictors of acute cardiac injury and normalization of troponin after hospital discharge from COVID-19. eBioMedicine 2022, 76, 103821. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.Y.; Wilson, J.; Hou, W.; Fleysher, R.; Herold, B.C.; Herold, K.C.; Duong, T.Q. Incidence of new-onset in-hospital and persistent diabetes in COVID-19 patients: Comparison with influenza. eBioMedicine 2023, 90, 104487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, V.; Fisher, M.; Hou, W.; Zhang, L.; Duong, T.Q. Incidence of New-Onset Hypertension Post-COVID-19: Comparison With Influenza. Hypertension 2023, 80, 2135–2148. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, D.B.; Budhathoki, P.; Raut, S.; Adhikari, S.; Ghimire, P.; Thapaliya, S.; Rabaan, A.A.; Karki, B.J. New-onset diabetes in COVID-19 and clinical outcomes: A systematic review and meta-analysis. World J. Virol. 2021, 10, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Sathish, T.; Anton, M.C.; Sivakumar, T. New-onset diabetes in “long COVID”. J. Diabetes 2021, 13, 693–694. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.; Amiel, S.A.; Zimmet, P.; Alberti, G.; Bornstein, S.; Eckel, R.H.; Mingrone, G.; Boehm, B.; Cooper, M.E.; Chai, Z.; et al. New-Onset Diabetes in COVID-19. N. Engl. J. Med. 2020, 383, 789–790. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Al-Aly, Z. Risks and burdens of incident diabetes in long COVID: A cohort study. Lancet Diabetes Endocrinol. 2022, 10, 311–321. [Google Scholar] [CrossRef]
- Xu, A.Y.; Wang, S.H.; Duong, T.Q. Patients with prediabetes are at greater risk of developing diabetes 5 months postacute SARS-CoV-2 infection: A retrospective cohort study. BMJ Open Diabetes Res. Care 2023, 11, e003257. [Google Scholar] [CrossRef]
- Lu, J.Y.; Boparai, M.S.; Shi, C.; Henninger, E.M.; Rangareddy, M.; Veeraraghavan, S.; Mirhaji, P.; Fisher, M.C.; Duong, T.Q. Long-term outcomes of COVID-19 survivors with hospital AKI: Association with time to recovery from AKI. Nephrol. Dial. Transplant. 2023, 38, 2160–2169. [Google Scholar] [CrossRef]
- Akbarialiabad, H.; Taghrir, M.H.; Abdollahi, A.; Ghahramani, N.; Kumar, M.; Paydar, S.; Razani, B.; Mwangi, J.; Asadi-Pooya, A.A.; Malekmakan, L.; et al. Long COVID, a comprehensive systematic scoping review. Infection 2021, 49, 1163–1186. [Google Scholar] [CrossRef] [PubMed]
- Jalaber, C.; Lapotre, T.; Morcet-Delattre, T.; Ribet, F.; Jouneau, S.; Lederlin, M. Chest CT in COVID-19 pneumonia: A review of current knowledge. Diagn. Interv. Imaging 2020, 101, 431–437. [Google Scholar] [CrossRef]
- Xu, X.; Yu, C.; Qu, J.; Zhang, L.; Jiang, S.; Huang, D.; Chen, B.; Zhang, Z.; Guan, W.; Ling, Z.; et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Ullah, N.; Khan, J.A.; El-Sappagh, S.; El-Rashidy, N.; Khan, M.S. A Holistic Approach to Identify and Classify COVID-19 from Chest Radiographs, ECG, and CT-Scan Images Using ShuffleNet Convolutional Neural Network. Diagnostics 2023, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Ullah, N.; Khan, J.A.; Almakdi, S.; Khan, M.S.; Alshehri, M.; Alboaneen, D.; Raza, A. A Novel CovidDetNet Deep Learning Model for Effective COVID-19 Infection Detection Using Chest Radiograph Images. Appl. Sci. 2022, 12, 6269. [Google Scholar] [CrossRef]
- Hussain, L.; Nguyen, T.; Li, H.; Abbasi, A.A.; Lone, K.J.; Zhao, Z.; Zaib, M.; Chen, A.; Duong, T.Q. Machine-learning classification of texture features of portable chest X-ray accurately classifies COVID-19 lung infection. Biomed. Eng. Online 2020, 19, 88. [Google Scholar] [CrossRef] [PubMed]
