Abstract
The scoring systems for disseminated intravascular coagulation (DIC) criteria require several adequate cutoff values, vary, and are complicated. Accordingly, a simpler and quicker diagnostic method for DIC is needed. Under such circumstances, soluble C-type lectin-like receptor 2 (sCLEC-2) received attention as a biomarker for platelet activation. Materials and Methods: The diagnostic usefulness of sCLEC-2 and several formulas, including sCLEC-2xD-dimer, sCLEC-2/platelet count (sCLEC-2/PLT), and sCLEC-2/PLT × D-dimer (sCLEC-2xD-dimer/PLT), were evaluated among 38 patients with DIC, 39 patients with pre-DIC and 222 patients without DIC or pre-DIC (non-DIC). Results: Although the plasma level of sCLEC-2 alone was not a strong biomarker for the diagnosis of DIC or pre-DIC, the sCLEC-2xD-dimer/PLT values in patients with DIC were significantly higher than those in patients without DIC, and in a receiver operating characteristic (ROC) analysis for the diagnosis of DIC, sCLEC-2xD-dimer/PLT showed the highest AUC, sensitivity, and odds ratio. This formula is useful for the diagnosis of both pre-DIC and DIC. sCLEC-2xD-dimer/PLT values were significantly higher in non-survivors than in survivors. Conclusion: The sCLEC-2xD-dimer/PLT formula is simple, easy, and highly useful for the diagnosis of DIC and pre-DIC without the use of a scoring system.
1. Introduction
Disseminated intravascular coagulation (DIC) is a fatal disease that is often complicated by major bleeding or organ failure [1,2,3], although there are many guidelines for the management of DIC [4,5,6]. The main underlying diseases are infectious diseases, hematological malignancy, solid cancer, obstetrics, trauma, and aortic aneurysm [1,2,3]. DIC patients are generally treated for underlying diseases, and early treatment with anticoagulants such as antithrombin [1,4,7,8] or recombinant thrombomodulin [9,10,11] has been recommended for DIC in Japan.
As there is no gold standard for the diagnosis of DIC, many diagnostic criteria for DIC have been established by the Japanese Ministry of Health, Labour and Welfare (JMHLW) [12], the International Society of Thrombosis Haemostasis (ISTH) [13], the Japanese Association for Acute Medicine (JAAM) DIC [14], and the Japanese Society of Thrombosis Hemostasis [15]. Most diagnostic criteria for DIC are based on scoring systems for global coagulation tests, such as fibrin-related markers (FRMs), prothrombin time (PT), platelet count, and fibrinogen [15,16,17,18]. FRMs, including fibrinogen and fibrin degradation products (FDPs), D-dimer or soluble fibrin (SF), and D-dimer, require standardization [19,20,21]. The use of scoring systems for DIC is complicated, and multiple cutoff values for the parameters of DIC vary among the four diagnostic criteria for DIC [12,13,14,15]. Four DIC groups that were diagnosed using each of the diagnostic criteria showed variation in their disease severity and outcomes [22].
Simpler diagnostic criteria are required to facilitate the early treatment of DIC in the emergency room (ER) or intensive care unit (ICU). Therefore, the sepsis-induced coagulopathy (SIC) score [23], which includes the platelet count and sequential organ failure assessment score [24] or quick DIC score, which includes the D-dimer levels, platelet count, PT ratio, and underlying disease [25], which were recently developed, may be simpler and easier scoring systems and may be useful for diagnosing DIC or coagulopathy in patients with sepsis [26]. However, these scoring systems need to use adequate cutoff values and cannot be compared to other diagnostic criteria. Accordingly, these scoring systems are still complicated. In addition, there is no useful biomarker for platelet activation, which plays an important role in the progression of various pathogenic states [27,28]. Recently, elevated soluble C-type lectin-like receptor 2 (sCLEC-2) levels have been reported in patients with DIC [29], thrombotic microangiopathy (TMA) [30], acute myocardial infarction [31,32], acute cerebral infarction [33], and coronavirus disease 2019 [34].
In this study, we developed a simple formula for the diagnosis of DIC using sCLEC-2, platelet count, and D-dimer to examine the agreement with the JMHLW diagnostic criteria.
2. Materials and Methods
The study population included patients with the following conditions who were managed at Mie Prefectural General Medical Center from 1 September 2019 to 28 December 2022: infectious diseases (n = 215), solid cancer (n = 27), aortic aneurysm (n = 37), hematological disorders (n = 11), trauma (n = 41), cardiopulmonary arrest (n = 25), and unidentified clinical syndrome (n = 75) (Table 1). DIC was diagnosed using the Japanese Ministry of Health Labour and Welfare criteria for DIC [12]. Patients with a DIC score of ≥7 points, 5 or 6 points, and ≤4 points were diagnosed with DIC, pre-DIC, and non-DIC, respectively.

Table 1.
The subject study protocol (2019-K9) was approved by the Human Ethics Review Committee of Mie Prefectural General Medical Center, and informed consent was obtained from each participant. This study was carried out in accordance with the principles of the Declaration of Helsinki.
Plasma was prepared by two centrifugations at 3000 rpm for 15 min (the platelet count was less than 0.5 × 1010 platelet count/L). Plasma sCLEC-2 levels were measured by a chemiluminescent enzyme immunoassay (CLEIA) using previously described monoclonal antibodies and the STACIA CLEIA system (LSI Medience, Tokyo, Japan) [31,33]. FDP, D-dimer, and SF were measured using LPIA FDP-P, LPIA-Genesis, and Iatro SF II (LSI Medience, Tokyo, Japan), respectively, with the STACIA system (LSI Medience). The activated partial thromboplastin time (APTT) and PT-international normalized ratio (INR) were measured by a Thrombocheck APTT-SLA and Thromborel S (Sysmex Co., Kobe, Japan, respectively) using an automatic coagulation analyzer CS-5100 (Sysmex Co.). The platelet counts were measured using a fully automatic blood cell counter XN-3000 (Sysmex Co.). “sCLEC-2xD-dimer”, “sCLEC-2/platelet number (sCLEC-2/PLT)” and “sCLEC-2xD-dimer/platelet number (sCLEC-2xD-dimer/PLT)” were calculated using sCLEC-2 (ng/L), platelet number (×1010/L) and D-dimer (μg/mL).
Statistical Analyses
The data are expressed as median (25th–75th percentiles). The significance of differences between groups was examined using the Mann–Whitney U-test. The cutoff values, areas under the curve (AUCs), sensitivity, specificity, and odds ratios were determined by a receiver operating characteristic (ROC) analysis; p-values < 0.05 were considered to indicate a statistically significant difference. All statistical analyses were performed using the Stat-Flex software program (version 7; Artec Co., Ltd., Osaka, Japan).
3. Results
The mortality rate was highest in CPA and was > 10.0% in cases with solid cancer and infection and 0% in cases with hematological malignancy and UCS (Table 1). The APTT was significantly longer in cases with infection and CPA than in UCS, and the PT was significantly higher in cases with solid cancer, aortic aneurysm, infection, and CPA than UCS. Platelet counts were significantly lower in cases with aortic aneurysm, infection, and CPA than in those with UCS. In all underlying diseases, the DIC score, FDP, D-dimer, SF, and sCLEC-2 were significantly higher than in UCS.
