The Utility of Mitochondrial Detection Methods Applied as an Additional Tool for the Differentiation of Renal Cell Tumors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tissue Samples
2.2. Clinical Patient Data and Gross Tumor Examination
2.3. Routine Light Microscopy Tissue Analyses
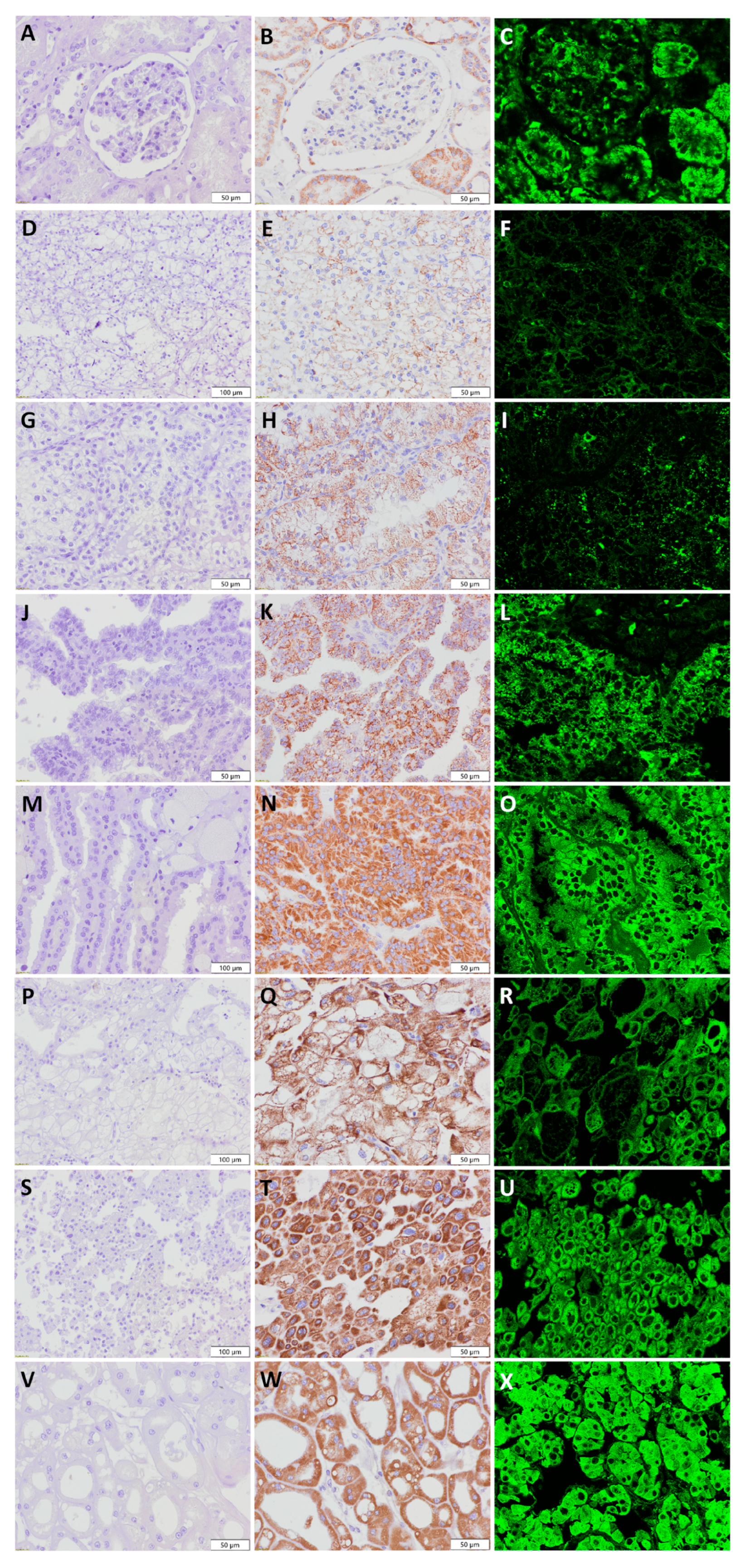
2.4. Immunohistochemical Staining and Analysis
2.5. Immunofluorescence Staining and Analysis
2.6. Transmission Electron Microscopy
3. Results
3.1. Expression Pattern of Anti-Mitochondrial Antibody in Renal Cell Tumors—Description of Patient Cohort
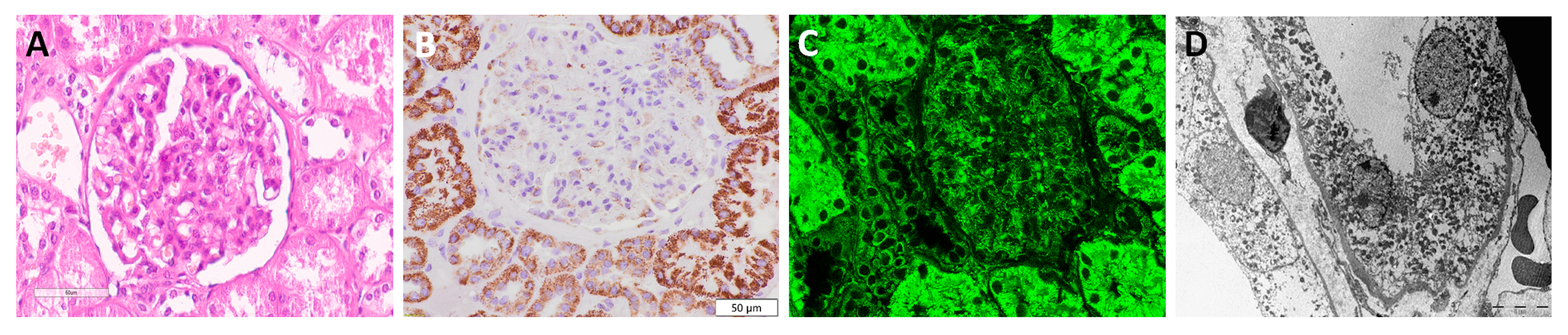
3.2. Visualization of Mitochondria in Non-Tumor Kidney Samples
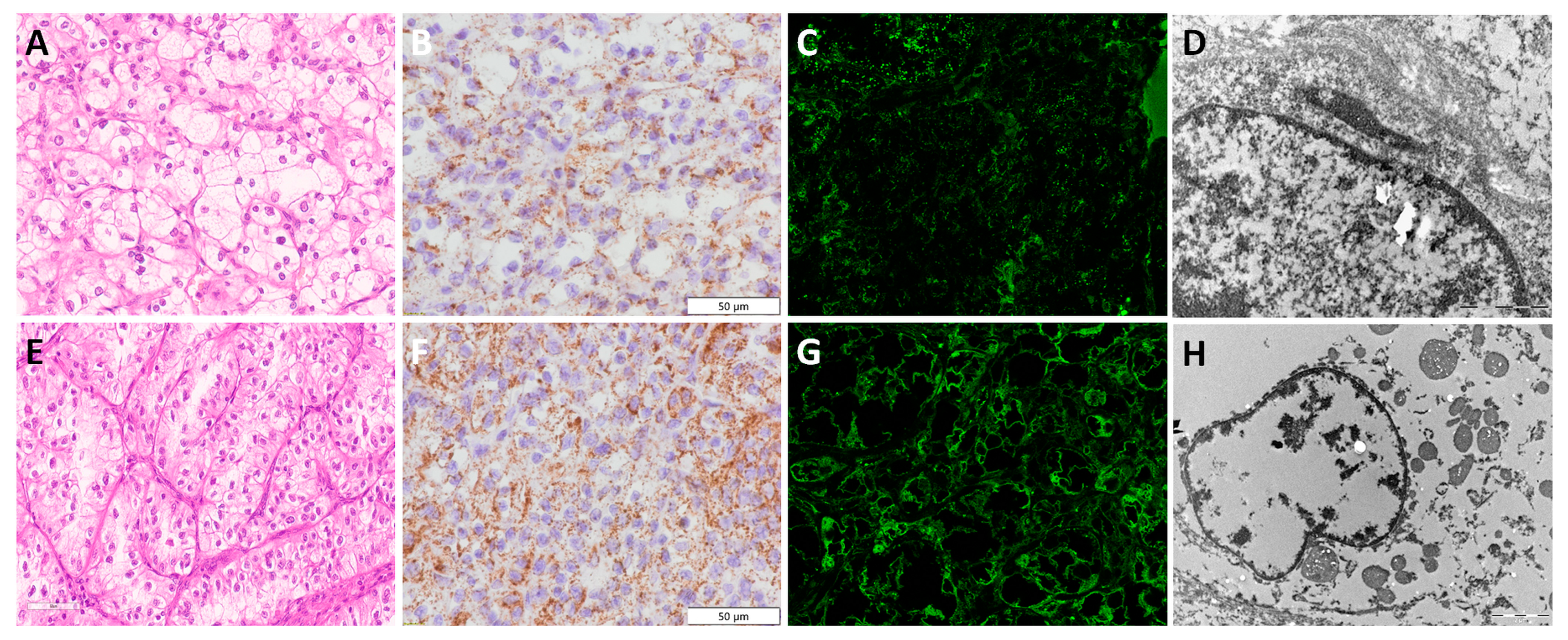
3.3. Clear Cell Renal Cell Carcinoma—ccRCC
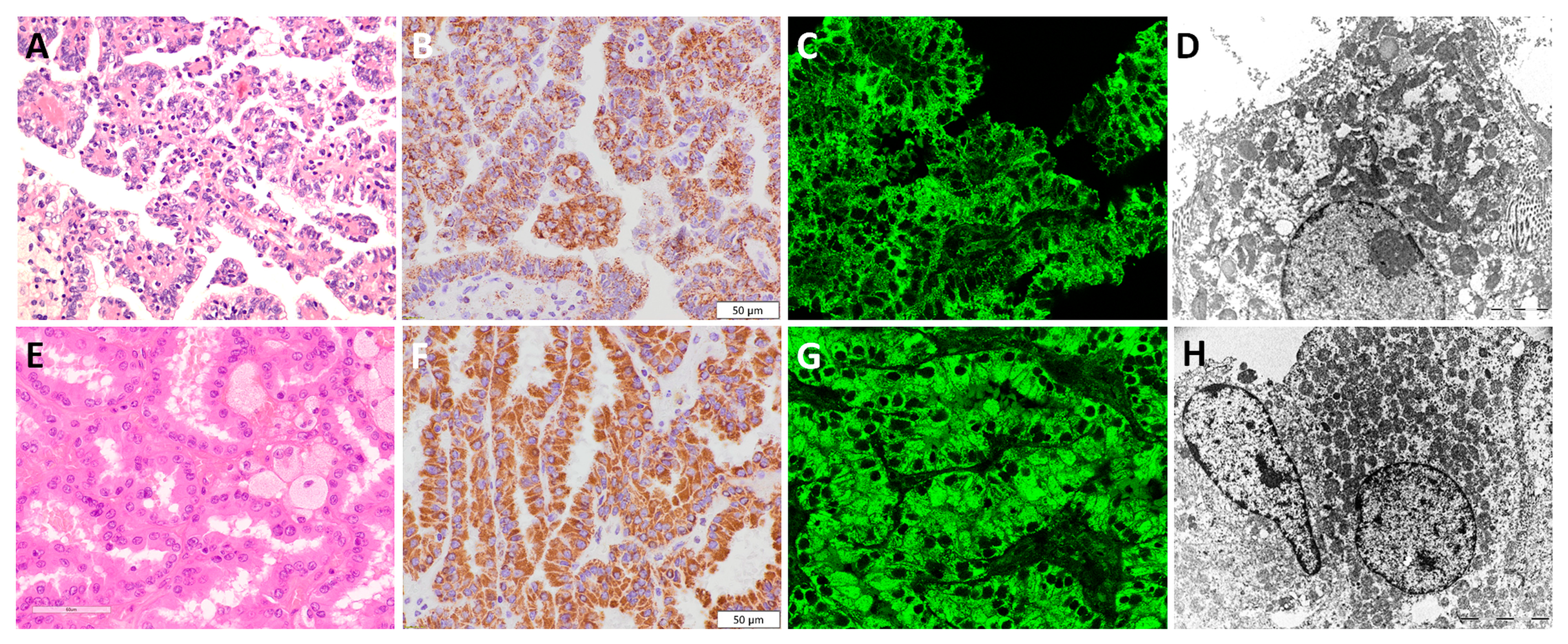
3.4. Papillary Renal Cell Carcinoma—pRCC
3.5. Chromophobe Renal Cell Carcinoma—chRCC
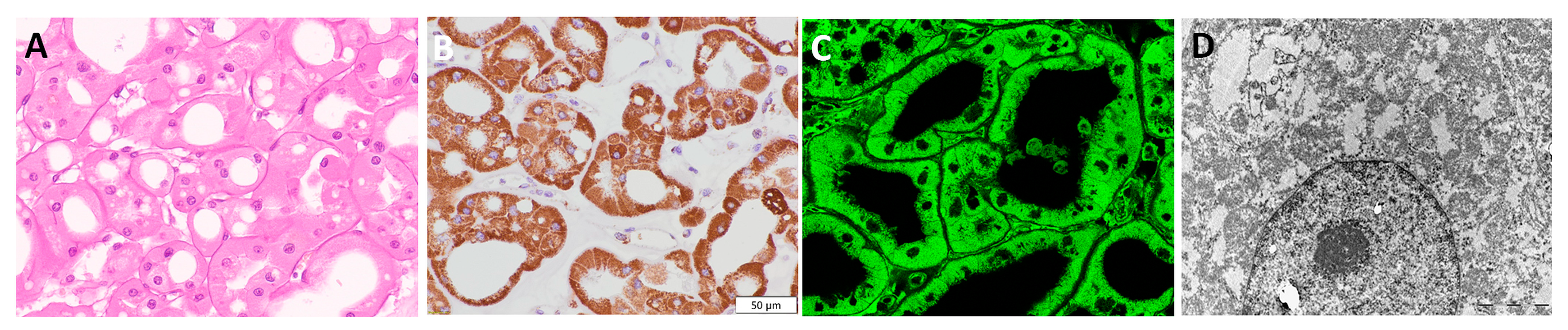
3.6. Renal Oncocytoma—RO
3.7. Summary of Immunohistochemistry and Immunofluorescence Applying Anti-Mitochondrial Antibody in Renal Cell Tumors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moch, H.; Amin, M.B.; Berney, D.M.; Compérat, E.M.; Gill, A.J.; Hartmann, A.; Menon, S.; Raspollini, M.R.; Rubin, M.A.; Srigley, J.R.; et al. The 2022 World Health Organization Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2022, 82, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Störkel, S.; Steart, P.V.; Drenckhahn, D.; Thoenes, W. The human chromophobe cell renal carcinoma: Its probable relation to intercalated cells of the collecting duct. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1989, 56, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Trpkov, K.; Hes, O.; Williamson, S.R.; Adeniran, A.J.; Agaimy, A.; Alaghehbandan, R.; Amin, M.B.; Argani, P.; Chen, Y.B.; Cheng, L.; et al. New developments in existing WHO entities and evolving molecular concepts: The Genitourinary Pathology Society (GUPS) update on renal neoplasia. Mod. Pathol. 2021, 34, 1392–1424. [Google Scholar] [CrossRef]
