Could Endothelin-1 Be a Promising Neurohormonal Biomarker in Acute Heart Failure?
Abstract
:1. Introduction
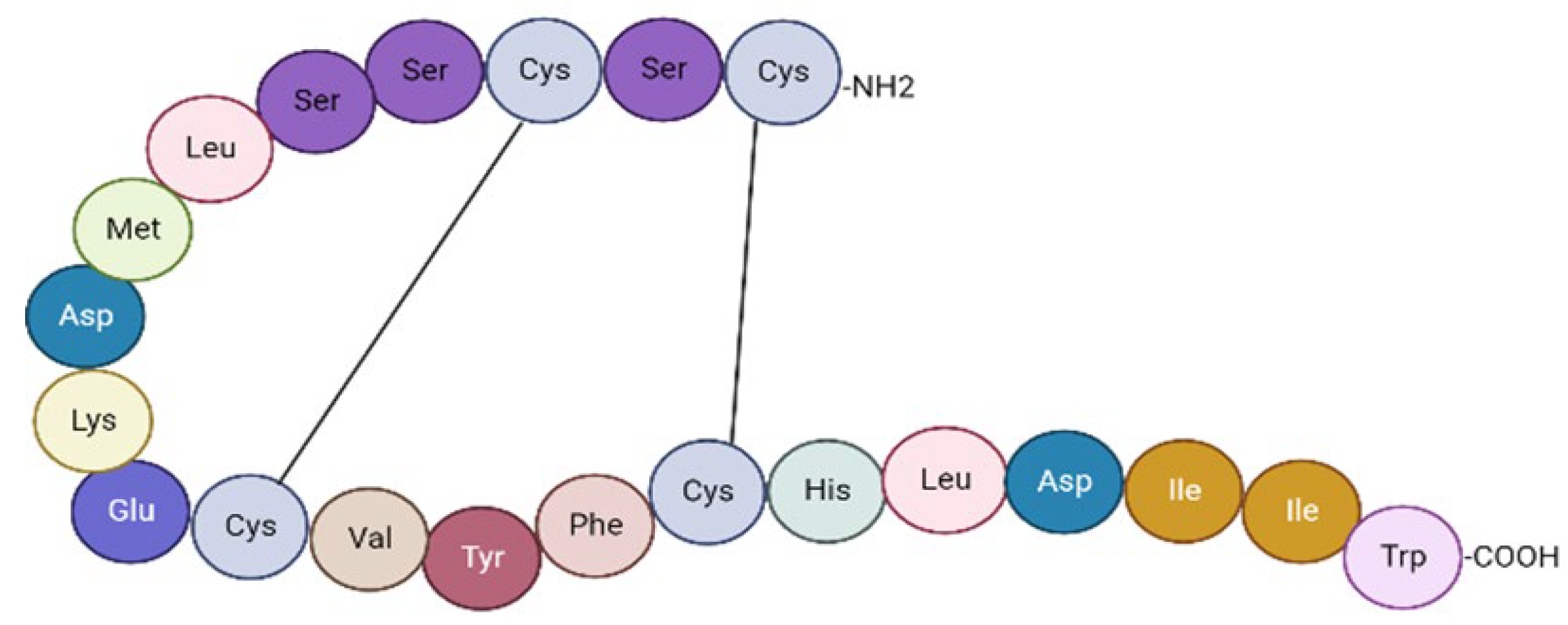
2. The Endothelin System: Morphofunctional Considerations
3. ET-1 Implications in the Pathophysiological Mechanisms of AHF
3.1. Endothelial Dysfunction
3.2. Venous Congestion
3.3. Atherosclerosis and Inflammation
3.4. Cardiac Remodeling
3.5. Worsening Renal Function
3.6. Neurohormonal Activation
4. Endothelin-1: A Promising Biomarker in AHF
5. Multimarker Panels Incorporating ET-1 and Conventional Biomarkers in AHF
5.1. Natriuretic Peptides and ET-1
5.2. Cardiac Troponin and ET-1
6. Multimarker Panel Incorporating ET-1 and Novel Biomarkers in AHF
6.1. Growth Differentiation Factor-15 and ET-1
6.2. Soluble Suppression of Tumorigenicity 2 and ET-1
7. ET Receptor Antagonist Therapy in AHF
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mentz, R.J.; O’Connor, C.M. Pathophysiology and clinical evaluation of acute heart failure. Nat. Rev. Cardiol. 2016, 13, 28–35. [Google Scholar] [CrossRef]
- Miftode, R.-S.; Costache, I.-I.; Cianga, P.; Petris, A.O.; Cianga, C.-M.; Maranduca, M.-A.; Miftode, I.-L.; Constantinescu, D.; Timpau, A.-S.; Crisan, A.; et al. The Influence of Socioeconomic Status on the Prognosis and Profile of Patients Admitted for Acute Heart Failure during COVID-19 Pandemic: Overestimated Aspects or a Multifaceted Hydra of Cardiovascular Risk Factors? Healthcare 2021, 9, 1700. [Google Scholar] [CrossRef]
- Teerlink, J.R.; McMurray, J.J.; Bourge, R.C.; Cleland, J.G.; Cotter, G.; Jondeau, G.; Krum, H.; Metra, M.; O’Connor, C.M.; Parker, J.D.; et al. Tezosentan in patients with acute heart failure: Design of the Value of Endothelin Receptor Inhibition with Tezosentan in Acute heart failure Study (VERITAS). Am. Heart J. 2005, 150, 46–53. [Google Scholar] [CrossRef]
- Tomasoni, D.; Lombardi, C.M.; Sbolli, M.; Cotter, G.; Metra, M. Acute heart failure: More questions than answers. Prog. Cardiovasc. Dis. 2020, 63, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Pourafkari, L.; Tajlil, A.; Nader, N.D. Biomarkers in diagnosing and treatment of acute heart failure. Biomark. Med. 2019, 13, 1235–1249. [Google Scholar] [CrossRef]
- Arrigo, M.; Jessup, M.; Mullens, W.; Reza, N.; Shah, A.M.; Sliwa, K.; Mebazaa, A. Acute heart failure. Nat. Rev. Dis. Primers 2020, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [PubMed] [Green Version]
- Sinnenberg, L.; Givertz, M.M. Acute heart failure. Trends Cardiovasc. Med. 2020, 30, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Wettersten, N. Biomarkers in Acute Heart Failure: Diagnosis, Prognosis, and Treatment. Int. J. Heart Fail. 2021, 3, 81–105. [Google Scholar] [CrossRef]
- Maisel, A.S.; Choudhary, R. Biomarkers in acute heart failure-state of the art. Nat. Rev. Cardiol. 2012, 9, 478–490. [Google Scholar] [CrossRef]
- Mallick, A.; Januzzi, J.L. Biomarkers in acute heart failure. Rev. Esp. Cardiol. 2015, 68, 514–525. [Google Scholar] [CrossRef]
- Miftode, R.-S.; Constantinescu, D.; Cianga, C.-M.; Petris, A.-O.; Costache, I.-I.; Mitu, O.; Miftode, I.-L.; Mitu, I.; Timpau, A.-S.; Duca, S.-T.; et al. A Rising Star of the Multimarker Panel: Growth Differentiation Factor-15 Levels Are an Independent Predictor of Mortality in Acute Heart Failure Patients Admitted to an Emergency Clinical Hospital from Eastern Europe. Life 2022, 12, 1948. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, M.; Kurihara, H.; Kimura, S.; Tomobe, Y.; Kobayashi, M.; Mitsui, Y.; Yazaki, Y.; Goto, K.; Masaki, T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988, 332, 411–415. [Google Scholar] [CrossRef] [Green Version]
- Jankowich, M.; Choudhary, G. Endothelin-1 levels and cardiovascular events. Trends Cardiovasc. Med. 2020, 30, 1–8. [Google Scholar] [CrossRef]
- Matsubara, T.J.; Fujiu, K. Endothelin-1 and Atrial Cardiomyopathy. Int. Heart J. 2019, 60, 238–240. [Google Scholar] [CrossRef] [Green Version]
- Omland, T. Targeting the endothelin system: A step towards a precision medicine approach in heart failure with preserved ejection fraction. Eur. Heart J. 2019, 40, 3718–3720. [Google Scholar] [CrossRef] [PubMed]
- Ern Yeoh, S.; Docherty, K.F.; Campbell, R.T.; Jhund, P.S.; Hammarstedt, A.; Heerspink, H.J.L.; Jarolim, P.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; et al. Endothelin-1, Outcomes in Patients with Heart Failure and Reduced Ejection Fraction, and Effects of Dapagliflozin: Findings From DAPA-HF. Circulation 2023, 147, 1670–1683. [Google Scholar] [CrossRef] [PubMed]