Regarding evaluation using the JMHLW diagnostic criteria, 38, 39, and 222 patients were diagnosed with DIC, pre-DIC, and non-DIC, respectively. (Table 2). FDP, D-dimer, SF PT-INR, and sCLEC-2 levels were significantly higher in DIC and pre-DIC than in non-DIC, platelet counts were significantly lower in DIC and pre-DIC than in non-DIC, and APTT was significantly longer in DIC and pre-DIC than in non-DIC.

Table 2.
Hemostatic markers in patients with DIC, pre-DIC, or non-DIC.
In the ROC analysis (Table 3), the cutoff values for FDP and D-dimer showed the highest AUC and sensitivity (both DIC vs. non-DIC and pre-DIC vs. non-DIC). With regard to the PT-INR, APTT, platelet count, and SF, the AUC for DIC was ≥0.89 but the AUC for pre-DIC was ≤0.75. Using sCLEC-2, the AUCs for DIC (0.801) and pre-DIC (0.748) were not significantly different.

Table 3.
The ROC analysis of hemostatic biomarkers for the diagnosis of DIC, pre-DIC, or DIC + pre-DIC vs. non-DIC.
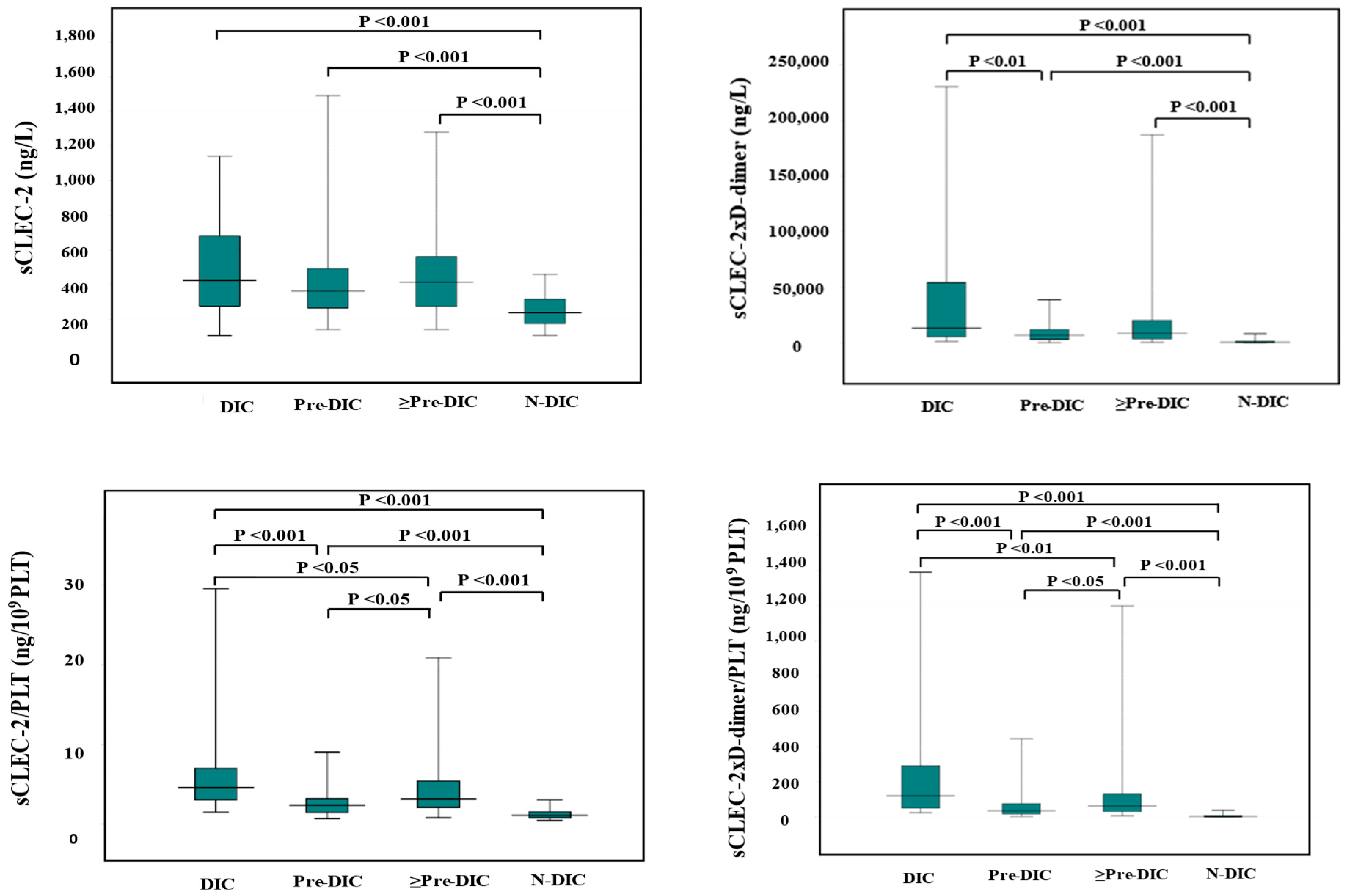
Although the difference of sCLEC-2 alone between DIC (median value, 423 ng/L) and non-DIC (median value, 237 ng/L) and between pre-DIC (median value 361 ng/L) and non-DIC were significant, the difference of sCLEC-2 alone between DIC and pre-DIC was not significant. The differences in sCLEC-2xD-dimer/PLT (median value of DIC, Pre-DIC, and non-DIC; 128, 35.6, and 3.45, respectively), sCLEC-2/PLT (median value; 4.78, 2.37, and 1.12, respectively), or sCLEC-2xD-dimer (median value; 13,198, 6923, and 657, respectively) among DIC, pre-DIC, and non-DIC were significant. (Table 4 and Figure 1).

Table 4.
sCLEC-2, sCLEC-2/PLT, sCLEC-2xD-dimer, and sCLEC-2xD-dimer/PLT.
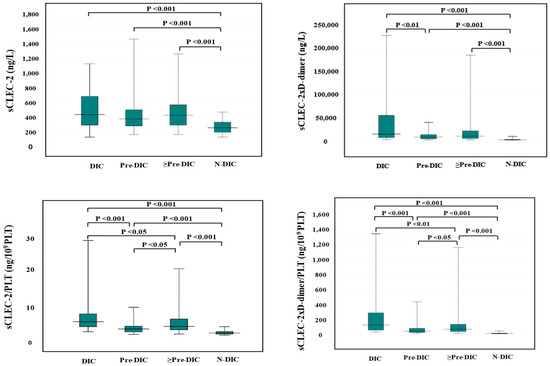
Figure 1.
sCLEC-2, sCLEC-2xD-dimer, sCLEC-2/PLT, and sCLEC-2xD-dimer/PLT in DIC, pre-DIC, ≥Pre-DIC and N-DIC. DIC, disseminated intravascular coagulation; PLT, platelet count; sCLEC-2, soluble C-type lectin-like receptor 2; ≥Pre-DIC, DIC + pre-DIC; N-DIC, non-DIC.
