Brain-Derived Neurotrophic Factor (BDNF) and Translocator Protein (TSPO) as Diagnostic Biomarkers for Acute Ischemic Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Ethics
2.3. Data Collection
2.4. Variable Definitions
2.5. Blood Collection
2.6. TSPO and BDNF Measurement
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Participants
3.2. Biomarker Level
3.3. Sampling Time Analysis in Stroke Patients
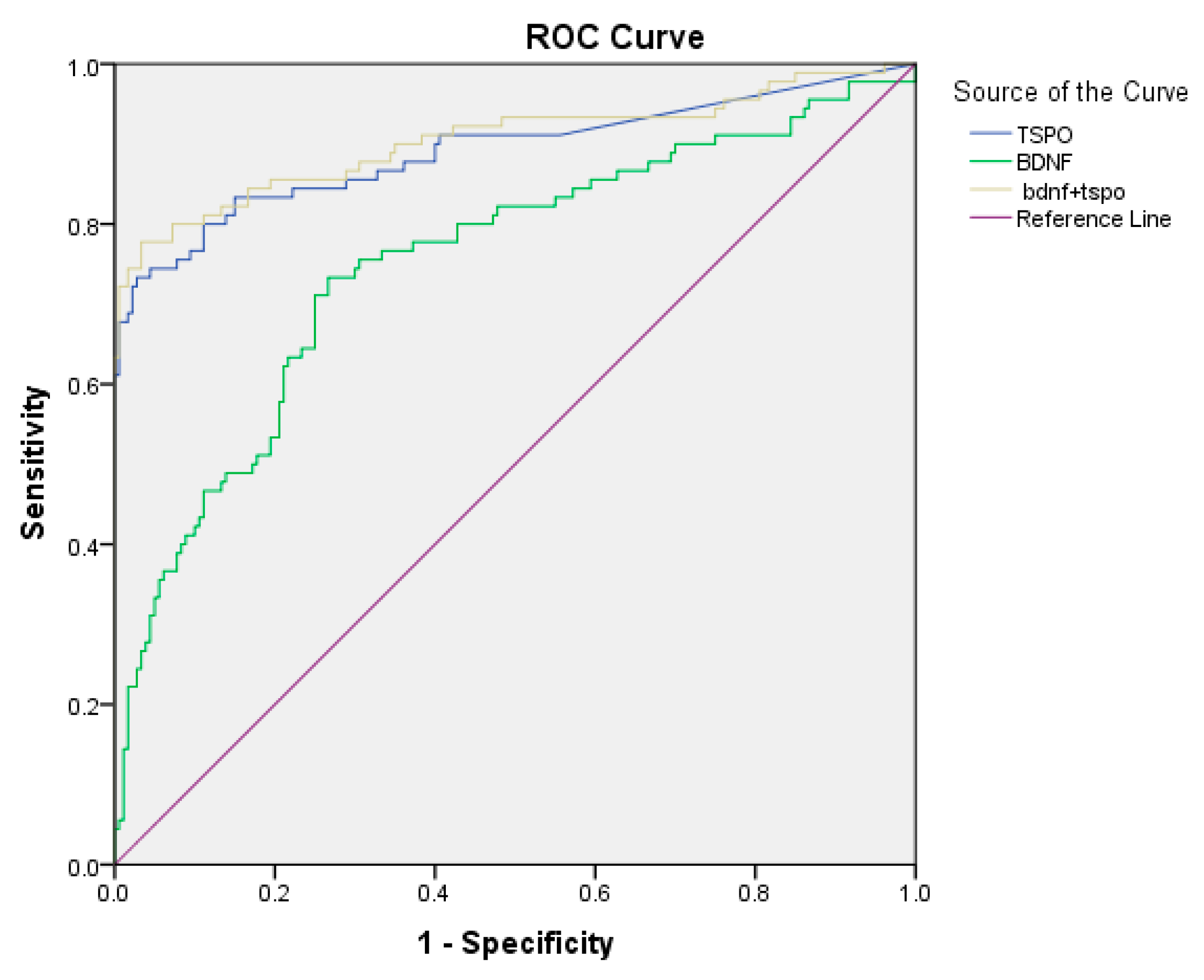
3.4. ROC Analysis of Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, C.O.; Nguyen, M.; Roth, G.A.; Nichols, E.; Alam, T.; Abate, D.; Abd-Allah, F.; Abdelalim, A.; Abraha, H.N.; Abu-Rmeileh, N.M.; et al. Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Rockman, C.B.; Hoang, H.; Guo, Y.; Maldonado, T.S.; Jacobowitz, G.R.; Talishinskiy, T.; Riles, T.S.; Berger, J.S. The prevalence of carotid artery stenosis varies significantly by race. J. Vasc. Surg. 2013, 57, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wu, B.; Liu, M.; Chen, Z.; Wang, W.; Anderson, C.S.; Sandercock, P.; Wang, Y.; Huang, Y.; Cui, L. Stroke in China: Advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019, 18, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, A.; Liberale, L.; Vecchié, A.; Casula, M.; Carbone, F.; Dallegri, F.; Montecucco, F. Update on inflammatory biomarkers and treatments in ischemic stroke. Int. J. Mol. Sci. 2016, 17, 1967. [Google Scholar] [CrossRef]
- Han, J.; Choi, Y.K.; Leung, W.K.; Hui, M.T.; Leung, M.K.W. Long term clinical outcomes of patients with ischemic stroke in primary care–a 9-year retrospective study. BMC Fam. Pract. 2021, 22, 164. [Google Scholar] [CrossRef]
- Donkor, E.S. Stroke in the 21st Century: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Res. Treat. 2018, 2018, 3238165. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in Neuronal Development and Function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef]
- Ishii, T.; Warabi, E.; Mann, G.E. Circadian control of BDNF-mediated Nrf2 activation in astrocytes protects dopaminergic neurons from ferroptosis. Free. Radic. Biol. Med. 2019, 133, 169–178. [Google Scholar] [CrossRef]