- Thorin, E.; Clozel, M. The cardiovascular physiology and pharmacology of endothelin-1. Adv. Pharmacol. 2010, 60, 1–26. [Google Scholar]
- Attinà, T.; Camidge, R.; Newby, D.E.; Webb, D.J. Endothelin antagonism in pulmonary hypertension, heart failure, and beyond. Heart 2005, 91, 825–831. [Google Scholar] [CrossRef] [Green Version]
- Kostov, K. The Causal Relationship between Endothelin-1 and Hypertension: Focusing on Endothelial Dysfunction, Arterial Stiffness, Vascular Remodeling, and Blood Pressure Regulation. Life 2021, 11, 986. [Google Scholar] [CrossRef]
- Khimji, A.K.; Rockey, D.C. Endothelin—Biology and disease. Cell. Signal. 2010, 22, 1615–1625. [Google Scholar] [CrossRef]
- Böhm, F.; Pernow, J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc. Res. 2007, 76, 8–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boerrigter, G.; Burnett, J.C. Endothelin in neurohormonal activation in heart failure. Coron. Artery Dis. 2003, 14, 495–500. [Google Scholar] [CrossRef]
- Mo, R.; Yang, Y.M.; Yu, L.T.; Tan, H.Q.; Zhu, J. Elevated Plasma Big Endothelin-1 at Admission Is Associated With Poor Short-Term Outcomes in Patients With Acute Decompensated Heart Failure. Front. Cardiovasc. Med. 2021, 8, 629268. [Google Scholar] [CrossRef] [PubMed]
- Alem, M.M. Endothelial Dysfunction in Chronic Heart Failure: Assessment, Findings, Significance, and Potential Therapeutic Targets. Int. J. Mol. Sci. 2019, 20, 3198. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, E.; Flammer, A.J.; Lerman, L.O.; Elízaga, J.; Lerman, A.; Fernández-Avilés, F. Endothelial dysfunction over the course of coronary artery disease. Eur. Heart J. 2013, 34, 3175–3181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marti, C.N.; Gheorghiade, M.; Kalogeropoulos, A.P.; Georgiopoulou, V.V.; Quyyumi, A.A.; Butler, J. Endothelial dysfunction, arterial stiffness, and heart failure. J. Am. Coll. Cardiol. 2012, 60, 1455–1469. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef] [Green Version]
- Hartopo, A.B.; Sukmasari, I.; Puspitawati, I.; Setianto, B.Y. Serum Endothelin-1 Correlates with Myocardial Injury and Independently Predicts Adverse Cardiac Events in Non-ST-Elevation Acute Myocardial Infarction. Int. J. Vasc. Med. 2020, 2020, 9260812. [Google Scholar] [CrossRef]
- Zuchi, C.; Tritto, I.; Carluccio, E.; Mattei, C.; Cattadori, G.; Ambrosio, G. Role of endothelial dysfunction in heart failure. Heart Fail. Rev. 2020, 25, 21–30. [Google Scholar] [CrossRef]
- Martens, P.; Mullens, W. How to tackle congestion in acute heart failure. Korean J. Intern. Med. 2018, 33, 462–473. [Google Scholar]
- Ganda, A.; Onat, D.; Demmer, R.T.; Wan, E.; Vittorio, T.J.; Sabbah, H.N.; Colombo, P.C. Venous congestion and endothelial cell activation in acute decompensated heart failure. Curr. Heart Fail. Rep. 2010, 7, 66–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Chudasama, N.; Hayashi, Y.; Hawk, C.; Ramnauth, S.D.; Wong, K.Y.; Harxhi, A.; Onat, D.; Wakabayashi, M.; Uriel, N.; et al. Peripheral venous congestion causes time- and dose-dependent release of endothelin-1 in humans. Physiol. Rep. 2017, 5, e13118. [Google Scholar]
- Colombo, P.C.; Onat, D.; Harxhi, A.; Demmer, R.T.; Hayashi, Y.; Jelic, S.; LeJemtel, T.H.; Bucciarelli, L.; Kebschull, M.; Papapanou, P.; et al. Peripheral venous congestion causes inflammation, neurohormonal, and endothelial cell activation. Eur. Heart J. 2014, 35, 448–454. [Google Scholar]
- Roger, V.L. Epidemiology of heart failure. Circ. Res. 2013, 113, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Del Buono, M.G.; Moroni, F.; Montone, R.A.; Azzalini, L.; Sanna, T.; Abbate, A. Ischemic Cardiomyopathy and Heart Failure After Acute Myocardial Infarction. Curr. Cardiol. Rep. 2022, 24, 1505–1515. [Google Scholar] [PubMed]
- Freixa, X.; Heras, M.; Ortiz, J.T.; Argiró, S.; Guasch, E.; Doltra, A.; Jiménez, M.; Betriu, A.; Masotti, M. Usefulness of endothelin-1 assessment in acute myocardial infarction. Rev. Esp. Cardiol. 2011, 64, 105–110. [Google Scholar]
- Setianto, B.Y.; Hartopo, A.B.; Sukmasari, I.; Puspitawati, I. On-admission high endothelin-1 level independently predicts in-hospital adverse cardiac events following ST-elevation acute myocardial infarction. Int. J. Cardiol. 2016, 220, 72–76. [Google Scholar] [CrossRef]
- Jenča, D.; Melenovský, V.; Stehlik, J.; Staněk, V.; Kettner, J.; Kautzner, J.; Adámková, V.; Wohlfahrt, P. Heart failure after myocardial infarction: Incidence and predictors. ESC Heart Fail. 2021, 8, 222–237. [Google Scholar] [CrossRef]