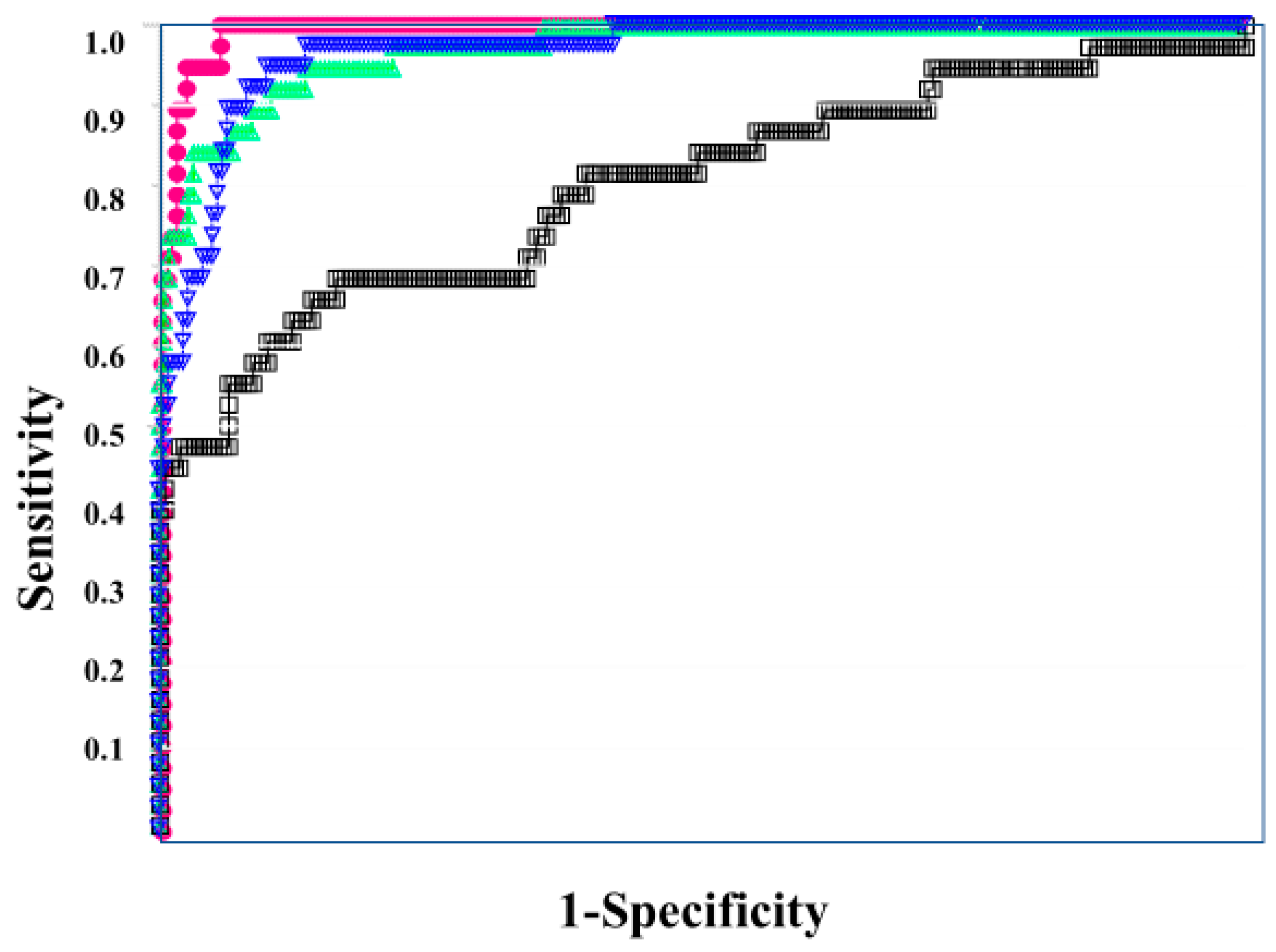
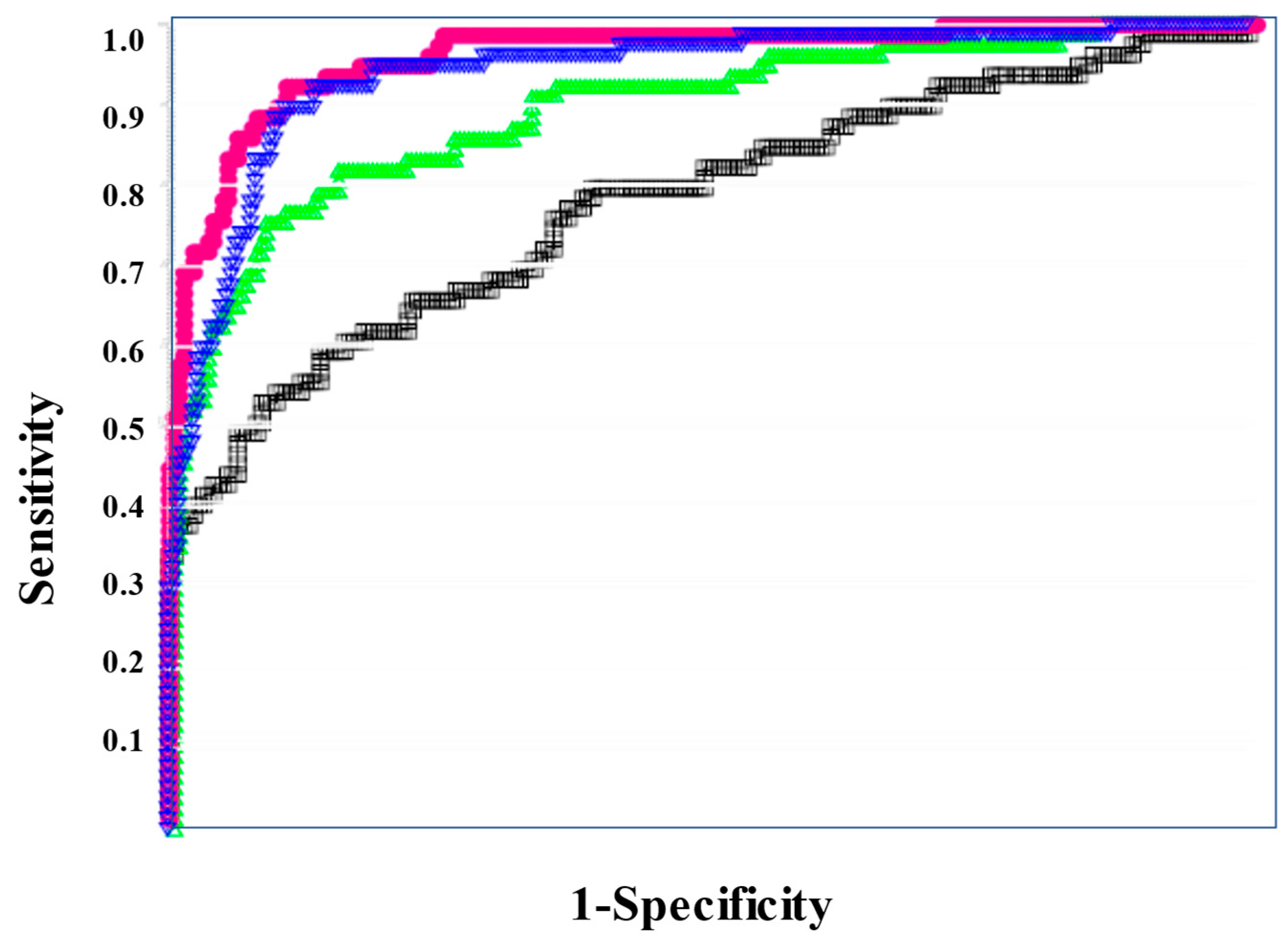
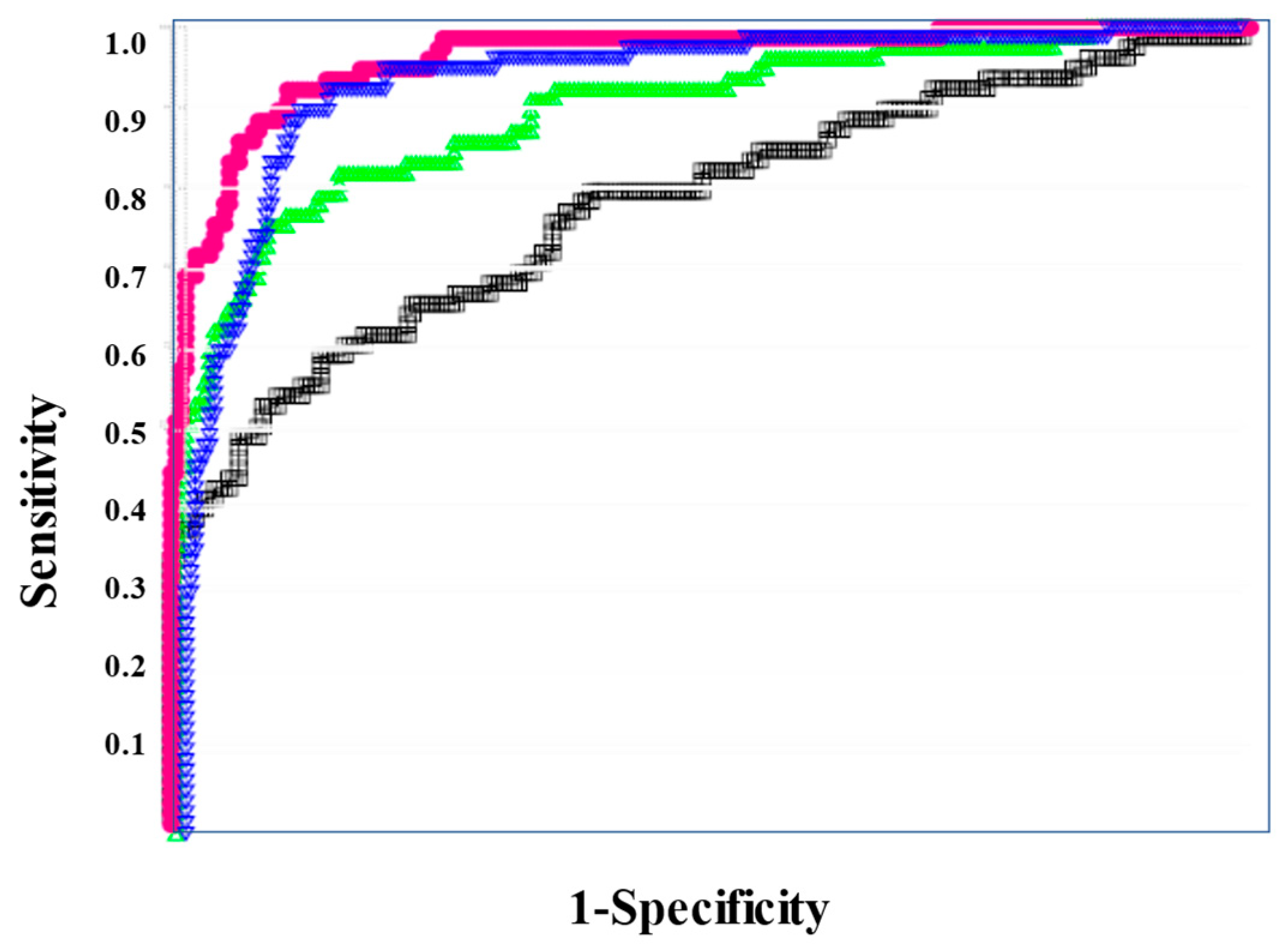
In the ROC for both DIC vs. non-DIC (Figure 2) and DIC + pre-DIC vs. non-DIC (Figure 3), sCLEC-2xD-dimer/PLT showed the highest curve, and sCLEC-2xD-dimer and sCLEC-2/PLT showed high curves, while sCLEC-2 alone showed the lowest curve.
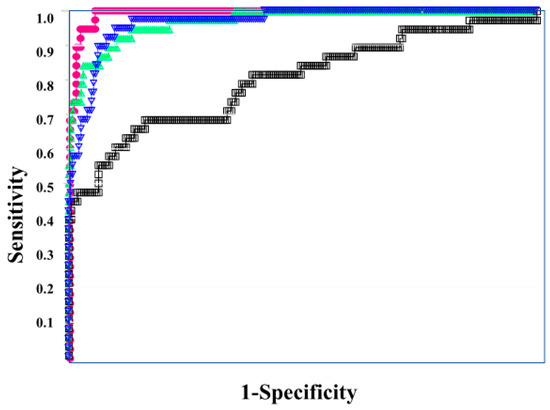