- Liu, Y.J.; Ussakli, C.; Antic, T.; Liu, Y.; Wu, Y.; True, L.; Tretiakova, M.S. Sporadic oncocytic tumors with features intermediate between oncocytoma and chromophobe renal cell carcinoma: Comprehensive clinicopathological and genomic profiling. Hum. Pathol. 2020, 104, 18–29. [Google Scholar] [CrossRef]
- Wu, A. Oncocytic Renal Neoplasms on Resections and Core Biopsies: Our Approach to This Challenging Differential Diagnosis. Arch. Pathol. Lab. Med. 2017, 141, 1336–1341. [Google Scholar] [CrossRef] [Green Version]
- Williamson, S.R.; Gadde, R.; Trpkov, K.; Hirsch, M.S.; Srigley, J.R.; Reuter, V.E.; Cheng, L.; Kunju, L.P.; Barod, R.; Rogers, C.G.; et al. Diagnostic criteria for oncocytic renal neoplasms: A survey of urologic pathologists. Hum. Pathol. 2017, 63, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Mete, O.; Kilicaslan, I.; Gulluoglu, M.G.; Uysal, V. Can renal oncocytoma be differentiated from its renal mimics? The utility of anti-mitochondrial, caveolin 1, CD63 and cytokeratin 14 antibodies in the differential diagnosis. Virchows Arch. 2005, 44, 938–946. [Google Scholar] [CrossRef]
- Kuroda, N.; Tanaka, A.; Yamaguchi, T.; Kasahara, K.; Naruse, K.; Yamada, Y.; Hatanaka, K.; Shinohara, N.; Nagashima, Y.; Mikami, S.; et al. Chromophobe renal cell carcinoma, oncocytic variant: A proposal of a new variant giving a critical diagnostic pitfall in diagnosing renal oncocytic tumors. Med. Mol. Morphol. 2013, 46, 49–55. [Google Scholar] [CrossRef]