- Mraiche, F.; Cena, J.; Das, D.; Vollrath, B. Effects of statins on vascular function of endothelin-1. Br. J. Pharmacol. 2005, 144, 715–726. [Google Scholar]
- Schiffrin, E.L.; Hayoz, D. How to assess vascular remodelling in small and medium-sized muscular arteries in humans. J. Hypertens. 1997, 15, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Ruetten, H.; Thiemermann, C. Endothelin-1 stimulates the biosynthesis of tumour necrosis factor in macrophages: ET-receptors, signal transduction and inhibition by dexamethasone. J. Physiol. Pharmacol. 1997, 48, 675–688. [Google Scholar] [PubMed]
- Hofman, F.M.; Chen, P.; Jeyaseelan, R.; Incardona, F.; Fisher, M.; Zidovetzki, R. Endothelin-1 induces production of the neutrophil chemotactic factor interleukin-8 by human brain-derived endothelial cells. Blood 1998, 92, 3064–3072. [Google Scholar] [CrossRef] [PubMed]
- Browatzki, M.; Schmidt, J.; Kübler, W.; Kranzhöfer, R. Endothelin-1 induces interleukin-6 release via activation of the transcription factor NF-kappaB in human vascular smooth muscle cells. Basic Res. Cardiol. 2000, 95, 98–105. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef]
- Gonon, A.T.; Gourine, A.V.; Middelveld, R.J.; Alving, K.; Pernow, J. Limitation of infarct size and attenuation of myeloperoxidase activity by an endothelin A receptor antagonist following ischaemia and reperfusion. Basic Res. Cardiol. 2001, 96, 454–462. [Google Scholar] [CrossRef]
- Wassmann, S.; Stumpf, M.; Strehlow, K.; Schmid, A.; Schieffer, B.; Böhm, M.; Nickenig, G. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ. Res. 2004, 94, 534–541. [Google Scholar] [CrossRef]
- Verma, S.; Li, S.H.; Badiwala, M.V.; Weisel, R.D.; Fedak, P.W.; Li, R.K.; Dhillon, B.; Mickle, D.A. Endothelin antagonism and interleukin-6 inhibition attenuate the proatherogenic effects of C-reactive protein. Circulation 2002, 105, 1890–1896. [Google Scholar] [CrossRef] [Green Version]
- Meng, T.; Wang, P.; Ding, J.; Du, R.; Gao, J.; Li, A.; Yu, S.; Liu, J.; Lu, X.; He, Q. Global Research Trends on Ventricular Remodeling: A Bibliometric Analysis From 2012 to 2022. Curr. Probl. Cardiol. 2022, 47, 101332. [Google Scholar] [CrossRef]
- Aimo, A.; Gaggin, H.K.; Barison, A.; Emdin, M.; Januzzi, J.L. Imaging, Biomarker, and Clinical Predictors of Cardiac Remodeling in Heart Failure With Reduced Ejection Fraction. JACC Heart Fail. 2019, 7, 782–794. [Google Scholar] [CrossRef]
- Jen, H.L.; Yin, W.H.; Chen, J.W.; Lin, S.J. Endothelin-1-Induced Cell Hypertrophy in Cardiomyocytes is Improved by Fenofibrate: Possible Roles of Adiponectin. J. Atheroscler. Thromb. 2017, 24, 508–517. [Google Scholar] [CrossRef] [Green Version]
- Bupha-Intr, T.; Haizlip, K.M.; Janssen, P.M. Role of endothelin in the induction of cardiac hypertrophy in vitro. PLoS ONE 2012, 7, e43179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seccia, T.M.; Belloni, A.S.; Kreutz, R.; Paul, M.; Nussdorfer, G.G.; Pessina, A.C.; Rossi, G.P. Cardiac fibrosis occurs early and involves endothelin and AT-1 receptors in hypertension due to endogenous angiotensin II. J. Am. Coll. Cardiol. 2003, 41, 666–673. [Google Scholar] [CrossRef] [Green Version]
- Mulder, P.; Boujedaini, H.; Richard, V.; Derumeaux, G.; Henry, J.P.; Renet, S.; Wessale, J.; Opgenorth, T.; Thuillez, C. Selective endothelin-A versus combined endothelin-A/endothelin-B receptor blockade in rat chronic heart failure. Circulation 2000, 102, 491–493. [Google Scholar] [CrossRef]
- Drawnel, F.M.; Archer, C.R.; Roderick, H.L. The role of the paracrine/autocrine mediator endothelin-1 in regulation of cardiac contractility and growth. Br. J. Pharmacol. 2013, 168, 296–317. [Google Scholar] [CrossRef] [Green Version]
- Baltogiannis, G.G.; Tsalikakis, D.G.; Mitsi, A.C.; Hatzistergos, K.E.; Elaiopoulos, D.; Fotiadis, D.I.; Kyriakides, Z.S.; Kolettis, T.M. Endothelin receptor—A blockade decreases ventricular arrhythmias after myocardial infarction in rats. Cardiovasc. Res. 2005, 67, 647–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvais, P.L.; Robert, A.; Ahn, S.; van Linden, F.; Ketelslegers, J.M.; Pouleur, H.; Rousseau, M.F. Direct comparison between endothelin-1, N-terminal proatrial natriuretic factor, and brain natriuretic peptide as prognostic markers of survival in congestive heart failure. J. Card. Fail. 2000, 6, 201–207. [Google Scholar] [CrossRef]
- Rosei, E.A.; Rizzoni, D.; Castellano, M.; Porteri, E.; Zulli, R.; Muiesan, M.L.; Bettoni, G.; Salvetti, M.; Muiesan, P.; Giulini, S.M. Media: Lumen ratio in human small resistance arteries is related to forearm minimal vascular resistance. J. Hypertens. 1995, 13, 341–347. [Google Scholar] [CrossRef]
