Real-Life Diagnostic Performance of the Hypersensitivity Pneumonitis Guidelines: A Multicenter Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Data Collection and MDD Diagnosis
2.3. The ATS/JRS/ALAT Guidelines Diagnostic Criteria
2.4. Statistical Analysis
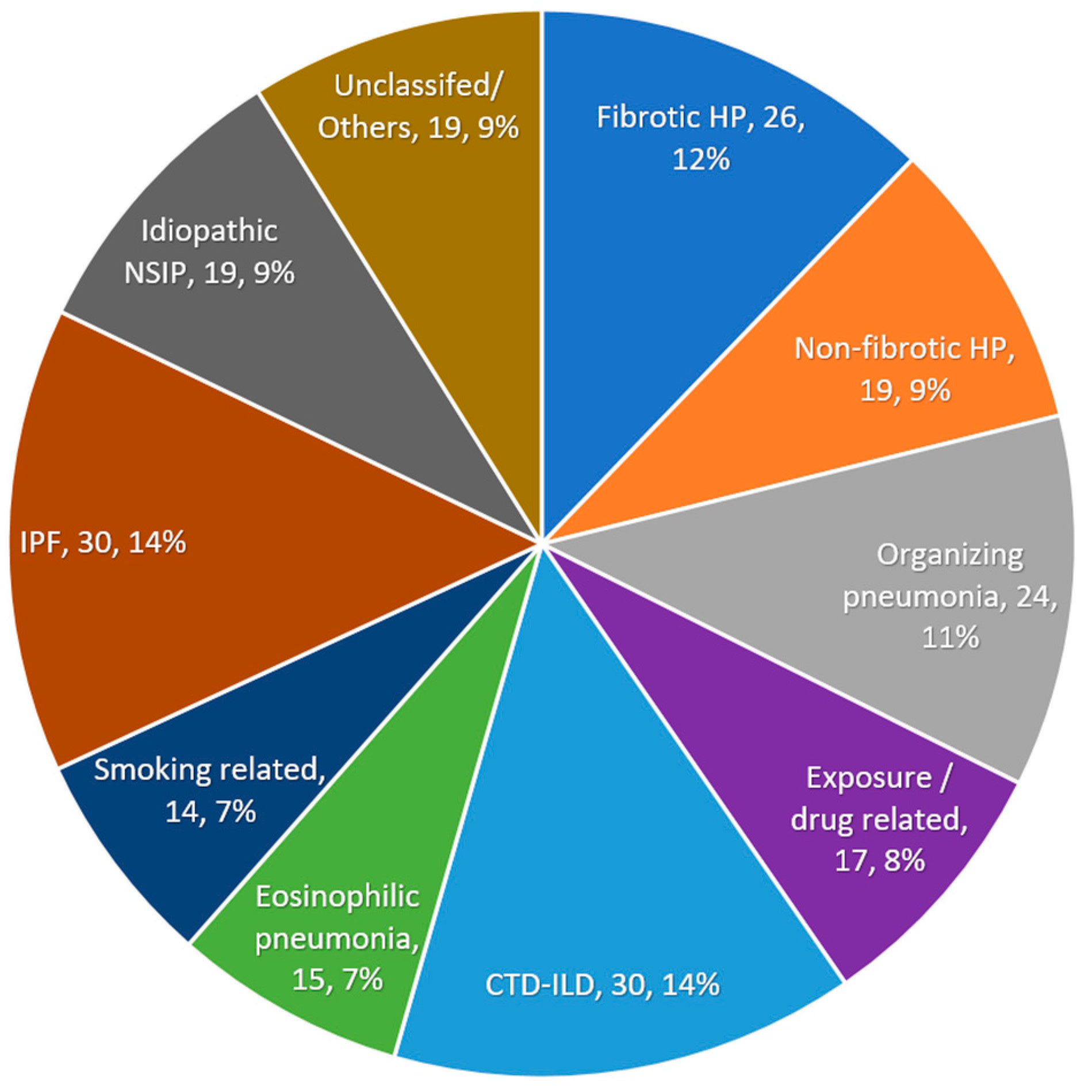
3. Results
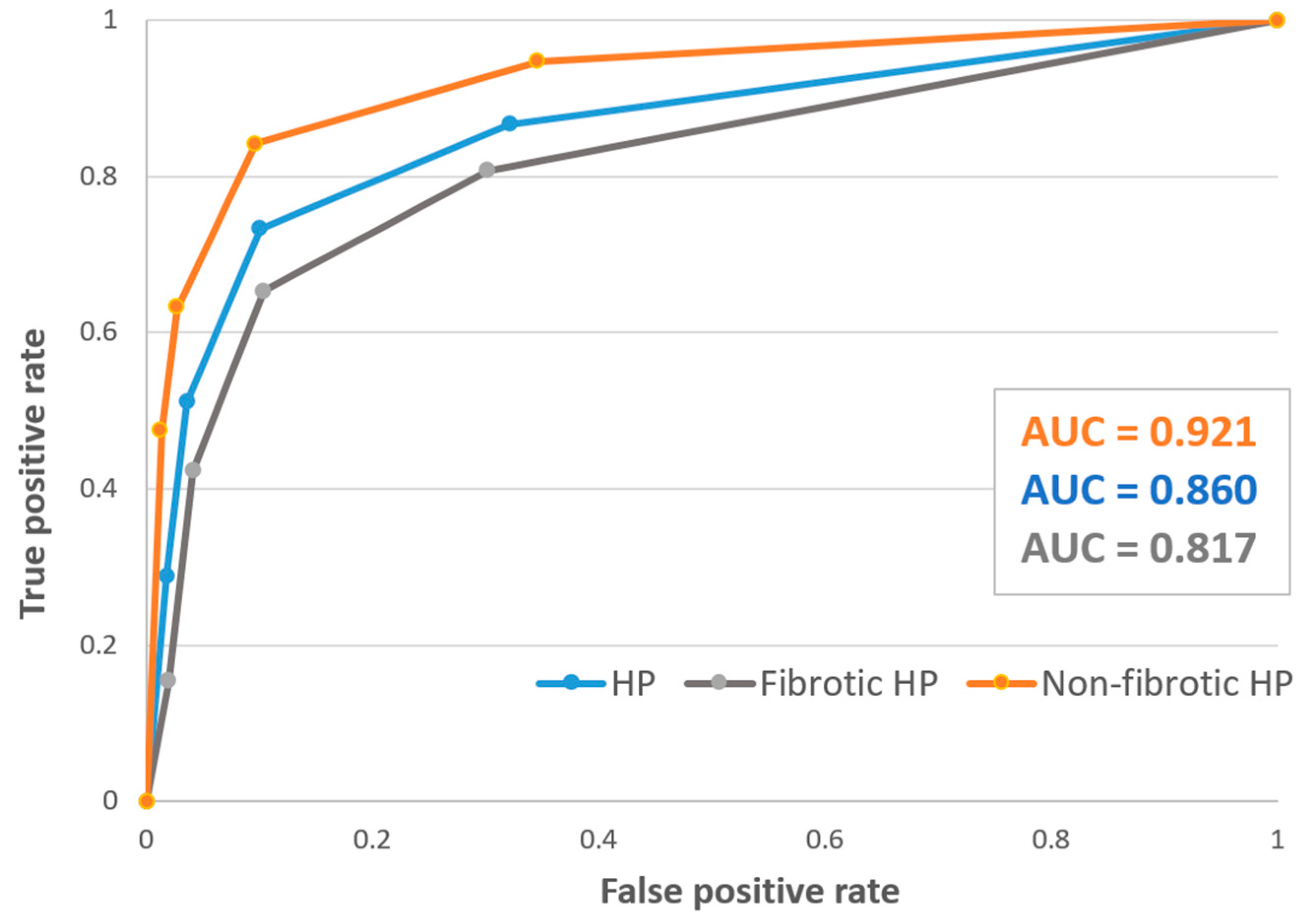
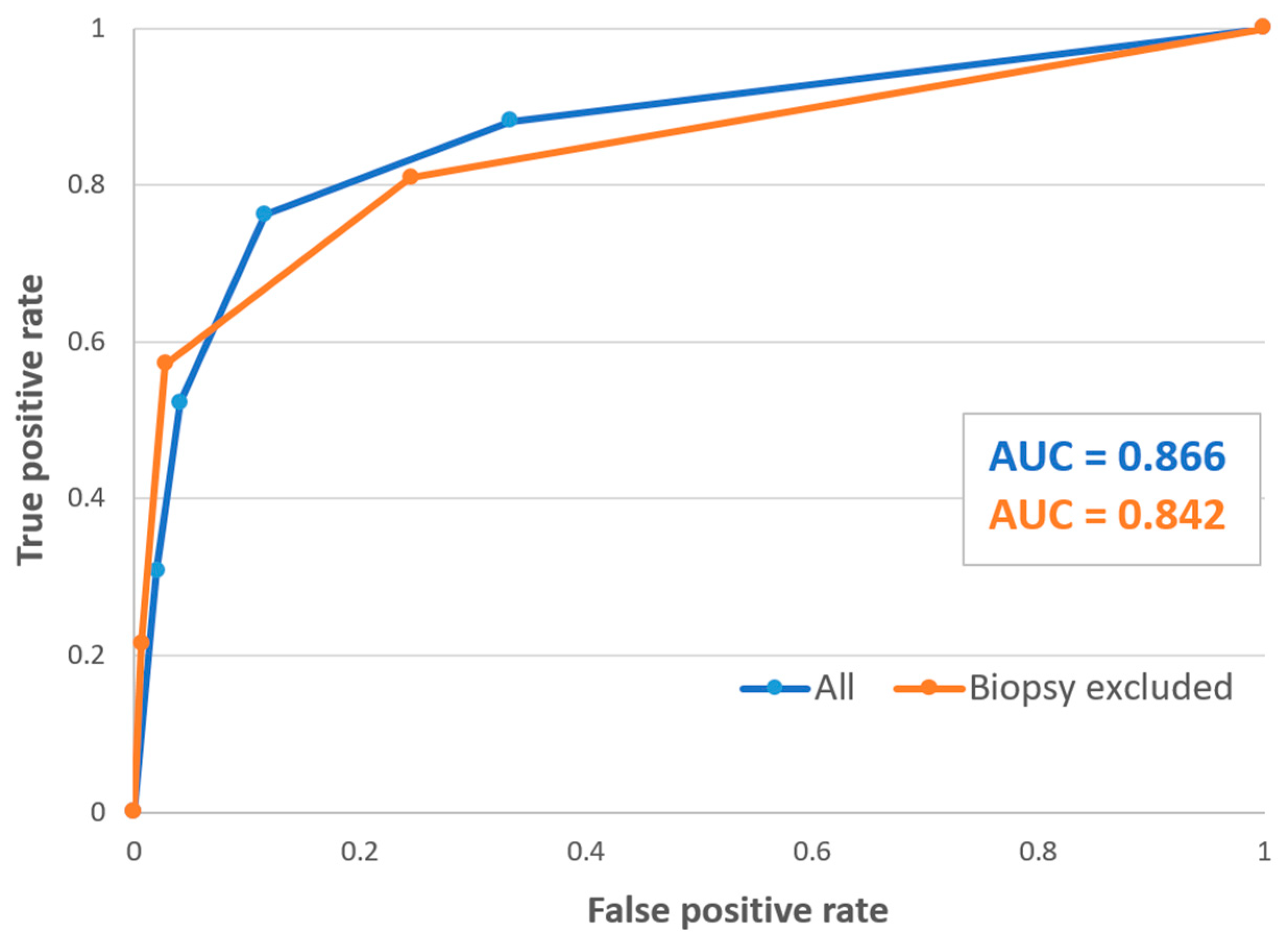
3.1. Diagnostic Performance of the Guidelines
3.2. Patient Characteristics and Predictors for MDD-HP Diagnosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lacasse, Y.; Selman, M.; Costabel, U.; Dalphin, J.C.; Ando, M.; Morell, F.; Erkinjuntti-Pekkanen, R.; Müller, N.; Colby, T.V.; Schuyler, M.; et al. Clinical Diagnosis of Hypersensitivity Pneumonitis. Am. J. Respir. Crit. Care Med. 2003, 168, 952–958. [Google Scholar] [CrossRef]
- Fernández Pérez, E.R.; Travis, W.D.; Lynch, D.A.; Brown, K.K.; Johannson, K.A.; Selman, M.; Ryu, J.H.; Wells, A.U.; Tony Huang, Y.-C.; Pereira, C.A.C.; et al. Diagnosis and Evaluation of Hypersensitivity Pneumonitis. Chest 2021, 160, e97–e156. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Wilson, K.C.; Bargagli, E.; Bendstrup, E.; Chami, H.A.; Chua, A.T.; Chung, J.H.; Collins, B.F.; Corte, T.J.; Dalphin, J.C.; et al. Diagnosis of Hypersensitivity Pneumonitis in Adults: An Official ATS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020, 202, e36–e69. [Google Scholar] [CrossRef]
- Selman, M.; Pardo, A.; King, T.E. Hypersensitivity pneumonitis: Insights in diagnosis and pathobiology. Am. J. Respir. Crit. Care Med. 2012, 186, 314–324. [Google Scholar] [CrossRef]