- Boulle, F.; Kenis, G.; Cazorla, M.; Hamon, M.; Steinbusch, H.W.M.; Lanfumey, L.; Van Den Hove, D.L.A. TrkB inhibition as a therapeutic target for CNS-related disorders. Prog. Neurobiol. 2012, 98, 197–206. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; O’Connor, M.; Wang, G.; Han, F. Brain-derived neurotrophic factor and its potential therapeutic role in stroke comorbidities. Neural Plast. 2020, 2020, 1969482. [Google Scholar] [CrossRef]
- Karantali, E.; Kazis, D.; Papavasileiou, V.; Prevezianou, A.; Chatzikonstantinou, S.; Petridis, F.; McKenna, J.; Luca, A.-C.; Trus, C.; Ciobica, A. Serum BDNF levels in acute stroke: A systematic review and meta-analysis. Medicina 2021, 57, 297. [Google Scholar] [CrossRef] [PubMed]
- Mirowska-Guzel, D.; Litwin, T.; Gromadzka, G.; Czlonkowski, A.; Czlonkowska, A. Influence of BDNF polymorphisms on Wilson’s disease susceptibility and clinical course. Metab. Brain Dis. 2013, 28, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Bawari, S.; Tewari, D.; Argüelles, S.; Sah, A.N.; Nabavi, S.F.; Xu, S.; Vacca, R.A.; Nabavi, S.M.; Shirooie, S. Targeting BDNF signaling by natural products: Novel synaptic repair therapeutics for neurodegeneration and behavior disorders. Pharmacol. Res. 2019, 148, 104458. [Google Scholar] [CrossRef] [PubMed]
- McEnery, M.W.; Snowman, A.M.; Trifiletti, R.R.; Snyder, S.H. Isolation of the mitochondrial benzodiazepine receptor: Association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc. Natl. Acad. Sci. USA 1992, 89, 3170–3174. [Google Scholar] [CrossRef]
- Betlazar, C.; Middleton, R.J.; Banati, R.; Liu, G.J. The Translocator Protein (TSPO) in Mitochondrial Bioenergetics and Immune Processes. Cells 2020, 9, 512. [Google Scholar] [CrossRef]
- Nutma, E.; Ceyzériat, K.; Amor, S.; Tsartsalis, S.; Millet, P.; Owen, D.R.; Papadopoulos, V.; Tournier, B.B. Cellular sources of TSPO expression in healthy and diseased brain. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 146–163. [Google Scholar] [CrossRef]
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapère, J.-J.; Lindemann, P.; Norenberg, M.D.; Nutt, D.; Weizman, A.; Zhang, M.-R. Translocator protein (18 kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006, 27, 402–409. [Google Scholar] [CrossRef]