- Johnson, N.B.; Johnson, M.M.; Selig, M.K.; Nielsen, G.P. Use of electron microscopy in core biopsy diagnosis of oncocytic renal tumors. Ultrastruct. Pathol. 2010, 34, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Pusiol, T.; Franceschetti, I.; Scialpi, M.; Piscioli, I.; Tardio, M.L. Electron microscopy: The gold standard in the differential diagnosis of chromophobe renal cell carcinoma and oncocytoma. Anal. Quant. Cytol. Histol. 2010, 32, 58–60. [Google Scholar]
- Abrahams, N.A.; MacLennan, G.T.; Khoury, J.D.; Ormsby, A.H.; Tamboli, P.; Doglioni, C.; Schumacher, B.; Tickoo, S.K. Chromophobe renal cell carcinoma: A comparative study of histological, immunohistochemical and ultrastructural features using high throughput tissue microarray. Histopathology 2004, 45, 593–602. [Google Scholar] [CrossRef]
- Pradhan, D.; Kakkar, N.; Bal, A.; Singh, S.K.; Joshi, K. Sub-typing of renal cell tumours; contribution of ancillary techniques. Diagn. Pathol. 2009, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, H.; Lindgren, D.; Mandahl Forsberg, A.; Mulder, H.; Axelson, H.; Johansson, M.E. Primary clear cell renal carcinoma cells display minimal mitochondrial respiratory capacity resulting in pronounced sensitivity to glycolytic inhibition by 3-Bromopyruvate. Cell Death Dis. 2015, 6, e1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messias, N.C.; Walker, P.D.; Larsen, C.P. Paraffin immunofluorescence in the renal pathology laboratory: More than a salvage technique. Mod. Pathol. 2015, 28, 854–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasr, S.H.; Fidler, M.E.; Said, S.M. Paraffin Immunofluorescence: A Valuable Ancillary Technique in Renal Pathology. Kidney Int. Rep. 2018, 3, 1260–1266. [Google Scholar] [CrossRef] [Green Version]