- Pérez, E.R.F.; Swigris, J.J.; Forssén, A.V.; Tourin, O.; Solomon, J.J.; Huie, T.J.; Olson, A.L.; Brown, K.K. Identifying an inciting antigen is associated with improved survival in patients with chronic hypersensitivity pneumonitis. Chest 2013, 144, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Riario Sforza, G.G.; Marinou, A. Hypersensitivity pneumonitis: A complex lung disease. Clin. Mol. Allergy 2017, 15, 6. [Google Scholar] [CrossRef]
- Varone, F.; Iovene, B.; Sgalla, G.; Calvello, M.; Calabrese, A.; Larici, A.R.; Richeldi, L. Fibrotic Hypersensitivity Pneumonitis: Diagnosis and Management. Lung 2020, 198, 429–440. [Google Scholar] [CrossRef]
- Kalra, S.S.; Jaber, J.F.; Alzghoul, B.; Jansen, B.; Innabi, A.; Tran, A.B.; Fu, K.; Reddy, R.; Gomez Manjarres, D.C.; Patel, D. Hypersensitivity Pneumonitis With and Without Autoimmune Features: A Clinical Comparative Analysis. Lung 2022, 200, 763–771. [Google Scholar] [CrossRef]
- Lynch, D.A.; Newell, J.D.; Logan, P.M.; King, T.E.; Muller, N.L. Can CT distinguish hypersensitivity pneumonitis from idiopathic pulmonary fibrosis? Am. J. Roentgenol. 1995, 165, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, M.L.; Myers, J.L.; Belloli, E.A.; Kazerooni, E.A.; Martinez, F.J.; Flaherty, K.R. Diagnosis and treatment of fibrotic hypersensitivity pneumonia: Where we stand and where we need to go. Am. J. Respir. Crit. Care Med. 2017, 196, 690–699. [Google Scholar] [CrossRef]
- Perluk, T.M.; Friedman Regev, I.; Freund, O.; Kleinhendler, E.; Hershko, T.; Ben-Ami, S.; Bar-Shai, A.; Unterman, A. Importance of physician history taking in complementing patient-reported interstitial lung disease questionnaire. BMC Pulm. Med. 2022, 22, 489. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.N.; Newton, C.A.; Batra, K.; Abu-Hijleh, M.; Barbera, T.; Torrealba, J.; Glazer, C.S. Utility of Bronchoalveolar Lavage and Transbronchial Biopsy in Patients with Hypersensitivity Pneumonitis. Lung 2018, 196, 617–622. [Google Scholar] [CrossRef]