- Rupprecht, R.; Papadopoulos, V.; Rammes, G.; Baghai, T.C.; Fan, J.; Akula, N.; Groyer, G.; Adams, D.; Schumacher, M. Translocator protein (18 kDa)(TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2010, 9, 971–988. [Google Scholar] [CrossRef]
- Cosenza-Nashat, M.; Zhao, M.L.; Suh, H.S.; Morgan, J.; Natividad, R.; Morgello, S.; Lee, S.C. Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol. Appl. Neurobiol. 2009, 35, 306–328. [Google Scholar] [CrossRef]
- Herrera-Rivero, M.; Heneka, M.T.; Papadopoulos, V. Translocator protein and new targets for neuroinflammation. Clin. Transl. Imaging 2015, 3, 391–402. [Google Scholar] [CrossRef]
- Venneti, S.; Lopresti, B.J.; Wiley, C.A. The peripheral benzodiazepine receptor (translocator protein 18 kDa) in microglia: From pathology to imaging. Prog. Neurobiol. 2006, 80, 308–322. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; Yan, J.; Csurhes, P.; Greer, J.; McCombe, P. Circulating brain derived neurotrophic factor (BDNF) and frequency of BDNF positive T cells in peripheral blood in human ischemic stroke: Effect on outcome. J. Neuroimmunol. 2015, 286, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Mojtabavi, H.; Shaka, Z.; Momtazmanesh, S.; Ajdari, A.; Rezaei, N. Circulating brain-derived neurotrophic factor as a potential biomarker in stroke: A systematic review and meta-analysis. J. Transl. Med. 2022, 20, 126. [Google Scholar] [CrossRef]
- Øverberg, L.T.; Lugg, E.F.; Gaarder, M.; Langhammer, B.; Thommessen, B.; Rønning, O.M.; Morland, C. Plasma levels of BDNF and EGF are reduced in acute stroke patients. Heliyon 2022, 8, e09661. [Google Scholar] [CrossRef] [PubMed]
- Algin, A.; Erdogan, M.O.; Aydin, I.; Poyraz, M.K.; Sirik, M. Clinical usefulness of brain-derived neurotrophic factor and visinin-like protein-1 in early diagnostic tests for acute stroke. Am. J. Emerg. Med. 2019, 37, 2051–2054. [Google Scholar] [CrossRef]
- Dimitrova-Shumkovska, J.; Krstanoski, L.; Veenman, L. Diagnostic and Therapeutic Potential of TSPO Studies Regarding Neurodegenerative Diseases, Psychiatric Disorders, Alcohol Use Disorders, Traumatic Brain Injury, and Stroke: An Update. Cells 2020, 9, 870. [Google Scholar] [CrossRef]