- Aalkjaer, C.; Heagerty, A.M.; Petersen, K.K.; Swales, J.D.; Mulvany, M.J. Evidence for increased media thickness, increased neuronal amine uptake, and depressed excitation-contraction coupling in isolated resistance vessels from essential hypertensives. Circ. Res. 1987, 61, 181–186. [Google Scholar] [CrossRef]
- Korsgaard, N.; Aalkjaer, C.; Heagerty, A.M.; Izzard, A.S.; Mulvany, M.J. Histology of subcutaneous small arteries from patients with essential hypertension. Hypertension 1993, 22, 523–526. [Google Scholar] [CrossRef] [Green Version]
- Russell, F.D.; Molenaar, P. The human heart endothelin system: ET-1 synthesis, storage, release and effect. Trends Pharmacol. Sci. 2000, 21, 353–359. [Google Scholar] [CrossRef]
- Hasegawa, K.; Fujiwara, H.; Koshiji, M.; Inada, T.; Ohtani, S.; Doyama, K.; Tanaka, M.; Matsumori, A.; Fujiwara, T.; Shirakami, G.; et al. Endothelin-1 and its receptor in hypertrophic cardiomyopathy. Hypertension 1996, 27, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Dmour, B.A.; Miftode, R.S.; Iliescu Halitchi, D.; Anton-Paduraru, D.T.; Iliescu Halitchi, C.O.; Miftode, I.L.; Mitu, O.; Costache, A.D.; Stafie, C.S.; Costache, I.I. Latest Insights into Mechanisms behind Atrial Cardiomyopathy: It Is Not always about Ventricular Function. Diagnostics 2021, 11, 449. [Google Scholar] [CrossRef] [PubMed]
- Hathaway, C.K.; Grant, R.; Hagaman, J.R.; Hiller, S.; Li, F.; Xu, L.; Chang, A.S.; Madden, V.J.; Bagnell, C.R.; Rojas, M.; et al. Endothelin-1 critically influences cardiac function via superoxide-MMP9 cascade. Proc. Natl. Acad. Sci. USA 2015, 112, 5141–5146. [Google Scholar] [CrossRef]
- Burstein, B.; Libby, E.; Calderone, A.; Nattel, S. Differential behaviors of atrial versus ventricular fibroblasts: A potential role for platelet-derived growth factor in atrial-ventricular remodeling differences. Circulation 2008, 117, 1630–1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayyas, F.; Niebauer, M.; Zurick, A.; Barnard, J.; Gillinov, A.M.; Chung, M.K.; Van Wagoner, D.R. Association of left atrial endothelin-1 with atrial rhythm, size, and fibrosis in patients with structural heart disease. Circ. Arrhythm. Electrophysiol. 2010, 3, 369–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronco, C.; Haapio, M.; House, A.A.; Anavekar, N.; Bellomo, R. Cardiorenal syndrome. J. Am. Coll. Cardiol. 2008, 52, 1527–1539. [Google Scholar] [CrossRef] [Green Version]
- Liang, K.V.; Williams, A.W.; Greene, E.L.; Redfield, M.M. Acute decompensated heart failure and the cardiorenal syndrome. Crit. Care Med. 2008, 36, S75–S88. [Google Scholar] [CrossRef]
- Ronco, C.; House, A.A.; Haapio, M. Cardiorenal syndrome: Refining the definition of a complex symbiosis gone wrong. Intensive Care Med. 2008, 34, 957–962. [Google Scholar] [CrossRef]
- Rangaswami, J.; Bhalla, V.; Blair, J.E.A.; Chang, T.I.; Costa, S.; Lentine, K.L.; Lerma, E.V.; Mezue, K.; Molitch, M.; Mullens, W.; et al. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement from the American Heart Association. Circulation 2019, 139, e840–e878. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; Rossignol, P. Cardiorenal Syndrome Revisited. Circulation 2018, 138, 929–944. [Google Scholar] [CrossRef]
- Metra, M.; Nodari, S.; Parrinello, G.; Bordonali, T.; Bugatti, S.; Danesi, R.; Fontanella, B.; Lombardi, C.; Milani, P.; Verzura, G.; et al. Worsening renal function in patients hospitalised for acute heart failure: Clinical implications and prognostic significance. Eur. J. Heart Fail. 2008, 10, 188–195. [Google Scholar] [CrossRef] [Green Version]
- Damman, K.; Navis, G.; Voors, A.A.; Asselbergs, F.W.; Smilde, T.D.; Cleland, J.G.; van Veldhuisen, D.J.; Hillege, H.L. Worsening renal function and prognosis in heart failure: Systematic review and meta-analysis. J. Card. Fail. 2007, 13, 599–608. [Google Scholar] [CrossRef]
- Wattad, M.; Darawsha, W.; Solomonica, A.; Hijazi, M.; Kaplan, M.; Makhoul, B.F.; Abassi, Z.A.; Azzam, Z.S.; Aronson, D. Interaction between worsening renal function and persistent congestion in acute decompensated heart failure. Am. J. Cardiol. 2015, 115, 932–937. [Google Scholar] [CrossRef]
- Berra, G.; Garin, N.; Stirnemann, J.; Jannot, A.S.; Martin, P.Y.; Perrier, A.; Carballo, S. Outcome in acute heart failure: Prognostic value of acute kidney injury and worsening renal function. J. Card. Fail. 2015, 21, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Freda, B.J.; Knee, A.B.; Braden, G.L.; Visintainer, P.F.; Thakar, C.V. Effect of Transient and Sustained Acute Kidney Injury on Readmissions in Acute Decompensated Heart Failure. Am. J. Cardiol. 2017, 119, 1809–1814. [Google Scholar] [CrossRef]