- Elicker, B.M.; Jones, K.D.; Henry, T.S.; Collard, H.R. Multidisciplinary Approach to Hypersensitivity Pneumonitis. J. Thorac. Imaging 2016, 31, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Morisset, J.; Johannson, K.A.; Jones, K.D.; Wolters, P.J.; Collard, H.R.; Walsh, S.L.F.; Ley, B.; Antoniou, K.M.; Assayag, D.; Behr, J.; et al. Identification of diagnostic criteria for chronic hypersensitivity pneumonitis: An international modified Delphi survey. Am. J. Respir. Crit. Care Med. 2018, 197, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.L.F.; Wells, A.U.; Desai, S.R.; Poletti, V.; Piciucchi, S.; Dubini, A.; Nunes, H.; Valeyre, D.; Brillet, P.Y.; Kambouchner, M.; et al. Multicentre evaluation of multidisciplinary team meeting agreement on diagnosis in diffuse parenchymal lung disease: A case-cohort study. Lancet Respir. Med. 2016, 4, 557–565. [Google Scholar] [CrossRef]
- Ohshimo, S.; Guzman, J.; Costabel, U.; Bonella, F. Differential diagnosis of granulomatous lung disease: Clues and pitfalls. Eur. Respir. Rev. 2017, 26, 170012. [Google Scholar] [CrossRef]
- Meyer, K.C.; Raghu, G.; Baughman, R.P.; Brown, K.K.; Costabel, U.; du Bois, R.M.; Drent, M.; Haslam, P.L.; Kim, D.S.; Nagai, S.; et al. An Official American Thoracic Society Clinical Practice Guideline: The Clinical Utility of Bronchoalveolar Lavage Cellular Analysis in Interstitial Lung Disease. Am. J. Respir. Crit. Care Med. 2012, 185, 1004–1014. [Google Scholar] [CrossRef]
- Lee, C.T. Multidisciplinary Meetings in Interstitial Lung Disease: Polishing the Gold Standard. Ann. Am. Thorac. Soc. 2022, 19, 7–9. [Google Scholar] [CrossRef]