- Lasek-Bal, A.; Jędrzejowska-Szypułka, H.; Różycka, J.; Bal, W.; Holecki, M.; Duława, J.; Lewin-Kowalik, J. Low concentration of BDNF in the acute phase of ischemic stroke as a factor in poor prognosis in terms of functional status of patients. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 3900. [Google Scholar] [CrossRef]
- Chen, W.-H.; Yeh, H.-L.; Tsao, C.-W.; Lien, L.-M.; Chiwaya, A.; Alizargar, J.; Bai, C.-H. Plasma Translocator Protein Levels and Outcomes of Acute Ischemic Stroke: A Pilot Study. Dis. Markers 2018, 2018, 9831079. [Google Scholar] [CrossRef]
- Sharma, V.K.; Singh, T.G.; Mehta, V.; Mannan, A. Biomarkers: Role and Scope in Neurological Disorders. Neurochem. Res. 2023, 48, 2029–2058. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Price, C.J.; Wang, D.; Menon, D.K.; Guadagno, J.V.; Cleij, M.; Fryer, T.; Aigbirhio, F.; Baron, J.-C.; Warburton, E.A. Intrinsic activated microglia map to the peri-infarct zone in the subacute phase of ischemic stroke. Stroke 2006, 37, 1749–1753. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Muzik, O.; Shandal, V.; Chugani, D.; Chakraborty, P.; Chugani, H.T. Evaluation of age-related changes in translocator protein (TSPO) in human brain using 11C-[R]-PK11195 PET. J. Neuroinflamm. 2012, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Dedeurwaerdere, S.; Callaghan, P.D.; Pham, T.; Rahardjo, G.L.; Amhaoul, H.; Berghofer, P.; Quinlivan, M.; Mattner, F.; Loc’h, C.; Katsifis, A.; et al. PET imaging of brain inflammation during early epileptogenesis in a rat model of temporal lobe epilepsy. EJNMMI Res. 2012, 2, 60. [Google Scholar] [CrossRef] [PubMed]
- Betlazar, C.; Harrison-Brown, M.; Middleton, R.J.; Banati, R.; Liu, G.-J. Cellular sources and regional variations in the expression of the neuroinflammatory marker translocator protein (TSPO) in the normal brain. Int. J. Mol. Sci. 2018, 19, 2707. [Google Scholar] [CrossRef]
- Kim, J.-M.; Stewart, R.; Kim, S.-W.; Yang, S.-J.; Shin, I.-S.; Kim, Y.-H.; Yoon, J.-S. BDNF genotype potentially modifying the association between incident stroke and depression. Neurobiol. Aging 2008, 29, 789–792. [Google Scholar] [CrossRef]