- Bosic, M.; Brasanac, D.; Stojkovic-Filipovic, J.; Zaletel, I.; Gardner, J.; Cirovic, S. Expression of p300 and p300/CBP associated factor (PCAF) in actinic keratosis and squamous cell carcinoma of the skin. Exp. Mol. Pathol. 2016, 100, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Vujicic, M.; Saksida, T.; Despotovic, S.; Bajic, S.S.; Lalić, I.; Koprivica, I.; Gajic, D.; Golic, N.; Tolinacki, M.; Stojanovic, I. The Role of Macrophage Migration Inhibitory Factor in the Function of Intestinal Barrier. Sci. Rep. 2018, 8, 6337. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.W.; Roh, J.; Park, C.S. Immunohistochemistry for Pathologists: Protocols, Pitfalls, and Tips. J. Pathol. Transl. Med. 2016, 50, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Libard, S.; Cerjan, D.; Alafuzoff, I. Characteristics of the tissue section that influence the staining outcome in immunohistochemistry. Histochem. Cell Biol. 2019, 151, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Tickoo, S.K.; Lee, M.W.; Eble, J.N.; Amin, M.; Christopherson, T.; Zarbo, R.J.; Amin, M.B. Ultrastructural observations on mitochondria and microvesicles in renal oncocytoma, chromophobe renal cell carcinoma, and eosinophilic variant of conventional (clear cell) renal cell carcinoma. Am. J. Surg. Pathol. 2000, 24, 1247–1256. [Google Scholar] [CrossRef]
- Mai, K.T.; Burns, B.F. Chromophobe cell carcinoma and renal cell neoplasms with mucin-like changes. Acta Histochem. 2000, 102, 103–113. [Google Scholar] [CrossRef]
- Yusenko, M.V. Molecular pathology of chromophobe renal cell carcinoma: A review. Int. J. Urol. 2010, 17, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Inkson, B.J. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) for Materials Characterization. In Materials Characterization Using Nondestructive Evaluation (NDE) Methods; Woodhead Publishing: Sawston, UK, 2016; pp. 17–43. [Google Scholar] [CrossRef]