- Patolia, S.; Tamae Kakazu, M.; Chami, H.A.; Chua, A.; Diaz-Mendoza, J.; Duggal, A.; Jenkins, A.R.; Knight, S.L.; Raghu, G.; Wilson, K.C. Bronchoalveolar Lavage Lymphocytes in the Diagnosis of Hypersensitivity Pneumonitis among Patients with Interstitial Lung Disease. Ann. Am. Thorac. Soc. 2020, 17, 1455–1467. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef]
- Ryerson, C.J.; Corte, T.J.; Lee, J.S.; Richeldi, L.; Walsh, S.L.F.; Myers, J.L.; Behr, J.; Cottin, V.; Danoff, S.K.; Flaherty, K.R.; et al. A standardized diagnostic ontology for fibrotic interstitial lung disease an international working group perspective. Am. J. Respir. Crit. Care Med. 2017, 196, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Hosmer Jr, D.W.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; John Wiley & Sons: Hoboken, NJ, USA, 2013; Volume 398. [Google Scholar]
- Buendia-Roldan, I.; Aguilar-Duran, H.; Johannson, K.A.; Selman, M. Comparing the Performance of Two Recommended Criteria for Establishing a Diagnosis for Hypersensitivity Pneumonitis. Am. J. Respir. Crit. Care Med. 2021, 204, 865–868. [Google Scholar] [CrossRef]
- Morell, F.; Villar, A.; Montero, M.-Á.; Muñoz, X.; Colby, T.V.; Pipvath, S.; Cruz, M.-J.; Raghu, G. Chronic hypersensitivity pneumonitis in patients diagnosed with idiopathic pulmonary fibrosis: A prospective case-cohort study. Lancet Respir. Med. 2013, 1, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Takei, R.; Yamano, Y.; Kataoka, K.; Yokoyama, T.; Matsuda, T.; Kimura, T.; Ozasa, M.; Fukuoka, J.; Johkoh, T.; Kondoh, Y. New Guideline Diagnosis of Fibrotic Hypersensitivity Pneumonitis in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2021, 204, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Adderley, N.; Humphreys, C.J.; Barnes, H.; Ley, B.; Premji, Z.A.; Johannson, K.A. Bronchoalveolar lavage fluid lymphocytosis in chronic hypersensitivity pneumonitis: A systematic review and meta-analysis. Eur. Respir. J. 2020, 56, 20000206. [Google Scholar] [CrossRef] [PubMed]
- Sobiecka, M.; Szturmowicz, M.; Lewandowska, K.B.; Barańska, I.; Zimna, K.; Łyżwa, E.; Dybowska, M.; Langfort, R.; Radwan-Röhrenschef, P.; Roży, A.; et al. Bronchoalveolar Lavage Cell Count and Lymphocytosis Are the Important Discriminators between Fibrotic Hypersensitivity Pneumonitis and Idiopathic Pulmonary Fibrosis. Diagnostics 2023, 13, 935. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.; Silva, M.; Rolo, R. The role of Bronchoalveolar lavage in Interstitial Lung Diseases. Rev. Port. Pneumol. 2017, 23, 360–362. [Google Scholar] [CrossRef]
| Guidelines Diagnostic Confidence * | Sensitivity % (95% CI) | Specificity † % (95% CI) | PPV † % (95% CI) | NPV † % (95% CI) | Accuracy † % (95% CI) | J-Index † |
|---|---|---|---|---|---|---|
| Low | 87 (73–95) | 68 (60–75) | 42 (36–48) | 95 (90–98) | 72 (65–78) | 0.55 |
| Moderate | ||||||
| Overall | 73 (58–85) | 89 (84–94) | 66 (55–76) | 93 (86–95) | 86 (81–91) | 0.62 |
| Fibrotic HP | 65 (44–83) | 90 (82–95) | 63 (47–77) | 90 (85–94) | 84 (77–90) | 0.55 |
| Non-fibrotic HP | 84 (60–96) | 89 (79–95) | 67 (50–80) | 96 (88–98) | 88 (80–94) | 0.73 |
| High | 51 (36–66) | 96 (92–98) | 79 (62–90) | 88 (85–91) | 86 (82–91) | 0.47 |
| Definite | 29 (16–44) | 98 (95–100) | 81 (56–94) | 84 (81–86) | 84 (78–88) | 0.27 |
| Variable | MDD-HP * n = 45 (%) | MDD-NonHP * n = 168 (%) | p Value |
|---|---|---|---|