- Sailunaz, K.; Ozyer, T.; Rokne, J.; Alhajj, R. A survey of machine learning-based methods for COVID-19 medical image analysis. Med. Biol. Eng. Comput. 2023, 61, 1257–1297. [Google Scholar] [CrossRef]
- Majrashi, N.A.A. The value of chest X-ray and CT severity scoring systems in the diagnosis of COVID-19: A review. Front. Med. 2022, 9, 1076184. [Google Scholar] [CrossRef]
- Cohen, J.P.; Morrison, P.; Dao, L.; Roth, K.; Duong, T.Q.; Ghassemi, M. COVID-19 image data collection: Prospective predictions are the future. J. Mach. Learn. Biomed. Imaging (MELBA) 2020, 2, 1–38. [Google Scholar] [CrossRef]
- Cohen, J.P.; Dao, L.; Roth, K.; Morrison, P.; Bengio, Y.; Abbasi, A.F.; Shen, B.; Mahsa, H.K.; Ghassemi, M.; Li, H.; et al. Predicting COVID-19 Pneumonia Severity on Chest X-ray With Deep Learning. Cureus 2020, 12, e9448. [Google Scholar] [CrossRef]
- Eligulashvili, A.; Darrell, M.; Miller, C.; Lee, J.; Congdon, S.; Lee, J.S.; Hsu, K.; Yee, J.; Hou, W.; Islam, M.; et al. COVID-19 Patients in the COVID-19 Recovery and Engagement (CORE) Clinics in the Bronx. Diagnostics 2022, 13, 119. [Google Scholar] [CrossRef]
- Kikkisetti, S.; Zhu, J.; Shen, B.; Li, H.; Duong, T.Q. Deep-learning convolutional neural networks with transfer learning accurately classify COVID-19 lung infection on portable chest radiographs. PeerJ 2020, 8, e10309. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Hoshmand-Kochi, M.; Abbasi, A.; Glass, S.; Jiang, Z.; Singer, A.J.; Thode, H.C.; Li, H.; Hou, W.; Duong, T.Q. Initial chest radiograph scores inform COVID-19 status, intensive care unit admission and need for mechanical ventilation. Clin. Radiol. 2021, 76, 473.e1–473.e7. [Google Scholar] [CrossRef]
- Zhu, J.; Shen, B.; Abbasi, A.; Hoshmand-Kochi, M.; Li, H.; Duong, T.Q. Deep transfer learning artificial intelligence accurately stages COVID-19 lung disease severity on portable chest radiographs. PLoS ONE 2020, 15, e0236621. [Google Scholar] [CrossRef]
- Duanmu, H.; Ren, T.; Li, H.; Mehta, N.; Singer, A.J.; Levsky, J.M.; Lipton, M.L.; Duong, T.Q. Deep learning of longitudinal chest X-ray and clinical variables predicts duration on ventilator and mortality in COVID-19 patients. Biomed. Eng. Online 2022, 21, 77. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Zhang, Y.; Wang, Y.; Huang, Z.; Song, B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): A pictorial review. Eur. Radiol. 2020, 30, 4381–4389. [Google Scholar] [CrossRef]
- Li, J.; Yan, R.; Zhai, Y.; Qi, X.; Lei, J. Chest CT findings in patients with coronavirus disease 2019 (COVID-19): A comprehensive review. Diagn. Interv. Radiol. 2021, 27, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Bocchino, M.; Rea, G.; Capitelli, L.; Lieto, R.; Bruzzese, D. Chest CT Lung Abnormalities 1 Year after COVID-19: A Systematic Review and Meta-Analysis. Radiology 2023, 308, e230535. [Google Scholar] [CrossRef]
- Guinto, E.; Gerayeli, F.V.; Eddy, R.L.; Lee, H.; Milne, S.; Sin, D.D. Post-COVID-19 dyspnoea and pulmonary imaging: A systematic review and meta-analysis. Eur. Respir. Rev. 2023, 32, 220253. [Google Scholar] [CrossRef]
- Watanabe, A.; So, M.; Iwagami, M.; Fukunaga, K.; Takagi, H.; Kabata, H.; Kuno, T. One-year follow-up CT findings in COVID-19 patients: A systematic review and meta-analysis. Respirology 2022, 27, 605–616. [Google Scholar] [CrossRef]
- Lee, J.H.; Yim, J.J.; Park, J. Pulmonary function and chest computed tomography abnormalities 6-12 months after recovery from COVID-19: A systematic review and meta-analysis. Respir. Res. 2022, 23, 233. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Covidence Systematic Review Software. Available online: www.covidence.org (accessed on 23 May 2023).