Figure 2.
The ROC analysis of sCLEC-2, sCLEC-2xD-dimer, sCLEC-2/PLT, and sCLEC-2xD-dimer/PLT for DIC vs. N-DIC. ROC, receiver operating characteristic; DIC, disseminated intravascular coagulation; PLT, platelet count; sCLEC-2, soluble C-type lectin-like receptor 2; N-DIC, non-DIC. sCLEC-2 (□); sCLEC-2xD-dimer (▽); sCLEC-2/PLT (△); sCLEC-2xD-dimer/PLT (●).
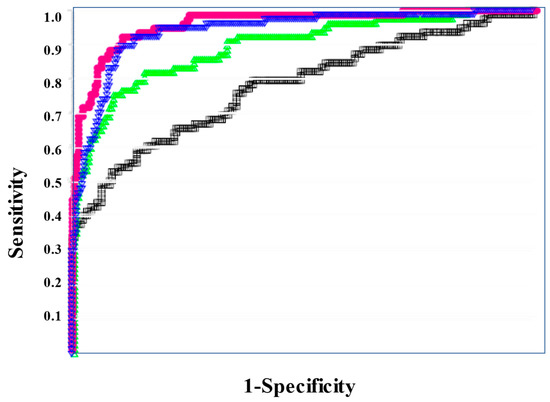
Figure 3.