- Tickoo, S.K.; Amin, M.B.; Linden, M.D.; Lee, M.W.; Zarbo, R.J. Antimitochondrial antibody (113-1) in the differential diagnosis of granular renal cell tumors. Am. J. Surg. Pathol. 1997, 21, 922–930. [Google Scholar] [CrossRef]
- Piña, R.; Santos-Díaz, A.I.; Orta-Salazar, E.; Aguilar-Vazquez, A.R.; Mantellero, C.A.; Acosta-Galeana, I.; Estrada-Mondragon, A.; Prior-Gonzalez, M.; Martinez-Cruz, J.I.; Rosas-Arellano, A. Ten Approaches That Improve Immunostaining: A Review of the Latest Advances for the Optimization of Immunofluorescence. Int. J. Mol. Sci. 2022, 23, 1426. [Google Scholar] [CrossRef] [PubMed]
- Honvo-Houéto, E.; Truchet, S. Indirect Immunofluorescence on Frozen Sections of Mouse Mammary Gland. J. Vis. Exp. 2015, 106, 53179. [Google Scholar] [CrossRef] [Green Version]
- Im, K.; Mareninov, S.; Diaz, M.F.P.; Yong, W.H. An Introduction to Performing Immunofluorescence Staining. Methods Mol. Biol. 2019, 1897, 299–311. [Google Scholar] [CrossRef]
- Available online: https://www.sinobiological.com/category/advantages-of-if-icc (accessed on 17 May 2023).
- Sheng, W.; Zhang, C.; Mohiuddin, T.M.; Al-Rawe, M.; Zeppernick, F.; Falcone, F.H.; Meinhold-Heerlein, I.; Hussain, A.F. Multiplex Immunofluorescence: A Powerful Tool in Cancer Immunotherapy. Int. J. Mol. Sci. 2023, 24, 3086. [Google Scholar] [CrossRef]
- Locke, D.; Hoyt, C.C. Companion diagnostic requirements for spatial biology using multiplex immunofluorescence and multispectral imaging. Front. Mol. Biosci. 2023, 10, 1051491. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.X.; Rabinowitz, J.D.; White, E. Mitochondria and Cancer. Mol. Cell. 2016, 61, 667–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Clinical and Pathological Characteristics | Distribution Pattern of Mitochondrial Staining | p | |||
|---|---|---|---|---|---|
| Coarse Scanty | Moderate Granular | Diffuse Granular | |||
| Gender | male | 28 (51.9 %) | 5 (9.3 %) | 21 (38.9 %) | p = 0.005 |