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 25 January 2024).
- Hoy, D.; Brooks, P.; Woolf, A.; Blyth, F.; March, L.; Bain, C.; Baker, P.; Smith, E.; Buchbinder, R. Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. J. Clin. Epidemiol. 2012, 65, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Barini, M.; Percivale, I.; Danna, P.; Longo, V.; Costantini, P.; Paladini, A.; Airoldi, C.; Bellan, M.; Saba, L.; Carriero, A. 18 months computed tomography follow-up after COVID-19 interstitial pneumonia. J. Public Health Res. 2022, 11, 2782. [Google Scholar] [CrossRef] [PubMed]
- Bellan, M.; Baricich, A.; Patrucco, F.; Zeppegno, P.; Gramaglia, C.; Balbo, P.E.; Carriero, A.; Amico, C.S.; Avanzi, G.C.; Barini, M.; et al. Long-term sequelae are highly prevalent one year after hospitalization for severe COVID-19. Sci. Rep. 2021, 11, 22666. [Google Scholar] [CrossRef] [PubMed]
- Bernardinello, N.; Cocconcelli, E.; Giraudo, C.; Daverio, M.; Castelli, G.; Petrarulo, S.; Bovo, M.; Fichera, G.; Cavinato, S.; Cattelan, A.M.; et al. Predictors of pulmonary sequelae after COVID-19 pneumonia: A 12-month follow-up study. Front. Med. 2023, 10, 1084002. [Google Scholar] [CrossRef] [PubMed]
- Bocchino, M.; Lieto, R.; Romano, F.; Sica, G.; Bocchini, G.; Muto, E.; Capitelli, L.; Sequino, D.; Valente, T.; Fiorentino, G.; et al. Chest CT-based Assessment of 1-year Outcomes after Moderate COVID-19 Pneumonia. Radiology 2022, 305, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, M.; Barilaro, G.; Bini, F. Twelve-month clinical, functional, and radiological outcomes in patients hospitalized for SARS-CoV-2 pneumonia. J. Med. Virol. 2023, 95, e28524. [Google Scholar] [CrossRef]
- Chen, Y.; Ding, C.; Yu, L.; Guo, W.; Feng, X.; Yu, L.; Su, J.; Xu, T.; Ren, C.; Shi, D.; et al. One-year follow-up of chest CT findings in patients after SARS-CoV-2 infection. BMC Med. 2021, 19, 191. [Google Scholar] [CrossRef]
- Corsi, A.; Caroli, A.; Bonaffini, P.A.; Conti, C.; Arrigoni, A.; Mercanzin, E.; Imeri, G.; Anelli, M.; Balbi, M.; Pace, M.; et al. Structural and Functional Pulmonary Assessment in Severe COVID-19 Survivors at 12 Months after Discharge. Tomography 2022, 8, 2588–2603. [Google Scholar] [CrossRef]
- Eberst, G.; Claudé, F.; Laurent, L.; Meurisse, A.; Roux-Claudé, P.; Barnig, C.; Vernerey, D.; Paget-Bailly, S.; Bouiller, K.; Chirouze, C.; et al. Result of one-year, prospective follow-up of intensive care unit survivors after SARS-CoV-2 pneumonia. Ann. Intensive Care 2022, 12, 23. [Google Scholar] [CrossRef]
- Faverio, P.; Luppi, F.; Rebora, P.; D’Andrea, G.; Stainer, A.; Busnelli, S.; Catalano, M.; Modafferi, G.; Franco, G.; Monzani, A.; et al. One-year pulmonary impairment after severe COVID-19: A prospective, multicenter follow-up study. Respir. Res. 2022, 23, 65. [Google Scholar] [CrossRef]
- Flor, N.; Leidi, F.; Casella, F.; Mariani, L.; Piazza, M.; Del Medico, M.; Cogliati, C.B. Two-years chest-CT follow-up after severe COVID-19 pneumonia. Intern. Emerg. Med. 2023, 18, 1243–1245. [Google Scholar] [CrossRef]