- Kohan, D.E. Endothelin, hypertension and chronic kidney disease: New insights. Curr. Opin. Nephrol. Hypertens. 2010, 19, 134–139. [Google Scholar] [CrossRef]
- Kohan, D.E.; Inscho, E.W.; Wesson, D.; Pollock, D.M. Physiology of endothelin and the kidney. Compr. Physiol. 2011, 1, 883–919. [Google Scholar] [PubMed] [Green Version]
- Abassi, Z.; Khoury, E.E.; Karram, T.; Aronson, D. Edema formation in congestive heart failure and the underlying mechanisms. Front. Cardiovasc. Med. 2022, 9, 933215. [Google Scholar] [CrossRef]
- Abukar, Y.; May, C.N.; Ramchandra, R. Role of endothelin-1 in mediating changes in cardiac sympathetic nerve activity in heart failure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R94–R99. [Google Scholar] [CrossRef] [Green Version]
- Kemp, C.D.; Conte, J.V. The pathophysiology of heart failure. Cardiovasc. Pathol. 2012, 21, 365–371. [Google Scholar] [CrossRef]
- Aronson, D.; Burger, A.J. Neurohumoral activation and ventricular arrhythmias in patients with decompensated congestive heart failure: Role of endothelin. Pacing Clin. Electrophysiol. 2003, 26, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Miyauchi, T.; Sakurai, T.; Kasuya, Y.; Ihara, M.; Yamaguchi, I.; Goto, K.; Sugishita, Y. Endogenous endothelin-1 participates in the maintenance of cardiac function in rats with congestive heart failure. Marked increase in endothelin-1 production in the failing heart. Circulation 1996, 93, 1214–1222. [Google Scholar] [CrossRef] [Green Version]
- Titus, A.; Marappa-Ganeshan, R. Physiology, Endothelin. 2022 May 8. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551627/ (accessed on 15 January 2023).
- Perez, A.L.; Grodin, J.L.; Wu, Y.; Hernandez, A.F.; Butler, J.; Metra, M.; Felker, G.M.; Voors, A.A.; McMurray, J.J.; Armstrong, P.W.; et al. Increased mortality with elevated plasma endothelin-1 in acute heart failure: An ASCEND-HF biomarker substudy. Eur. J. Heart Fail. 2016, 18, 290–297. [Google Scholar] [CrossRef] [Green Version]
- Gaggin, H.K.; Truong, Q.A.; Gandhi, P.U.; Motiwala, S.R.; Belcher, A.M.; Weiner, R.B.; Baggish, A.L.; Januzzi, J.L. Systematic Evaluation of Endothelin 1 Measurement Relative to Traditional and Modern Biomarkers for Clinical Assessment and Prognosis in Patients with Chronic Systolic Heart Failure: Serial Measurement and Multimarker Testing. Am. J. Clin. Pathol. 2017, 147, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Demissei, B.G.; Postmus, D.; Cleland, J.G.; O’Connor, C.M.; Metra, M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; Davison, B.A.; Givertz, M.M.; et al. Plasma biomarkers to predict or rule out early post-discharge events after hospitalization for acute heart failure. Eur. J. Heart Fail. 2017, 19, 728–738. [Google Scholar] [CrossRef] [Green Version]
- Massie, B.M.; O’Connor, C.M.; Metra, M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; Weatherley, B.D.; Cleland, J.G.; Givertz, M.M.; Voors, A.; et al. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N. Engl. J. Med. 2010, 363, 1419–1428. [Google Scholar] [CrossRef]
- Weatherley, B.D.; Cotter, G.; Dittrich, H.C.; DeLucca, P.; Mansoor, G.A.; Bloomfield, D.M.; Ponikowski, P.; O’Connor, C.M.; Metra, M.; Massie, B.M. Design and rationale of the PROTECT study: A placebo-controlled randomized study of the selective A1 adenosine receptor antagonist rolofylline for patients hospitalized with acute decompensated heart failure and volume overload to assess treatment effect on congestion and renal function. J. Card. Fail. 2010, 16, 25–35. [Google Scholar]
- Zymliński, R.; Sierpiński, R.; Metra, M.; Cotter, G.; Sokolski, M.; Siwołowski, P.; Garus, M.; Gajewski, P.; Tryba, J.; Samorek, M.; et al. Elevated plasma endothelin-1 is related to low natriuresis, clinical signs of congestion, and poor outcome in acute heart failure. ESC Heart Fail. 2020, 7, 3536–3544. [Google Scholar] [CrossRef] [PubMed]
- Pacher, R.; Stanek, B.; Hülsmann, M.; Koller-Strametz, J.; Berger, R.; Schuller, M.; Hartter, E.; Ogris, E.; Frey, B.; Heinz, G.; et al. Prognostic impact of big endothelin-1 plasma concentrations compared with invasive hemodynamic evaluation in severe heart failure. J. Am. Coll. Cardiol. 1996, 27, 633–641. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.M.; Lerman, A.; Rodeheffer, R.J.; McGregor, C.G.; Brandt, R.R.; Wright, S.; Heublein, D.M.; Kao, P.C.; Edwards, W.D.; Burnett, J.C. Endothelin in human congestive heart failure. Circulation 1994, 89, 1580–1586. [Google Scholar] [CrossRef] [Green Version]