| n (%) | female | 13 (31.0%) | 15 (35.7 %) | 14 (33.3 %) | |
| Age (average age ± SD) | 60.9 ± 9.0 | 61.6 ± 8.3 | 61.2 ± 10.3 | p = 0.960 | |
| Side | left | 23 (43.4 %) | 9 (17.0 %) | 21 (39.6%) | p = 0.478 |
| n (%) | right | 17 (41.5 %) | 11 (26.8%) | 13 (31.7%) | |
| Highest tumor dimension (average mm ± SD) | 64.8 ± 36.5 | 59.8 ±26.2 | 63.9 ± 34.2 | p = 0.865 | |
| Tumor type | Clear cell RCC | 41 (95.3 %) | 0 (0.0 %) | 2 (4.7 %) | p < 0.001 |
| n (%) | Papillary RCC | 0 (0.0 %) | 0 (0.0 %) | 15 (100 %) | |
| Chromophobe RCC | 0 (0.0 %) | 20 (100 %) | 0 (0.0 %) | ||
| Oncocytoma | 0 (0.0 %) | 0 (0.0 %) | 18 (100 %) | ||
| Nuclear grade- ccRCC # | Low grade (NG I. II) | 30 (100 %) | 0 (0.0%) | 0 (0.0%) | p = 0.005 |
| n (%) | High grade (NG III. IV) | 11 (84.6 %) | 0 (0.0%) | 2 (15.4 %) | |
| T staging | T1a | 8 (44.4 %) | 4 (22.2 %) | 6 (33.3 %) | p = 0.764 |
| n (%) | T1b | 20 (71.4 %) | 7 (25.0 %) | 1 (3.6 %) | |
| T2a | 5 (35.7 %) | 5 (35.7 %) | 4 (28.6 %) | ||
| T2b | 0 (0.0%) | 1 (50.0 %) | 1 (50.0%) | ||
| T3a | 6 (50.0 %) | 3 (25.0 %) | 3 (25.0 %) | ||
| T3b | 1 (100.0 %) | 0 (0.0%) | 0 (0.0%) | ||
| T4 | 1 (100.0 %) | 0 (0.0%) | 0 (0.0%) | ||
| Expression intensity of anti-mitochondrial antibody | mild moderate | 41 (95.3 %) 0 (0.0%) | 0 (0.0%) 0 (0.0%) | 0 (0.0%) 2 (4.7 %) | p < 0.001 |
| strong | 0 (0.0%) | 20 (37.7 %) | 33 (62.3 %) | ||
| Type of RCTs | IHC and IF Findings | IHC Score (Mean ± SD) | p | |
|---|---|---|---|---|
| ccRCC | low NG | Mild intensity with coarse scanty cytoplasmic distribution pattern | 2.14 ± 0.64 | <0.001 |
| high NG | Moderate intensity with diffuse granular cytoplasmic staining | |||
| pRCC | low-grade | Strong intensity with diffuse granular staining throughout the whole cytoplasm, with slight apical accentuation | 5.67 ± 0.49 | |