- Gamberini, L.; Mazzoli, C.A.; Prediletto, I.; Sintonen, H.; Scaramuzzo, G.; Allegri, D.; Colombo, D.; Tonetti, T.; Zani, G.; Capozzi, C.; et al. Health-related quality of life profiles, trajectories, persistent symptoms and pulmonary function one year after ICU discharge in invasively ventilated COVID-19 patients, a prospective follow-up study. Respir. Med. 2021, 189, 106665. [Google Scholar] [CrossRef]
- González, J.; Zuil, M.; Benítez, I.D.; de Gonzalo-Calvo, D.; Aguilar, M.; Santisteve, S.; Vaca, R.; Minguez, O.; Seck, F.; Torres, G.; et al. One Year Overview and Follow-Up in a Post-COVID Consultation of Critically Ill Patients. Front. Med. 2022, 9, 897990. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, H.; Xiao, M.; Guan, X.; Lei, Y.; Diao, T.; Long, P.; Zeng, R.; Lai, X.; Cai, H.; et al. Long-term outcomes of COVID-19 convalescents: An 18.5-month longitudinal study in Wuhan. Int. J. Infect. Dis. 2023, 127, 85–92. [Google Scholar] [CrossRef]
- Han, X.; Chen, L.; Fan, Y.; Alwalid, O.; Jia, X.; Zheng, Y.; Liu, J.; Li, Y.; Cao, Y.; Gu, J.; et al. Longitudinal Assessment of Chest CT Findings and Pulmonary Function after COVID-19 Infection. Radiology 2023, 307, e222888. [Google Scholar] [CrossRef]
- Han, X.; Fan, Y.; Alwalid, O.; Zhang, X.; Jia, X.; Zheng, Y.; Shi, H. Fibrotic Interstitial Lung Abnormalities at 1-year Follow-up CT after Severe COVID-19. Radiology 2021, 301, E438–E440. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, X.; Gu, X.; Zhang, H.; Ren, L.; Guo, L.; Liu, M.; Wang, Y.; Cui, D.; Wang, Y.; et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: A longitudinal cohort study. Lancet Respir. Med. 2022, 10, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, A.; Christe, A.; Ebner, L.; Beigelman-Aubry, C.; Bridevaux, P.O.; Brutsche, M.; Clarenbach, C.; Erkosar, B.; Garzoni, C.; Geiser, T.; et al. Pulmonary Recovery 12 Months after Non-Severe and Severe COVID-19: The Prospective Swiss COVID-19 Lung Study. Respiration 2023, 102, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Lerum, T.V.; Meltzer, C.; Rodriguez, J.R.; Aaløkken, T.M.; Brønstad, E.; Aarli, B.B.; Aarberg-Lund, K.M.; Durheim, M.T.; Ashraf, H.; Einvik, G.; et al. A prospective study of pulmonary outcomes and chest computed tomography in the first year after COVID-19. ERJ Open Res. 2023, 9, 00575-2022. [Google Scholar] [CrossRef]
- Li, Y.; Han, X.; Huang, J.; Alwalid, O.; Jia, X.; Yuan, M.; Cao, Y.; Shao, G.; Cui, Y.; Liu, J.; et al. Follow-up study of pulmonary sequelae in discharged COVID-19 patients with diabetes or secondary hyperglycemia. Eur. J. Radiol. 2021, 144, 109997. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.; Meng, D.; Xiong, L.; Wu, S.; Yang, L.; Wang, S.; Zhou, M.; He, X.; Cao, X.; Xiong, H.; et al. Long-Term Effects of COVID-19 on Health Care Workers 1-Year Post-Discharge in Wuhan. Infect. Dis. Ther. 2022, 11, 145–163. [Google Scholar] [CrossRef]
- Liu, T.; Wu, D.; Yan, W.; Wang, X.; Zhang, X.; Ma, K.; Chen, H.; Zeng, Z.; Qin, Y.; Wang, H.; et al. Twelve-Month Systemic Consequences of Coronavirus Disease 2019 (COVID-19) in Patients Discharged From Hospital: A Prospective Cohort Study in Wuhan, China. Clin. Infect. Dis. 2022, 74, 1953–1965. [Google Scholar] [CrossRef] [PubMed]