- Béjot, Y.; Prigent-Tessier, A.; Cachia, C.; Giroud, M.; Mossiat, C.; Bertrand, N.; Garnier, P.; Marie, C. Time-dependent contribution of non neuronal cells to BDNF production after ischemic stroke in rats. Neurochem. Int. 2011, 58, 102–111. [Google Scholar] [CrossRef]
- Mourão, A.M.; Vicente, L.C.C.; Abreu, M.N.S.; Sant’Anna, R.V.; Vieira, E.L.M.; de Souza, L.C.; de Miranda, A.S.; Rachid, M.A.; Teixeira, A.L. Plasma levels of brain-derived neurotrophic factor are associated with prognosis in the acute phase of ischemic stroke. J. Stroke Cerebrovasc. Dis. 2019, 28, 735–740. [Google Scholar] [CrossRef]
- Eyileten, C.; Sharif, L.; Wicik, Z.; Jakubik, D.; Jarosz-Popek, J.; Soplinska, A.; Postula, M.; Czlonkowska, A.; Kaplon-Cieslicka, A.; Mirowska-Guzel, D. The Relation of the Brain-Derived Neurotrophic Factor with MicroRNAs in Neurodegenerative Diseases and Ischemic Stroke. Mol. Neurobiol. 2021, 58, 329–347. [Google Scholar] [CrossRef]
- Schäbitz, W.-R.; Sommer, C.; Zoder, W.; Kiessling, M.; Schwaninger, M.; Schwab, S. Intravenous brain-derived neurotrophic factor reduces infarct size and counterregulates Bax and Bcl-2 expression after temporary focal cerebral ischemia. Stroke 2000, 31, 2212–2217. [Google Scholar] [CrossRef]
- Chen, H.H.; Zhang, N.; Li, W.Y.; Fang, M.R.; Zhang, H.; Fang, Y.S.; Ding, M.X.; Fu, X.Y. Overexpression of brain-derived neurotrophic factor in the hippocampus protects against post-stroke depression. Neural Regen. Res. 2015, 10, 1427. [Google Scholar]
- Latchaw, R.E.; Alberts, M.J.; Lev, M.H.; Connors, J.J.; Harbaugh, R.E.; Higashida, R.T.; Hobson, R.; Kidwell, C.S.; Koroshetz, W.J.; Mathews, V. Recommendations for imaging of acute ischemic stroke: A scientific statement from the American Heart Association. Stroke 2009, 40, 3646–3678. [Google Scholar] [CrossRef] [PubMed]
- Chalela, J.A.; Kidwell, C.S.; Nentwich, L.M.; Luby, M.; Butman, J.A.; Demchuk, A.M.; Hill, M.D.; Patronas, N.; Latour, L.; Warach, S. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: A prospective comparison. Lancet 2007, 369, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, Z.; Kan, P.; Zhang, B. The diagnostic value of serum miRNA-221-3p, miRNA-382-5p, and miRNA-4271 in ischemic stroke. J. Stroke Cerebrovasc. Dis. 2017, 26, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Zivin, J.A. Acute Stroke Therapy with Tissue Plasminogen Activator (tPA) Since It Was Approved by the US Food and Drug Administration (FDA); Wiley Online Library: Hoboken, NJ, USA, 2009; Volume 66, pp. 6–10. [Google Scholar]
- Sims, S.-K.; Wilken-Resman, B.; Smith, C.J.; Mitchell, A.; McGonegal, L.; Sims-Robinson, C. Brain-Derived Neurotrophic Factor and Nerve Growth Factor Therapeutics for Brain Injury: The Current Translational Challenges in Preclinical and Clinical Research. Neural Plast. 2022, 2022, 3889300. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kang, S.Y.; Kim, M.C.; Lee, W.I. Plasma biomarkers in the diagnosis of acute ischemic stroke. Ann. Clin. Lab. Sci. 2010, 40, 336–341. [Google Scholar] [PubMed]
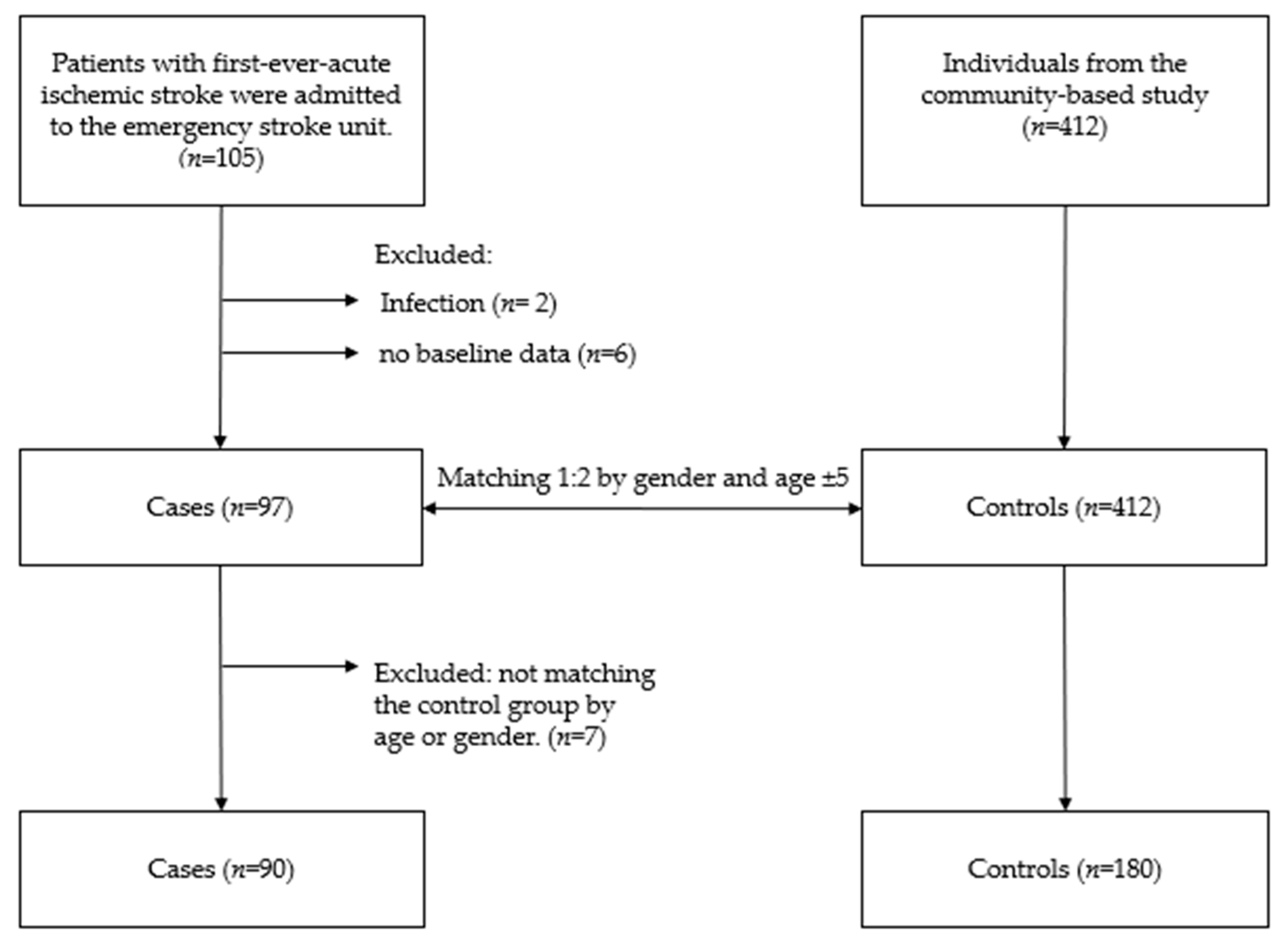

| Characteristics | Cases (n = 90) N (%)/M ± SD | Control (n = 180) N (%)/M ± SD | p-Value |
|---|---|---|---|
| Age | 67.7 ± 12.88 | 66.63 ± 11.25 | 0.484 |
| Gender (Male) | 61 (67.8) | 122 (67.8) | 1.0 |
| Systolic blood pressure | 163.04 ± 41.98 | 124.63 ± 17.62 | <0.001 |
| Diastolic blood pressure | 90.62 ± 26.99 | 73.4 ± 8.99 | <0.001 |
| Hypertension | 64 (71.1%) | 33 (18.3%) | <0.001 |
| Diabetes mellitus | 44 (48.9%) | 30 (16.7%) | <0.001 |
| Hyperlipidemia | 37 (41.1%) | 85 (47.2%) | 0.366 |
| Platelet (103/uL) | 236.76 ± 99.47 | 229.18 ± 60.94 | 0.437 |
| White blood cell (103/uL) | 10.3 ± 4.33 | 5.85 ± 1.4 | <0.001 |
| Hematocrit (%) | 41.80 ± 5.66 | 42.03 ± 3.56 | 0.683 |
| Blood urea nitrogen (mg/dL) | 20.31 ± 9.57 | 17.0 ± 6.1 | <0.001 |
| Hs C-reactive protein (mg/dL) | 5.72 ± 6.41 | 0.22 ± 0.63 | <0.001 |
| Creatinine (mg/dL) | 1.37 ± 1.31 | 0.87 ± 0.24 | <0.001 |
| Hemoglobin A1c (%) | 6.59 ± 1.68 | 6.0 ± 0.87 | <0.001 |
| Glucose (mg/dL) | 127.53 ± 47.59 | 96.24 ± 29.74 | <0.001 |
| Triglycerides (mg/dL) | 124.05 ± 67.55 | 123.15 ± 141.94 | 0.955 |
| Total cholesterol (mg/dL) | 178.55 ± 46.46 | 183.16 ± 33.59 | 0.353 |
| High-density lipoprotein cholesterol (mg/dL) | 45.39 ± 10.79 | 50.54 ± 12.72 | <0.001 |