| high-grade | Strong intensity with diffuse granular staining throughout the whole cytoplasm, with prominent apical accentuation | |||
| chRCC | classic pattern | Strong intensity with mild to moderate granular distribution staining pattern, localized mostly along the cell membrane | 4.80 ± 0.41 | |
| eosinophilic pattern | Strong intensity with moderate granular distribution staining pattern, localized mostly along the cell membrane | |||
| RO | eosinophilic pattern | Strong intensity with diffuse granular staining throughout the whole cytoplasm | 6.00 ± 0.00 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolic, G.; Zivotic, M.; Cirovic, S.; Despotovic, S.; Dundjerovic, D.; Radojevic Skodric, S. The Utility of Mitochondrial Detection Methods Applied as an Additional Tool for the Differentiation of Renal Cell Tumors. Diagnostics 2023, 13, 2319. https://doi.org/10.3390/diagnostics13142319
Nikolic G, Zivotic M, Cirovic S, Despotovic S, Dundjerovic D, Radojevic Skodric S. The Utility of Mitochondrial Detection Methods Applied as an Additional Tool for the Differentiation of Renal Cell Tumors. Diagnostics. 2023; 13(14):2319. https://doi.org/10.3390/diagnostics13142319
Chicago/Turabian StyleNikolic, Gorana, Maja Zivotic, Sanja Cirovic, Sanja Despotovic, Dusko Dundjerovic, and Sanja Radojevic Skodric. 2023. "The Utility of Mitochondrial Detection Methods Applied as an Additional Tool for the Differentiation of Renal Cell Tumors" Diagnostics 13, no. 14: 2319. https://doi.org/10.3390/diagnostics13142319
APA StyleNikolic, G., Zivotic, M., Cirovic, S., Despotovic, S., Dundjerovic, D., & Radojevic Skodric, S. (2023). The Utility of Mitochondrial Detection Methods Applied as an Additional Tool for the Differentiation of Renal Cell Tumors. Diagnostics, 13(14), 2319. https://doi.org/10.3390/diagnostics13142319