- Masson, S.; Latini, R.; Anand, I.S.; Barlera, S.; Judd, D.; Salio, M.; Perticone, F.; Perini, G.; Tognoni, G.; Cohn, J.N. The prognostic value of big endothelin-1 in more than 2300 patients with heart failure enrolled in the Valsartan Heart Failure Trial (Val-HeFT). J. Card. Fail. 2006, 12, 375–380. [Google Scholar] [CrossRef]
- Van Beneden, R.; Gurné, O.; Selvais, P.L.; Ahn, S.A.; Robert, A.R.; Ketelslegers, J.M.; Pouleur, H.G.; Rousseau, M.F. Superiority of big endothelin-1 and endothelin-1 over natriuretic peptides in predicting survival in severe congestive heart failure: A 7-year follow-up study. J. Card. Fail. 2004, 10, 490–495. [Google Scholar] [CrossRef]
- Alvarez-Cienfuegos, A.; Cantero-Nieto, L.; García-Gómez, J.A.; Ríos-Fernández, R.; Martin, J.; González-Gay, M.A.; Ortego-Centeno, N. Endothelin-1 serum levels in women with Rheumatoid Arthritis. Acta Reumatol. Port. 2019, 44, 250–257. [Google Scholar]
- Mostmans, Y.; Cutolo, M.; Giddelo, C.; Decuman, S.; Melsens, K.; Declercq, H.; Vandecasteel, E.; De Keyser, F.; Distler, O.; Gutermuth, J.; et al. The role of endothelial cells in the vasculopathy of systemic sclerosis: A systematic review. Autoimmun. Rev. 2017, 16, 774–786. [Google Scholar] [CrossRef]
- Kotyla, P.J.; Owczarek, A.; Rakoczy, J.; Lewicki, M.; Kucharz, E.J.; Emery, P. Infliximab treatment increases left ventricular ejection fraction in patients with rheumatoid arthritis: Assessment of heart function by echocardiography, endothelin 1, interleukin 6, and NT-pro brain natriuretic peptide. J. Rheumatol. 2012, 39, 701–706. [Google Scholar] [CrossRef]
- Pope, J. An update in pulmonary hypertension in systemic lupus erythematosus—Do we need to know about it? Lupus 2008, 17, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Safonova, J.; Kozhevnikova, M.; Danilogorskaya, Y.; Zheleznykh, E.; Zektser, V.; Ilgisonis, I.; Popova, L.; Khabarova, N.; Privalova, E.; Belenkov, Y. Angiotensin-Converting Enzyme Inhibitor Therapy Effects in Patients With Heart Failure with Preserved and Mid-Range Ejection Fraction. Cardiol. Res. 2021, 12, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Momose, N.; Fukuo, K.; Morimoto, S.; Ogihara, T. Captopril inhibits endothelin-1 secretion from endothelial cells through bradykinin. Hypertension 1993, 21 (6 Pt 2), 921–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desideri, G.; Grassi, D.; Croce, G.; Bocale, R.; Tiberti, S.; Evangelista, S.; Necozione, S.; Di Orio, F.; Ferri, C. Different effects of angiotensin converting enzyme inhibitors on endothelin-1 and nitric oxide balance in human vascular endothelial cells: Evidence of an oxidant-sensitive pathway. Mediat. Inflamm. 2008, 2008, 305087. [Google Scholar] [CrossRef]
- Brehm, B.R.; Bertsch, D.; von Fallois, J.; Wolf, S.C. Beta-blockers of the third generation inhibit endothelin-1 liberation, mRNA production and proliferation of human coronary smooth muscle and endothelial cells. J. Cardiovasc. Pharmacol. 2000, 36 (Suppl. S1), S401–S403. [Google Scholar] [CrossRef]
- Garlichs, C.D.; Zhang, H.; Mügge, A.; Daniel, W.G. Beta-blockers reduce the release and synthesis of endothelin-1 in human endothelial cells. Eur. J. Clin. Investig. 1999, 29, 12–16. [Google Scholar] [CrossRef]
- Fortuño, A.; Muñiz, P.; Zalba, G.; Fortuño, M.A.; Díez, J. The loop diuretic torasemide interferes with endothelin-1 actions in the aorta of hypertensive rats. Nephrol. Dial. Transpl. 2001, 16 (Suppl. S1), 18–21. [Google Scholar] [CrossRef] [Green Version]
- Vogt, S.; Winkler, E.; Hermsen, D.; Schott, M.; Schinner, S.; Scherbaum, W.A.; Willenberg, H.S. Endothelin-1 and adrenomedullin plasma levels after exposure to fludrocortisone, dexamethasone, and spironolactone. Clin. Exp. Hypertens. 2012, 34, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Aronson, D.; Burger, A.J. Intravenous nesiritide (human B-type natriuretic peptide) reduces plasma endothelin-1 levels in patients with decompensated congestive heart failure. Am. J. Cardiol. 2002, 90, 435–438. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Z.; Liu, S.; Ding, L.; Chen, K.; Hua, W.; Zhang, S. Association of baseline big endothelin-1 level with long-term prognosis among cardiac resynchronization therapy recipients. Clin. Biochem. 2018, 59, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, Z.; Hu, Y.; Jing, R.; Gu, M.; Niu, H.; Ding, L.; Xing, A.; Zhang, S.; Hua, W. A novel risk model for mortality and hospitalization following cardiac resynchronization therapy in patients with non-ischemic cardiomyopathy: The alpha-score. BMC Cardiovasc. Disord. 2020, 20, 205. [Google Scholar] [CrossRef]