| Low-density lipoprotein cholesterol (mg/dL) | 112.79 ± 36.51 | 113.0 ± 29.0 | 0.960 |
| Biomarker | All Median (IQR) | Cases Median (IQR) | Control Median (IQR) | p-Value |
|---|---|---|---|---|
| BDNF (ng/mL) | 1.615 (0.8339–3.705) | 3.227 (1.867–6.801) | 1.187 (0.640–2.546) | <0.001 |
| TSPO (ng/mL) | 0.194 (0.091–0.417) | 0.658 (0.333–0.964) | 0.133 (0.071–0.222) | <0.001 |
| Biomarkers (ng/mL) | Crude Model OR (95%CI) | Model 1 OR (95%CI) | Model 2 OR (95%CI) |
|---|---|---|---|
| BDNF (ng/mL) (≥1.615 vs. <1.615) * | 12.46(6.40–24.24) | 13.62 (6.83–27.14) | 6.77 (2.02–22.68) |
| TSPO (ng/mL) (≥0.194 vs. <0.194) * | 5.68 (3.19–10.09) | 5.82 (3.26–10.38) | 3.27 (1.09–9.82) |
| Biomarkers | AUC | Sensitivity | Specificity | PLR | Cut-Off | Y.I. | p-Value |
|---|---|---|---|---|---|---|---|
| BDNF | 0.76 | 73% | 73.3% | 2.70 | 2.171 | 0.467 | 0.001 |
| TSPO | 0.89 | 73.3% | 97.2% | 26.28 | 0.375 | 0.706 | 0.001 |
| BDNF + TSPO | 0.90 | 78% | 96.7% | 23.64 | 0.428 | 0.744 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuwar, M.N.; Chen, W.-H.; Chiwaya, A.M.; Yeh, H.-L.; Nguyen, M.H.; Bai, C.-H. Brain-Derived Neurotrophic Factor (BDNF) and Translocator Protein (TSPO) as Diagnostic Biomarkers for Acute Ischemic Stroke. Diagnostics 2023, 13, 2298. https://doi.org/10.3390/diagnostics13132298
Tuwar MN, Chen W-H, Chiwaya AM, Yeh H-L, Nguyen MH, Bai C-H. Brain-Derived Neurotrophic Factor (BDNF) and Translocator Protein (TSPO) as Diagnostic Biomarkers for Acute Ischemic Stroke. Diagnostics. 2023; 13(13):2298. https://doi.org/10.3390/diagnostics13132298
Chicago/Turabian StyleTuwar, Mayuri N., Wei-Hung Chen, Arthur M. Chiwaya, Hsu-Ling Yeh, Minh H. Nguyen, and Chyi-Huey Bai. 2023. "Brain-Derived Neurotrophic Factor (BDNF) and Translocator Protein (TSPO) as Diagnostic Biomarkers for Acute Ischemic Stroke" Diagnostics 13, no. 13: 2298. https://doi.org/10.3390/diagnostics13132298
APA StyleTuwar, M. N., Chen, W.-H., Chiwaya, A. M., Yeh, H.-L., Nguyen, M. H., & Bai, C.-H. (2023). Brain-Derived Neurotrophic Factor (BDNF) and Translocator Protein (TSPO) as Diagnostic Biomarkers for Acute Ischemic Stroke. Diagnostics, 13(13), 2298. https://doi.org/10.3390/diagnostics13132298