| Female sex | 28 (62) | 71 (42) | 0.017 |
| Age, years (SD) | 62 (±13) | 62 (±14) | 0.939 |
| Current or former smokers | 20 (44) | 85 (50) | 0.464 |
| Smoking pack years, median (IQR) | 0 (0–20) | 5 (0–28) | 0.651 |
| Hypertension | 17 (38) | 59 (35) | 0.741 |
| Diabetes mellitus | 9 (20) | 36 (21) | 0.835 |
| Additional (non-ILD) lung disease | 4 (9) | 18 (11) | 0.781 |
| Gastroesophageal reflux disease | 12 (27) | 33 (20) | 0.305 |
| Fibrotic lung disease | 26 (58) | 96 (57) | 0.939 |
| Lung function tests: | |||
| FEV1%—predicted (SD) | 75 (±17) | 78 (±16) | 0.436 |
| FVC%—predicted (SD) | 75 (±15) | 79 (±15) | 0.332 |
| FEV1/FVC (SD) | 0.80 (±0.1) | 0.82 (±0.1) | 0.496 |
| TLC%—predicted (SD) | 83 (±20) | 83 (±22) | 0.889 |
| DLCO%—predicted (SD) | 54 (±18) | 56 (±17) | 0.601 |
| MDD-HP * n = 45 (%) | MDD-NonHP * n = 168 (%) | p Value | |
|---|---|---|---|
| (1) HP-related antigen exposure | 29 (64) | 35 (21) | <0.001 |
| (2) Typical or compatible HRCT pattern for HP | 36 (80) | 65 (39) | <0.001 |
| (3) Bronchoalveolar lavage: † | |||
| Lymphocytes above 20% | 19 (49) | 24 (20) | <0.001 |
| Lymphocytes above 30% | 12 (31) | 15 (12.5) | 0.008 |
| Lymphocytes % (IQR) | 18 (11–37) | 8 (4–17) | <0.001 |
| Macrophages % (IQR) | 43 (17–64) | 39 (16–63) | 0.923 |
| Neutrophils % (IQR) | 18 (6–36) | 27 (10–49) | 0.083 |
| Eosinophils % (IQR) | 2.2 (1–4) | 2.4 (0.5–5) | 0.838 |
| CD4/CD8 ratio (IQR) | 1.7 (1–3) | 1.2 (0.5–2) | 0.163 |
| (4) Trans-bronchial biopsy **: | |||
| Probable or typical for HP | 18 (40) | 17 (10) | <0.001 |
| Indeterminate | 12 (27) | 68 (40) | 0.089 |
| Non-indicative or missing | 10 (22) | 51 (30) | 0.284 |
| Other diagnosis | 5 (11) | 32 (19) | 0.212 |
| Variable | AOR | 95% CI | p |
|---|---|---|---|