The ROC analysis of sCLEC-2, sCLEC-2xD-dimer, sCLEC-2/PLT, and sCLEC-2xD-dimer/PLT for ≥Pre-DIC vs. N-DIC. ROC, receiver operating characteristic; DIC, disseminated intravascular coagulation; PLT, platelet count; sCLEC-2, soluble C-type lectin-like receptor 2; ≥Pre-DIC, DIC + pre-DIC; N-DIC, non-DIC; sCLEC-2 (□); sCLEC-2xD-dimer (▽); sCLEC-2/PLT (△); sCLEC-2xD-dimer/PLT (●).
Regarding the ROC analysis (Table 5), sCLEC-2xD-dimer/PLT showed the highest AUC, sensitivity (specificity), and odds ratio for DIC (0.993, 94.7%, and 315.0, respectively), DIC + pre-DIC (0.961, 89.6%, and 74.6), and pre-DIC (0.929, 85.6%, and 32.7, respectively). sCLEC-2xD-dimer and sCLEC-2/PLT also showed high AUC, sensitivity (specificity), and odds ratio for DIC, DIC + pre-DIC, and pre-DIC.

Table 5.
The ROC analysis of sCLEC-2, sCLEC2/PLT, sCLEC2xD-dimer, and sCLEC-2xD-dimer/PLT for the diagnosis of DIC, pre-DIC, or DIC + pre-DIC vs. non-DIC.
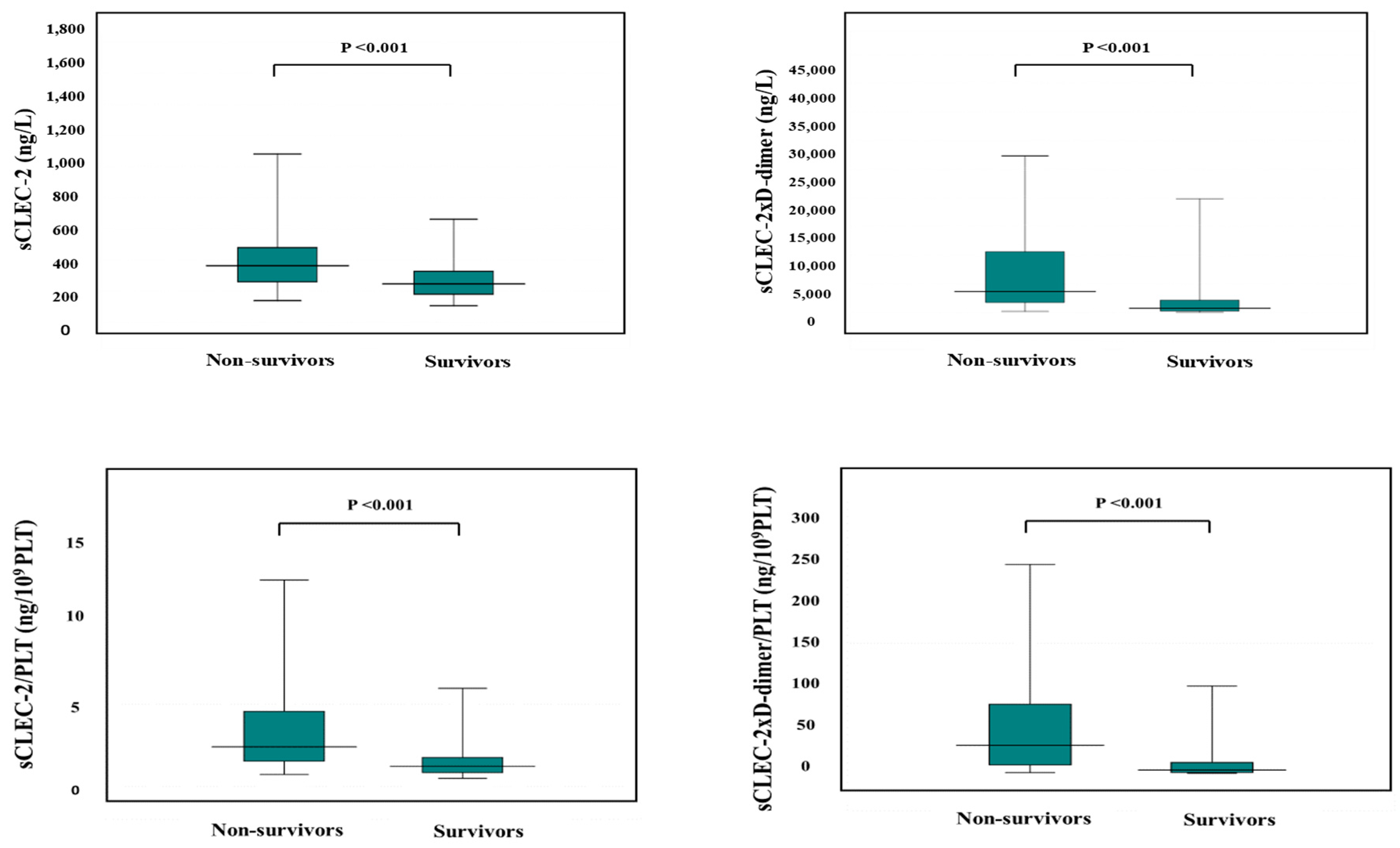
The APTT was significantly longer in non-survivors than in survivors, and PT-INR, FDP, D-dimer, SF, and sCLEC-2 were significantly higher in non-survivors than in survivors, while platelet counts were significantly higher in non-survivors than in survivors (Table 6). sCLEC-2xD-dimer/PLT (median value, 46.3 vs. 4.29), sCLEC-2/PLT (median value, 2.53 vs. 1.25), sCLEC-2xD-dimer (4962 vs. 771), and sCLEC-2 (365 ng/L vs. 247 ng/L) were significantly higher in non-survivors than in survivors (Figure 4).

Table 6.
Hemostatic markers in survivors and non-survivors.
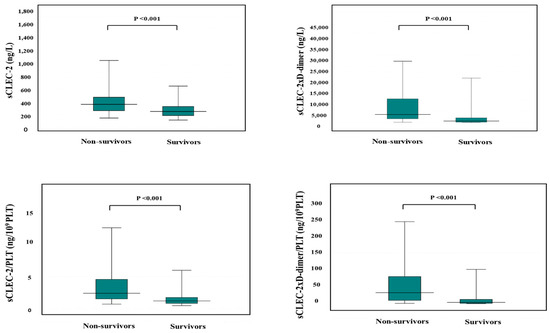
Figure 4.
sCLEC-2, sCLEC-2xD-dimer, sCLEC-2/PLT, and sCLEC-2xD-dimer/PLT in non-survivors and survivors. PLT, platelet count; sCLEC-2, soluble C-type lectin-like receptor 2.
According to the ROC curve, the usefulness of the formulas for predicting a poor outcome was ranked in the order of sCLEC-2xD-dimer/PLT, sCLEC-2xD-dimer, sCLEC-2/PLT, and sCLEC-2 (Figure 5). The ROC analysis revealed that FDP, sCLEC-2xD-dimer/PLT, and PT-INR had high AUC (0.840, 0.816, and 0.817) and sensitivity (76.8%, 76.8%, and 70.6%) for predicting a poor outcome (Table 7).