- Lorent, N.; Vande Weygaerde, Y.; Claeys, E.; Guler Caamano Fajardo, I.; De Vos, N.; De Wever, W.; Salhi, B.; Gyselinck, I.; Bosteels, C.; Lambrecht, B.N.; et al. Prospective longitudinal evaluation of hospitalised COVID-19 survivors 3 and 12 months after discharge. ERJ Open Res. 2022, 8, 00004-2022. [Google Scholar] [CrossRef] [PubMed]
- Luger, A.K.; Sonnweber, T.; Gruber, L.; Schwabl, C.; Cima, K.; Tymoszuk, P.; Gerstner, A.K.; Pizzini, A.; Sahanic, S.; Boehm, A.; et al. Chest CT of Lung Injury 1 Year after COVID-19 Pneumonia: The CovILD Study. Radiology 2022, 304, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Marando, M.; Fusi-Schmidhauser, T.; Tamburello, A.; Grazioli Gauthier, L.; Rigamonti, E.; Argentieri, G.; Puligheddu, C.; Pagnamenta, A.; Valenti, A.; Pons, M.; et al. 1-year radiological, functional and quality-of-life outcomes in patients with SARS-CoV-2 pneumonia—A prospective observational study. NPJ Prim. Care Respir. Med. 2022, 32, 8. [Google Scholar] [CrossRef] [PubMed]
- Martino, G.P.; Benfaremo, D.; Bitti, G.; Valeri, G.; Postacchini, L.; Marchetti, A.; Angelici, S.; Moroncini, G. 6 and 12 month outcomes in patients following COVID-19-related hospitalization: A prospective monocentric study. Intern. Emerg. Med. 2022, 17, 1641–1649. [Google Scholar] [CrossRef]
- Mulet, A.; Tarrasó, J.; Rodríguez-Borja, E.; Carbonell-Asins, J.A.; Lope-Martínez, A.; Martí-Martinez, A.; Murria, R.; Safont, B.; Fernandez-Fabrellas, E.; Ros, J.A.; et al. Biomarkers of Fibrosis in Patients with COVID-19 One Year After Hospital Discharge: A Prospective Cohort Study. Am. J. Respir. Cell Mol. Biol. 2023, 69, 321–327. [Google Scholar] [CrossRef]
- Noureddine, S.; Roux-Claudé, P.; Laurent, L.; Ritter, O.; Dolla, P.; Karaer, S.; Claudé, F.; Eberst, G.; Westeel, V.; Barnig, C. Evaluation of long-term sequelae by cardiopulmonary exercise testing 12 months after hospitalization for severe COVID-19. BMC Pulm. Med. 2023, 23, 13. [Google Scholar] [CrossRef]
- Núñez-Fernández, M.; Ramos-Hernández, C.; García-Río, F.; Pérez-González, A.; Tilve-Gómez, A.; Rodríguez-Fernández, P.; Nodar-Germiñas, A.; Fernández-García, A.; Ruano-Raviña, A.; Fernández-Villar, A. Evolution and long-term respiratory sequelae after severe COVID-19 pneumonia: Nitric oxide diffusion measurement value. Respir. Res. 2023, 24, 48. [Google Scholar] [CrossRef]
- Pan, F.; Yang, L.; Liang, B.; Ye, T.; Li, L.; Li, L.; Liu, D.; Wang, J.; Hesketh, R.L.; Zheng, C. Chest CT Patterns from Diagnosis to 1 Year of Follow-up in Patients with COVID-19. Radiology 2022, 302, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Rigoni, M.; Torri, E.; Nollo, G.; Donne, L.D.; Rizzardo, S.; Lenzi, L.; Falzone, A.; Cozzio, S. “Long COVID” results after hospitalization for SARS-CoV-2 infection. Sci. Rep. 2022, 12, 9581. [Google Scholar] [CrossRef]
- Sahanic, S.; Tymoszuk, P.; Luger, A.K.; Hüfner, K.; Boehm, A.; Pizzini, A.; Schwabl, C.; Koppelstätter, S.; Kurz, K.; Asshoff, M.; et al. COVID-19 and its continuing burden after 12 months: A longitudinal observational prospective multicentre trial. ERJ Open Res. 2023, 9, 00317-2022. [Google Scholar] [CrossRef] [PubMed]