- Li, X.Y.; Zhao, S.; Fan, X.H.; Chen, K.P.; Hua, W.; Liu, Z.M.; Xue, X.D.; Zhou, B.; Zhang, S. Plasma big endothelin-1 is an effective predictor for ventricular arrythmias and end-stage events in primary prevention implantable cardioverter-defibrillator indication patients. J. Geriatr. Cardiol. 2020, 17, 427–433. [Google Scholar] [PubMed]
- Meijers, W.C.; Bayes-Genis, A.; Mebazaa, A.; Bauersachs, J.; Cleland, J.G.F.; Coats, A.J.S.; Januzzi, J.L.; Maisel, A.S.; McDonald, K.; Mueller, T.; et al. Circulating heart failure biomarkers beyond natriuretic peptides: Review from the Biomarker Study Group of the Heart Failure Association (HFA), European Society of Cardiology (ESC). Eur. J. Heart Fail. 2021, 23, 1610–1632. [Google Scholar] [CrossRef]
- Castiglione, V.; Aimo, A.; Vergaro, G.; Saccaro, L.; Passino, C.; Emdin, M. Biomarkers for the diagnosis and management of heart failure. Heart Fail. Rev. 2022, 27, 625–643. [Google Scholar] [CrossRef]
- Ohmae, M. Endothelin-1 levels in chronic congestive heart failure. Wien. Klin. Wochenschr. 2011, 123, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Liu, X.; Li, Y. Correlation of serum BNP and ET-1 levels with cardiac pump function and ventricular remodeling in patients with heart failure. Cell. Mol. Biol. 2020, 66, 125–131. [Google Scholar] [CrossRef]
- Pascual-Figal, D.A.; Casas, T.; Ordonez-Llanos, J.; Manzano-Fernández, S.; Bonaque, J.C.; Boronat, M.; Muñoz-Esparza, C.; Valdés, M.; Januzzi, J.L. Highly sensitive troponin T for risk stratification of acutely destabilized heart failure. Am. Heart J. 2012, 163, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Harrison, N.; Favot, M.; Levy, P. The Role of Troponin for Acute Heart Failure. Curr. Heart Fail. Rep. 2019, 16, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Figal, D.A.; Manzano-Fernández, S.; Boronat, M.; Casas, T.; Garrido, I.P.; Bonaque, J.C.; Pastor-Perez, F.; Valdés, M.; Januzzi, J.L. Soluble ST2, high-sensitivity troponin T- and N-terminal pro-B-type natriuretic peptide: Complementary role for risk stratification in acutely decompensated heart failure. Eur. J. Heart Fail. 2011, 13, 718–725. [Google Scholar] [CrossRef]
- Grodin, J.L.; Butler, J.; Metra, M.; Felker, G.M.; Voors, A.A.; McMurray, J.J.; Armstrong, P.W.; Hernandez, A.F.; O’Connor, C.; Starling, R.C.; et al. Circulating Cardiac Troponin I Levels Measured by a Novel Highly Sensitive Assay in Acute Decompensated Heart Failure: Insights From the ASCEND-HF Trial. J. Card. Fail. 2018, 24, 512–519. [Google Scholar] [CrossRef] [Green Version]
- Felker, G.M.; Mentz, R.J.; Teerlink, J.R.; Voors, A.A.; Pang, P.S.; Ponikowski, P.; Greenberg, B.H.; Filippatos, G.; Davison, B.A.; Cotter, G.; et al. Serial high sensitivity cardiac troponin T measurement in acute heart failure: Insights from the RELAX-AHF study. Eur. J. Heart Fail. 2015, 17, 1262–1270. [Google Scholar] [CrossRef]
- Felker, G.M.; Hasselblad, V.; Tang, W.H.; Hernandez, A.F.; Armstrong, P.W.; Fonarow, G.C.; Voors, A.A.; Metra, M.; McMurray, J.J.; Butler, J.; et al. Troponin I in acute decompensated heart failure: Insights from the ASCEND-HF study. Eur. J. Heart Fail. 2012, 14, 1257–1264. [Google Scholar] [CrossRef] [Green Version]
- Peacock, W.F.; De Marco, T.; Fonarow, G.C.; Diercks, D.; Wynne, J.; Apple, F.S.; Wu, A.H. Cardiac troponin and outcome in acute heart failure. N. Engl. J. Med. 2008, 358, 2117–2126. [Google Scholar] [CrossRef] [Green Version]
- Lok, D.J.; Klip, I.T.; Lok, S.I.; de la Porte, P.W.B.-A.; Badings, E.; van Wijngaarden, J.; Voors, A.A.; de Boer, R.A.; van Veldhuisen, D.J.; van der Meer, P. Incremental prognostic power of novel biomarkers (growth-differentiation factor- 15, high-sensitivity C-reactive protein, galectin-3, and high-sensitivity troponin-T) in patients with advanced chronic heart failure. Am. J. Cardiol. 2013, 112, 831–837. [Google Scholar] [CrossRef]
- Biasucci, L.M.; Maino, A.; Grimaldi, M.C.; Cappannoli, L.; Aspromonte, N. Novel Biomarkers in Heart Failure: New Insight in Pathophysiology and Clinical Perspective. J. Clin. Med. 2021, 10, 2771. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.E.; Lyass, A.; Courchesne, P.; Chen, G.; Liu, C.; Yin, X.; Hwang, S.J.; Massaro, J.M.; Larson, M.G.; Levy, D. Protein biomarkers of cardiovascular disease and mortality in the community. J. Am. Heart Assoc. 2018, 7, e008108. [Google Scholar] [CrossRef] [Green Version]