| Female sex | 1.90 | 0.66–5.46 | 0.231 |
| Antigen exposure | 11.01 | 3.79–28.03 | <0.001 |
| Typical or compatible HRCT pattern for HP † | 5.14 | 1.68–13.69 | 0.004 |
| BAL lymphocytes > 20% | 2.23 | 0.75–6.63 | 0.148 |
| Probable or typical HP histological findings † | 5.99 | 1.65–18.70 | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freund, O.; Hadad, Y.; Shalmon, T.; Wand, O.; Schneer, S.; Perluk, T.M.; Kleinhendler, E.; Hershko, T.; Tiran, B.; Aviram, G.; et al. Real-Life Diagnostic Performance of the Hypersensitivity Pneumonitis Guidelines: A Multicenter Cohort Study. Diagnostics 2023, 13, 2335. https://doi.org/10.3390/diagnostics13142335
Freund O, Hadad Y, Shalmon T, Wand O, Schneer S, Perluk TM, Kleinhendler E, Hershko T, Tiran B, Aviram G, et al. Real-Life Diagnostic Performance of the Hypersensitivity Pneumonitis Guidelines: A Multicenter Cohort Study. Diagnostics. 2023; 13(14):2335. https://doi.org/10.3390/diagnostics13142335
Chicago/Turabian StyleFreund, Ophir, Yitzhac Hadad, Tamar Shalmon, Ori Wand, Sonia Schneer, Tal Moshe Perluk, Eyal Kleinhendler, Tzlil Hershko, Boaz Tiran, Galit Aviram, and et al. 2023. "Real-Life Diagnostic Performance of the Hypersensitivity Pneumonitis Guidelines: A Multicenter Cohort Study" Diagnostics 13, no. 14: 2335. https://doi.org/10.3390/diagnostics13142335
APA StyleFreund, O., Hadad, Y., Shalmon, T., Wand, O., Schneer, S., Perluk, T. M., Kleinhendler, E., Hershko, T., Tiran, B., Aviram, G., Gershman, E., Adir, Y., Shitrit, D., Bar-Shai, A., & Unterman, A. (2023). Real-Life Diagnostic Performance of the Hypersensitivity Pneumonitis Guidelines: A Multicenter Cohort Study. Diagnostics, 13(14), 2335. https://doi.org/10.3390/diagnostics13142335