- Sanna, A.; Pellegrino, D.; Messina, E.; Siena, L.M.; Baccolini, V.; D’Antoni, L.; Landini, N.; Baiocchi, P.; Villari, P.; Catalano, C.; et al. The Role of Pulmonary Function Testing and Lung Imaging in the Long-Term Follow-Up of Patients with COVID-19 Pneumonia Role of Pulmonary Function Tests and High-Resolution Computed Tomography in Post-COVID-19 Interstitial Lung Disease. Respiration 2023, 102, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Schlemmer, F.; Valentin, S.; Boyer, L.; Guillaumot, A.; Chabot, F.; Dupin, C.; Le Guen, P.; Lorillon, G.; Bergeron, A.; Basille, D.; et al. Respiratory recovery trajectories after severe-to-critical COVID-19: A 1-year prospective multicentre study. Eur. Respir. J. 2023, 61, 2201532. [Google Scholar] [CrossRef]
- Tarraso, J.; Safont, B.; Carbonell-Asins, J.A.; Fernandez-Fabrellas, E.; Sancho-Chust, J.N.; Naval, E.; Amat, B.; Herrera, S.; Ros, J.A.; Soler-Cataluña, J.J.; et al. Lung function and radiological findings 1 year after COVID-19: A prospective follow-up. Respir. Res. 2022, 23, 242. [Google Scholar] [CrossRef]
- van der Sar-van der Brugge, S.; Flikweert, A.; du Mee, A.; Gense, K.; Talman, S.; Kant, M.; De Backer, I. Recovery after admission with COVID-19 pneumonia—A follow-up study. Respir. Med. Res. 2023, 83, 101001. [Google Scholar] [CrossRef]
- van Raaij, B.F.M.; Stöger, J.L.; Hinnen, C.; Penfornis, K.M.; de Jong, C.M.M.; Klok, F.A.; Roukens, A.H.E.; Veldhuijzen, D.S.; Arbous, M.S.; Noordam, R.; et al. Fibrotic-like abnormalities notably prevalent one year after hospitalization with COVID-19. Respir. Med. Res. 2022, 82, 100973. [Google Scholar] [CrossRef]
- Vijayakumar, B.; Tonkin, J.; Devaraj, A.; Philip, K.E.J.; Orton, C.M.; Desai, S.R.; Shah, P.L. CT Lung Abnormalities after COVID-19 at 3 Months and 1 Year after Hospital Discharge. Radiology 2022, 303, 444–454. [Google Scholar] [CrossRef]
- Wu, X.; Liu, X.; Zhou, Y.; Yu, H.; Li, R.; Zhan, Q.; Ni, F.; Fang, S.; Lu, Y.; Ding, X.; et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: A prospective study. Lancet Respir. Med. 2021, 9, 747–754. [Google Scholar] [CrossRef]
- Zangrillo, A.; Belletti, A.; Palumbo, D.; Calvi, M.R.; Guzzo, F.; Fominskiy, E.V.; Ortalda, A.; Nardelli, P.; Ripa, M.; Baiardo Redaelli, M.; et al. One-Year Multidisciplinary Follow-Up of Patients With COVID-19 Requiring Invasive Mechanical Ventilation. J. Cardiothorac. Vasc. Anesth. 2022, 36, 1354–1363. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhu, Y.; Wang, S.; Jia, S.; Gao, Y.; Lu, Y.; Zhou, C.; Liang, R.; Sun, D.; Wang, X.; et al. SARS-CoV-2 immunity and functional recovery of COVID-19 patients 1-year after infection. Signal Transduct. Target. Ther. 2021, 6, 368. [Google Scholar] [CrossRef]
- Zhang, L.; Lei, J.; Zhang, J.; Yin, L.; Chen, Y.; Xi, Y.; Moreira, J.P. Undiagnosed Long COVID-19 in China Among Non-vaccinated Individuals: Identifying Persistent Symptoms and Impacts on Patients’ Health-Related Quality of Life. J. Epidemiol. Glob. Health 2022, 12, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, C.; An, X.; Xiong, Y.; Shang, Y.; He, J.; Qiu, Y.; Zhang, N.; Huang, L.; Jia, J.; et al. Follow-up study on COVID-19 survivors one year after discharge from hospital. Int. J. Infect. Dis. 2021, 112, 173–182. [Google Scholar] [CrossRef]