- Januzzi, J.L.; Peacock, W.F.; Maisel, A.S.; Chae, C.U.; Jesse, R.L.; Baggish, A.L.; O’Donoghue, M.; Sakhuja, R.; Chen, A.A.; van Kimmenade, R.R.; et al. Measurement of the interleukin family member ST2 in patients with acute dyspnea: Results from the PRIDE (Pro-Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) study. J. Am. Coll. Cardiol. 2007, 50, 607–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najjar, E.; Faxén, U.L.; Hage, C.; Donal, E.; Daubert, J.C.; Linde, C.; Lund, L.H. ST2 in heart failure with preserved and reduced ejection fraction. Scand. Cardiovasc. J. 2019, 53, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Xanthakis, V.; Larson, M.G.; Wollert, K.C.; Aragam, J.; Cheng, S.; Ho, J.; Coglianese, E.; Levy, D.; Colucci, W.S.; Michael Felker, G.; et al. Association of novel biomarkers of cardiovascular stress with left ventricular hypertrophy and dysfunction: Implications for screening. J. Am. Heart Assoc. 2013, 2, e000399. [Google Scholar] [CrossRef] [Green Version]
- Miftode, R.-S.; Constantinescu, D.; Cianga, C.M.; Petris, A.O.; Timpau, A.-S.; Crisan, A.; Costache, I.-I.; Mitu, O.; Anton-Paduraru, D.-T.; Miftode, I.-L.; et al. A Novel Paradigm Based on ST2 and Its Contribution towards a Multimarker Approach in the Diagnosis and Prognosis of Heart Failure: A Prospective Study during the Pandemic Storm. Life 2021, 11, 1080. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; Zamora, E.; de Antonio, M.; Galán, A.; Vila, J.; Urrutia, A.; Díez, C.; Coll, R.; Altimir, S.; Lupón, J. Soluble ST2 serum concentration and renal function in heart failure. J. Card. Fail. 2013, 19, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Haryono, A.; Ramadhiani, R.; Ryanto, G.R.T.; Emoto, N. Endothelin and the Cardiovascular System: The Long Journey and Where We Are Going. Biology 2022, 11, 759. [Google Scholar] [CrossRef]
- Liu, C.; Chen, J.; Gao, Y.; Deng, B.; Liu, K. Endothelin receptor antagonists for pulmonary arterial hypertension. Cochrane Database Syst. Rev. 2021, 3, CD004434. [Google Scholar]
- Zhang, Z.Q.; Zhu, S.K.; Wang, M.; Wang, X.A.; Tong, X.H.; Wan, J.Q.; Ding, J.W. New progress in diagnosis and treatment of pulmonary arterial hypertension. J. Cardiothorac. Surg. 2022, 17, 216. [Google Scholar] [CrossRef] [PubMed]


| ET-1 | ET-2 | ET-3 |
|---|---|---|
| Vascular smooth muscle cells | Gastrointestinal stromal cells | Gastrointestinal stromal cells |
| Endothelial cells | Kidney epithelial cells | Kidney epithelial cells |
| Cardiac myocytes | Neurons | |
| Kidney epithelial cells | Glia | |
| Inflammatory cells | ||
| Hepatocytes | ||
| Neurons |
| Drug | ET-1 Level |
|---|---|
| Loop diuretics -Furosemide -Torasemide | ↓ |
| Mineralocorticoid receptor antagonists -Spironolactone | ↔ |
| ACEI -Perindopril -Captopril -Lisinopril | ↓ |
| Angiotensin receptor blockers | ↔ |
| Beta-blockers -Metoprolol -Propranolol -Nebivolol -Carvedilol | ↓ |
| SGLT2 inhibitors -Dapagliflozin | ↓ |
| Vasodilators -Nesiritide | ↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dmour, B.-A.; Costache, A.D.; Dmour, A.; Huzum, B.; Duca, Ș.T.; Chetran, A.; Miftode, R.Ș.; Afrăsânie, I.; Tuchiluș, C.; Cianga, C.M.; et al. Could Endothelin-1 Be a Promising Neurohormonal Biomarker in Acute Heart Failure? Diagnostics 2023, 13, 2277. https://doi.org/10.3390/diagnostics13132277
Dmour B-A, Costache AD, Dmour A, Huzum B, Duca ȘT, Chetran A, Miftode RȘ, Afrăsânie I, Tuchiluș C, Cianga CM, et al. Could Endothelin-1 Be a Promising Neurohormonal Biomarker in Acute Heart Failure? Diagnostics. 2023; 13(13):2277. https://doi.org/10.3390/diagnostics13132277
Chicago/Turabian StyleDmour, Bianca-Ana, Alexandru Dan Costache, Awad Dmour, Bogdan Huzum, Ștefania Teodora Duca, Adriana Chetran, Radu Ștefan Miftode, Irina Afrăsânie, Cristina Tuchiluș, Corina Maria Cianga, and et al. 2023. "Could Endothelin-1 Be a Promising Neurohormonal Biomarker in Acute Heart Failure?" Diagnostics 13, no. 13: 2277. https://doi.org/10.3390/diagnostics13132277
APA StyleDmour, B.-A., Costache, A. D., Dmour, A., Huzum, B., Duca, Ș. T., Chetran, A., Miftode, R. Ș., Afrăsânie, I., Tuchiluș, C., Cianga, C. M., Botnariu, G., Șerban, L. I., Ciocoiu, M., Bădescu, C. M., & Costache, I. I. (2023). Could Endothelin-1 Be a Promising Neurohormonal Biomarker in Acute Heart Failure? Diagnostics, 13(13), 2277. https://doi.org/10.3390/diagnostics13132277