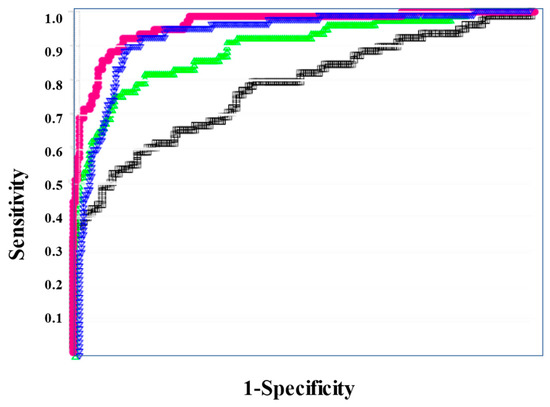
Figure 5.
ROC analysis of sCLEC-2, sCLEC-2xD-dimer, sCLEC-2/PLT, and sCLEC-2xD-dimer/PLT for non-survivors vs. survivors. ROC, receiver operating characteristic; DIC, disseminated intravascular coagulation; PLT, platelet count; sCLEC-2 (□), soluble C-type lectin-like receptor 2. sCLEC-2xD-dimer (▽); sCLEC-2/PLT (△); sCLEC-2xD-dimer/PLT (●).

Table 7.
The ROC analysis of hemostatic biomarkers for non-survivors vs. survivors.
4. Discussion
sCLEC-2 has been introduced as a new biomarker of platelet activation [35]. Elevated sCLEC-2 levels have been reported without thrombocytopenia in patients with acute coronary syndrome [31,32] or acute cerebral infarction [33], suggesting that sCLEC2 may reflect platelet activation in atherosclerotic thrombosis. In addition to reports related to platelet activation [34] such as DIC [29], TMA [30], and hypercoagulability such as nephrotic syndrome [36] and colorectal cancer [37], it was reported that CLEC-2 regulates inflammatory reactions [38,39,40] and CLEC-2 may be related to cancer progression with platelet activation and hypercoagulability [37,41]. In particular, the plasma sCLEC-2 levels in patients with COVID-19 infections were significantly higher than those in patients with other infections and reflected the progression of the severity of COVID-19 infections [34]. In particular, the sCLEC-2/platelet ratio is useful for evaluating the severity of COVID-19 infections. Furthermore, the plasma sCLEC-2 levels in patients with mild-stage COVID-19 infections were similar to those in patients with severe other pneumonia [34,42].
As there is no gold standard and many diagnostic criteria are used for DIC, this study evaluated hemostatic biomarkers for the diagnosis of DIC based on the diagnostic criteria of the JMHLW [12]. This is because JMHLW is the most frequently used and famous diagnostic criterion for DIC in Japan. The mortality rate of DIC diagnosed using the JMHLW criteria was higher in comparison to DIC diagnosed using the JAAM criteria [14] or SIC criteria [23], suggesting that the severity of pre-DIC in patients diagnosed according to the JMHLW criteria may be similar to the severity of that in patients diagnosed according to the JAAM or SIC criteria [22]. In addition, most of the previous diagnostic criteria for DIC have involved complicated scoring systems that require adequate cutoff values of biomarkers and scoring systems [12,13,14,15]. Biomarkers, especially D-dimer, strongly require standardization [19].
sCLEC-2 alone was not more useful than FDP, D-dimer, or PT-INR in the diagnosis of DIC or pre-DIC. In this study, the most useful diagnostic markers for DIC were FDP and D-dimer. These findings may depend on the underlying diseases, as this study included many patients with infectious diseases with non-DIC who had low FDP or D-dimer levels [22]. Hematological malignancy patients without DIC had relatively high FDP or D-dimer levels, suggesting that the usefulness of FDP or D-dimer for the diagnosis of DIC may be decreased in hematological malignancy [22]. Although the AUC of FDP was markedly high for both DIC and pre-DIC, the AUC of the platelet count and PT-INR was markedly high for DIC but not for pre-DIC. The AUC of sCLEC-2 was moderately high for both DIC and pre-DIC, suggesting that sCLEC-2 may be useful for the diagnosis of pre-DIC and early-stage DIC.
Regarding the formula of sCLEC-2, sCLEC-2/PLT or sCLEC-2xD-dimer increased the diagnostic for DIC or pre-DIC in comparison to sCLEC-2 alone, and sCLEC-2xD-dimer/PLT was the most useful for the diagnosis of both DIC and pre-DIC. As thrombocytopenia is frequently observed in DIC, the levels of plasma sCLEC-2 released from decreased platelets may not be sufficiently elevated in patients with DIC. Accordingly, the sCLEC-2/PLT ratio has been reported to be useful for the diagnosis of DIC [43]. An elevated sCLEC-2/PLT ratio was also reported in postoperative glioma patients with venous thromboembolism [44]. As D-dimer and FDP were the most useful biomarkers for DIC in this study [45], sCLEC-2xD-dimer, which reflects the activation of both platelets and coagulation, showed high diagnostic ability for DIC. In particular, the sCLEC-2xD-dimer/PLT ratio was more useful for the diagnosis of pre-DIC in comparison to FDP or D-dimer because the treatment of early phase DIC (e.g., pre-DIC or SIC) is recommended [23,26].
Regarding the outcome, the DIC score, FDP, sCLEC-2xD-dimer/PLT, and PT-INR were useful biomarkers for predicting a poor outcome. The complication of DIC is considered to be associated with poor outcomes [22]. The outcomes of DIC diagnosed using the JMHLW criteria were poorer in comparison to DIC diagnosed using JAAM [22], suggesting that the diagnostic criteria for the early phase of DIC cannot sufficiently predict a poor outcome. ROC analysis showed that the cutoff value of the JMHLW DIC score for a poor outcome was 3.3. These findings suggest that the treatment of DIC for the improvement of an outcome should start at a DIC score of four points before the diagnosis of DIC (DIC score of seven points). In addition, the outcome generally depends on underlying diseases; thus, survival should be examined in a large-scale study.
Although the comparison of the accuracy of the JMHLW diagnostic criteria and the super formula using sCLEC-2 in the diagnosis of DIC requires a gold standard definition for DIC, the super formula using sCLEC-2 is simpler and easier to apply than JMHLW diagnostic criteria. In addition, the present study demonstrates that the concordance between the JMHLW diagnostic criteria and the super formula using sCLEC-2 is significantly high and that the super formula using sCLEC-2 can be used in place of the JMHWD diagnostic criteria in the diagnosis of DIC.
5. Conclusions
sCLEC-2xD-dimer/PLT, which can be diagnosed without a complicated scoring system, is a simple and useful diagnostic formula for the diagnosis of pre-DIC as well as overt-DIC.
Author Contributions
Conceptualization, H.W.; methodology, Y.I. and M.E.; validation, T.I. and K.S.; formal analysis, H.W.; investigation, M.S.; resources, A.Y.; data curation, M.T.; writing—original draft preparation, A.Y.; writing—review and editing, M.K. and H.W.; visualization, K.S.-I.; supervision, H.S.; project administration, K.S.; funding acquisition, H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a Grant-in-Aid from the Ministry of Health, Labour and Welfare of Japan (21FC1008).