- Zhou, F.; Tao, M.; Shang, L.; Liu, Y.; Pan, G.; Jin, Y.; Wang, L.; Hu, S.; Li, J.; Zhang, M.; et al. Assessment of Sequelae of COVID-19 Nearly 1 Year After Diagnosis. Front. Med. 2021, 8, 717194. [Google Scholar] [CrossRef]
- Lassan, S.; Tesar, T.; Tisonova, J.; Lassanova, M. Pharmacological approaches to pulmonary fibrosis following COVID-19. Front. Pharmacol. 2023, 14, 1143158. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Z.; Li, J.W.; Tan, K.; Yang, W.; Zhao, H.; Wang, G.Q. Discharge may not be the end of treatment: Pay attention to pulmonary fibrosis caused by severe COVID-19. J. Med. Virol. 2021, 93, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Najjar-Debbiny, R.; Barnett-Griness, O.; Khoury, J.; Gronich, N.; Weber, G.; Adir, Y.; Steinberg, M.; Shneir, S.; Sharma, L.; Saliba, W. Association between COVID-19 infection and pulmonary fibrosis: A nested case-control study. Am. J. Med. 2023, 136, 1087–1093.e2. [Google Scholar] [CrossRef] [PubMed]
- Ojo, A.S.; Balogun, S.A.; Williams, O.T.; Ojo, O.S. Pulmonary Fibrosis in COVID-19 Survivors: Predictive Factors and Risk Reduction Strategies. Pulm. Med. 2020, 2020, 6175964. [Google Scholar] [CrossRef]
- Twomey, R.; DeMars, J.; Franklin, K.; Culos-Reed, S.N.; Weatherald, J.; Wrightson, J.G. Chronic Fatigue and Postexertional Malaise in People Living With Long COVID: An Observational Study. Phys. Ther. 2022, 102, pzac005. [Google Scholar] [CrossRef]
- Verveen, A.; Müller, F.; Lloyd, A.; Moss-Morris, R.; Omland, T.; Penninx, B.; Raijmakers, R.P.H.; van der Schaaf, M.; Sandler, C.X.; Stavem, K.; et al. A research agenda for post-COVID-19 fatigue. J. Psychosom. Res. 2022, 154, 110726. [Google Scholar] [CrossRef]
- Chiner-Vives, E.; Cordovilla-Pérez, R.; de la Rosa-Carrillo, D.; García-Clemente, M.; Izquierdo-Alonso, J.L.; Otero-Candelera, R.; Pérez-de Llano, L.; Sellares-Torres, J.; de Granda-Orive, J.I. Short and Long-Term Impact of COVID-19 Infection on Previous Respiratory Diseases. Arch. Bronconeumol. 2022, 58 (Suppl. S1), 39–50. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.L.; Helgeson, S.A.; Tatari, M.M.; Mallea, J.M.; Baig, H.Z.; Patel, N.M. COVID-19 and the effects on pulmonary function following infection: A retrospective analysis. eClinicalMedicine 2021, 39, 101079. [Google Scholar] [CrossRef]
- Tsampasian, V.; Elghazaly, H.; Chattopadhyay, R.; Debski, M.; Naing, T.K.P.; Garg, P.; Clark, A.; Ntatsaki, E.; Vassiliou, V.S. Risk Factors Associated With Post−COVID-19 Condition: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2023, 183, 566–580. [Google Scholar] [CrossRef]
- Hui, D.S.; Joynt, G.M.; Wong, K.T.; Gomersall, C.D.; Li, T.S.; Antonio, G.; Ko, F.W.; Chan, M.C.; Chan, D.P.; Tong, M.W.; et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax 2005, 60, 401–409. [Google Scholar] [CrossRef]
- Ng, C.K.; Chan, J.W.; Kwan, T.L.; To, T.S.; Chan, Y.H.; Ng, F.Y.; Mok, T.Y. Six month radiological and physiological outcomes in severe acute respiratory syndrome (SARS) survivors. Thorax 2004, 59, 889–891. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, J.; Liu, H.; Han, N.; Ju, J.; Kou, Y.; Chen, L.; Jiang, M.; Pan, F.; Zheng, Y.; et al. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: A 15-year follow-up from a prospective cohort study. Bone Res. 2020, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Antonio, G.E.; Wong, K.T.; Hui, D.S.; Wu, A.; Lee, N.; Yuen, E.H.; Leung, C.B.; Rainer, T.H.; Cameron, P.; Chung, S.S.; et al. Thin-section CT in patients with severe acute respiratory syndrome following hospital discharge: Preliminary experience. Radiology 2003, 228, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Das, K.M.; Lee, E.Y.; Singh, R.; Enani, M.A.; Al Dossari, K.; Van Gorkom, K.; Larsson, S.G.; Langer, R.D. Follow-up chest radiographic findings in patients with MERS-CoV after recovery. Indian J. Radiol. Imaging 2017, 27, 342–349. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.; Hao, S.; Yang, M.; Lu, X.; Chen, X.; Li, L. Long term outcomes in survivors of epidemic Influenza A (H7N9) virus infection. Sci. Rep. 2017, 7, 17275. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Al-Ahmed, S.H.; Haque, S.; Sah, R.; Tiwari, R.; Malik, Y.S.; Dhama, K.; Yatoo, M.I.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. SARS-CoV-2, SARS-CoV, and MERS-COV: A comparative overview. Infez. Med. 2020, 28, 174–184. [Google Scholar] [PubMed]
- Mylvaganam, R.J.; Bailey, J.I.; Sznajder, J.I.; Sala, M.A. Recovering from a pandemic: Pulmonary fibrosis after SARS-CoV-2 infection. Eur. Respir. Rev. 2021, 30, 210194. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Choi, T.; Al-Aly, Z. Long-term outcomes following hospital admission for COVID-19 versus seasonal influenza: A cohort study. Lancet Infect. Dis. 2024, 24, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Pormohammad, A.; Ghorbani, S.; Khatami, A.; Farzi, R.; Baradaran, B.; Turner, D.L.; Turner, R.J.; Bahr, N.C.; Idrovo, J.P. Comparison of confirmed COVID-19 with SARS and MERS cases—Clinical characteristics, laboratory findings, radiographic signs and outcomes: A systematic review and meta-analysis. Rev. Med. Virol. 2020, 30, e2112. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Singh, D.; Zia, A.; Umrao, J.; Srivastava, N.; Pandey, A.; Singh, S.; Bhattacharya, P.; Kumari, R.; Kushwaha, R.; et al. Clinical characterization of influenza A and human respiratory syncytial virus among patients with influenza like illness. J. Med. Virol. 2017, 89, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Franquet, T.; Jeong, Y.J.; Lam, H.Y.S.; Wong, H.Y.F.; Chang, Y.C.; Chung, M.J.; Lee, K.S. Imaging findings in coronavirus infections: SARS-CoV, MERS-CoV, and SARS-CoV-2. Br. J. Radiol. 2020, 93, 20200515. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 31 August 2023).
- Pustake, M.; Tambolkar, I.; Giri, P.; Gandhi, C. SARS, MERS and COVID-19: An overview and comparison of clinical, laboratory and radiological features. J. Fam. Med. Prim. Care 2022, 11, 10–17. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