Institutional Review Board Statement
The study protocol (2022-S25) was approved by the Human Ethics Review Committee of Mie Prefectural General Medical Center, and informed consent was obtained from each patient.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Conflicts of Interest
The measurements of sCLEC-2 and D-dimer levels were partially supported by LSI Medience. There are no other conflict of interest.
References
- Iba, T.; Levi, M.; Levy, J.H. Sepsis-Induced Coagulopathy and Disseminated Intravascular Coagulation. Semin. Thromb. Hemost. 2020, 46, 89–95. [Google Scholar] [PubMed]
- Adelborg, K.; Larsen, J.B.; Hvas, A.M. Disseminated intravascular coagulation: Epidemiology, biomarkers, and management. Br. J. Haematol. 2021, 192, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Sivapalaratnam, S. Disseminated intravascular coagulation: An update on pathogenesis and diagnosis. Expert. Rev. Hematol. 2018, 11, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Toh, C.H.; Thachil, J.; Watson, H.G. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br. J. Haematol. 2009, 145, 24–33. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Warkentin, T.E.; Thachil, J.; van der Poll, T.; Levi, M. Scientific and Standardization Committee on DIC, and the Scientific and Standardization Committee on Perioperative and Critical Care of the International Society on Thrombosis and Haemostasis: Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J. Thromb. Haemost. 2019, 17, 1989–1994. [Google Scholar]
- Di Nisio, M.; Baudo, F.; Cosmi, B.; D’Angelo, A.; De Gasperi, A.; Malato, A.; Schiavoni, M.; Squizzato, A. Diagnosis and treatment of disseminated intravascular coagulation: Guidelines of the Italian Society for Haemostasis and Thrombosis (SISET). Thromb. Res. 2012, 129, e177–e184. [Google Scholar] [CrossRef]
- Wiedermann, C.J.; Kaneider, N.C. A systematic review of antithrombin concentrate use in patients with disseminated intravascular coagulation of severe sepsis. Blood Coagul. Fibrinolysis 2006, 17, 521–526. [Google Scholar] [CrossRef]
- Iba, T.; Connors, J.M.; Nagaoka, I.; Levy, J.H. Recent advances in the research and management of sepsis-associated DIC. Int. J. Hematol. 2021, 113, 24–33. [Google Scholar] [CrossRef]
- Saito, H.; Maruyama, I.; Shimazaki, S.; Yamamoto, Y.; Aikawa, N.; Ohno, R.; Hirayama, A.; Matsuda, T.; Asakura, H.; Nakashima, M.; et al. Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: Results of a phase III, randomized, double-blind clinical trial. J. Thromb. Haemost. 2007, 5, 31–41. [Google Scholar] [CrossRef]
- Yamakawa, K.; Aihara, M.; Ogura, H.; Yuhara, H.; Hamasaki, T.; Shimazu, T. Recombinant human soluble thrombomodulin in severe sepsis: Systematic review and meta-analysis. J. Thromb. Haemost. 2015, 13, 508–519. [Google Scholar] [CrossRef]
- Ito, T.; Kakuuchi, M.; Maruyama, I. Endotheliopathy in septic conditions: Mechanistic insight into intravascular coagulation. Crit. Care 2021, 25, 95. [Google Scholar] [CrossRef]
- Kobayashi, N.; Maegawa, T.; Takada, M.; Tanaka, H.; Gonmori, H. Criteria for diagnosis of DIC based on the analysis of clinical and laboratory findings in 345 DIC patients collected by the Research Committee on DIC in Japan. Bibl. Haemotol. 1983, 49, 265–275. [Google Scholar]
- Hosseini, S.F.; Behnam-Roudsari, S.; Alavinia, G.; Emami, A.; Toghyani, A.; Moradi, S.; Zadeh, M.M.; Mohseni, S.; Shafiee, M.A. Diagnostic and prognostic value of Sepsis-Induced coagulopathy and International Society on Thrombosis and Hemostasis scoring systems in COVID-19-associated disseminated intravascular coagulopathy. J. Res. Med. Sci. 2021, 26, 102. [Google Scholar]
- Gando, S.; Iba, T.; Eguchi, Y.; Ohtomo, Y.; Okamoto, K.; Koseki, K.; Mayumi, T.; Murata, A.; Ikeda, T.; Ishikura, H.; et al. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: Comparing current criteria. Crit. Care Med. 2006, 34, 625–631. [Google Scholar] [CrossRef]
- Ikezoe, T. Advances in the diagnosis and treatment of disseminated intravascular coagulation in haematological malignancies. Int. J. Hematol. 2021, 113, 34–44. [Google Scholar] [CrossRef]
- Papageorgiou, C.; Jourdi, G.; Adjambri, E.; Walborn, A.; Patel, P.; Fareed, J.; Elalamy, I.; Hoppensteadt, D.; Gerotziafas, G.T. Disseminated Intravascular Coagulation: An Update on Pathogenesis, Diagnosis, and Therapeutic Strategies. Clin. Appl. Thromb. Hemost. 2018, 24, 8S–28S. [Google Scholar] [CrossRef]
- VanVooren, D.M.; Bradshaw, W.T.; Blake, S.M. Disseminated Intravascular Coagulation in the Neonate. Neonatal Netw. 2018, 37, 205–211. [Google Scholar] [CrossRef]
- Ha, S.O.; Park, S.H.; Hong, S.B.; Jang, S. Performance Evaluation of Five Different Disseminated Intravascular Coagulation (DIC) Diagnostic Criteria for Predicting Mortality in Patients with Complicated Sepsis. J. Korean Med. Sci. 2016, 31, 1838–1845. [Google Scholar] [CrossRef]
- Dempfle, C.E. D-dimer: Standardization versus harmonization. Thromb. Haemost. 2006, 95, 399–400. [Google Scholar] [CrossRef]
- Linkins, L.A.; Takach Lapner, S. Review of D-dimer testing: Good, Bad, and Ugly. Int. J. Lab. Hematol. 2017, 39, 98–103. [Google Scholar] [CrossRef]
- Johnson, E.D.; Schell, J.C.; Rodgers, G.M. The D-dimer assay. Am. J. Hematol. 2019, 94, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Takemitsu, T.; Wada, H.; Hatada, T.; Ohmori, Y.; Ishikura, K.; Takeda, T.; Sugiyama, T.; Yamada, N.; Maruyama, K.; Katayama, N.; et al. Prospective evaluation of three different diagnostic criteria for disseminated intravascular coagulation. Thromb. Haemost. 2011, 105, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Levy, J.H.; Raj, A.; Warkentin, T.E. Advance in the Management of Sepsis- Induced Coagulopathy and Disseminated Intravascular Coagulation. J. Clin. Med. 2019, 8, 728. [Google Scholar] [CrossRef] [PubMed]
- Lambden, S.; Laterre, P.F.; Levy, M.M.; Francois, B. The SOFA score-development, utility and challenges of accurate assessment in clinical trials. Crit. Care 2019, 23, 374. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Yamamoto, A.; Tomida, M.; Ichikawa, Y.; Ezaki, M.; Masuda, J.; Yoshida, M.; Fukui, S.; Moritani, I.; Inoue, H.; et al. Proposal of Quick Diagnostic Criteria for Disseminated Intravascular Coagulation. J. Clin. Med. 2022, 11, 1028. [Google Scholar] [CrossRef]
- Iba, T.; Levi, M.; Thachil, J.; Helms, J.; Scarlatescu, E.; Levy, J.H. Communication from the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis on sepsis-induced coagulopathy in the management of sepsis. J. Thromb. Haemost. 2023, 21, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Kazama, F.; Nakamura, J.; Osada, M.; Inoue, O.; Oosawa, M.; Tamura, S.; Tsukiji, N.; Aida, K.; Kawaguchi, A.; Takizawa, S.; et al. Measurement of soluble C-type lectin-like receptor 2 in human plasma. Platelets 2015, 26, 711–719. [Google Scholar] [CrossRef]
- Tanaka, K.; Tanaka, M.; Watanabe, N.; Ito, M.; Pastan, I.; Koizumi, M.; Matsusaka, T. C-type lectin-like receptor (CLEC)-2, the ligand of podoplanin, induces morphological changes in podocytes. Sci. Rep. 2022, 12, 22356. [Google Scholar] [CrossRef]
- Yamamoto, A.; Wada, H.; Ichkawa, Y.; Tanaka, M.; Tashiro, H.; Shiraki, K.; Shimpo, H.; Yamashita, Y.; Mastumoto, T.; Shimaoka, M.; et al. Soluble C-Type Lectin-Like Receptor 2 Is a Biomarker for Disseminated Intravascular Coagulation. J. Clin. Med. 2021, 10, 2860. [Google Scholar] [CrossRef]
- Yamashita, Y.; Suzuki, K.; Mastumoto, T.; Ikejiri, M.; Ohishi, K.; Katayama, N.; Suzuki-Inoue, K.; Wada, H. Elevated plasma levels of soluble C-type lectin-like receptor 2 (CLEC2) in patients with thrombotic microangiopathy. Thromb. Res. 2019, 178, 54–58. [Google Scholar] [CrossRef]
- Inoue, O.; Osada, M.; Nakamura, J.; Kazama, F.; Shirai, T.; Tsukiji, N.; Sasaki, T.; Yokomichi, H.; Dohi, T.; Kaneko, M.; et al. Soluble CLEC-2 is generated independently of ADAM10 and is increased in plasma in acute coronary syndrome: Comparison with soluble GPVI. Int. J. Hematol. 2019, 110, 285–294. [Google Scholar] [CrossRef]
- Fei, M.; Xiang, L.; Chai, X.; Jin, J.; You, T.; Zhao, Y.; Ruan, C.; Hao, Y.; Zhu, L. Plasma soluble C-type lectin-like receptor-2 is associated with the risk of coronary artery disease. Front. Med. 2020, 14, 81–90. [Google Scholar] [CrossRef]
- Nishigaki, A.; Ichikawa, Y.; Ezaki, E.; Yamamoto, A.; Suzuki, K.; Tachibana, K.; Kamon, T.; Horie, S.; Masuda, J.; Makino, K.; et al. Soluble C-type lectin-like receptor 2 elevation in patients with acute cerebral infarction. J. Clin. Med. 2021, 10, 3408. [Google Scholar] [CrossRef]
- Wada, H.; Ichikawa, Y.; Ezaki, M.; Yamamoto, A.; Tomida, M.; Yoshida, M.; Fukui, S.; Moritani, I.; Shiraki, K.; Shimaoka, M.; et al. Elevated Plasma Soluble C-Type Lectin-like Receptor 2 Is Associated with the Worsening of Coronavirus Disease 2019. J. Clin. Med. 2022, 11, 985. [Google Scholar] [CrossRef]
- Suzuki-Inoue, K.; Fuller, G.L.; Garcia, A.; Eble, J.A.; Pohlmann, S.; Inoue, O.; Gartner, T.K.; Hughan, S.C.; Pearce, A.C.; Laing, G.D.; et al. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor sCLEC-2. Blood 2006, 107, 542–549. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, Y.L.; Xu, F.; Jia, X.B.; Mu, S.H.; Lyu, H.Y.; Yuan, X.Y.; Na, S.P.; Bao, Y.S. Elevated Soluble Podoplanin Associates with Hypercoagulability in Patients with Nephrotic Syndrome. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221108967. [Google Scholar] [CrossRef]
- Zhang, M.L.; Huang, W.J.; Yue, C.X.; Li, M.M.; Li, N.; Wang, R.T.; Xie, R. Significant difference of c-type lectin-like receptor 2 between colorectal cancer and polyp subgroups. Cancer Biomark. 2021, 31, 99–105. [Google Scholar] [CrossRef]
- Bourne, J.H.; Beristain-Covarrubias, N.; Zuidscherwoude, M.; Campos, J.; Di, Y.; Garlick, E.; Colicchia, M.; Terry, L.V.; Thomas, S.G.; Brill, A.; et al. CLEC-2 Prevents Accumulation and Retention of Inflammatory Macrophages During Murine Peritonitis. Front. Immunol. 2021, 12, 693974. [Google Scholar] [CrossRef]
- Agrawal, S.; Ganguly, S.; Hajian, P.; Cao, J.N.; Agrawal, A. PDGF upregulates CLEC-2 to induce T regulatory cells. Oncotarget 2015, 6, 28621. [Google Scholar] [CrossRef]
- Chiarini, A.; Armato, U.; Hu, P.; Dal Prà, I. Danger-Sensing/Patten Recognition Receptors and Neuroinflammation in Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 9036. [Google Scholar] [CrossRef]
- Obermann, W.M.J.; Brockhaus, K.; Eble, J.A. Platelets, Constant and Cooperative Companions of Sessile and Disseminating Tumor Cells, Crucially Contribute to the Tumor Microenvironment. Front. Cell. Dev. Biol. 2021, 9, 674553. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Shiraki, K.; Suzuki-Inoue, K. “Unconventional CD147-dependent platelet activation elicited by SARS-CoV-2 in COVID-19”: Comment from Wada et al. J. Thromb. Haemost. 2022, 20, 2159–2160. [Google Scholar] [CrossRef] [PubMed]
- Ishikura, H.; Irie, Y.; Kawamura, M.; Hoshino, K.; Nakamura, Y.; Mizunuma, M.; Maruyama, J.; Nakashio, M.; Suzuki-Inoue, K.; Kitamura, T. Early recognition of sepsis-induced coagulopathy using the C2PAC index: A ratio of soluble type C lectin-like receptor 2 (sCLEC-2) level and platelet count. Platelets 2022, 33, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Natsumeda, M.; Kawamura, M.; Shirakawa, K.; Okada, M.; Tsukamoto, Y.; Eda, T.; Watanabe, J.; Saito, S.; Takahashi, H.; et al. Elevated ratio of C-type lectin-like receptor 2 level and platelet count (C2PAC) aids in the diagnosis of post-operative venous thromboembolism in IDH-wildtype gliomas. Thromb. Res. 2023, 223, 36–43. [Google Scholar] [CrossRef]
- Bick, R.L.; Baker, W.F. Diagnostic efficacy of the D-dimer assay in disseminated intravascular coagulation (DIC). Thromb. Res. 1992, 65, 785–790. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).