Prognostic Significance of Preoperative NLR, MLR, and PLR Values in Predicting the Outcome of Primary Cytoreductive Surgery in Serous Epithelial Ovarian Cancer
Abstract
1. Introduction
2. Materials and Methods
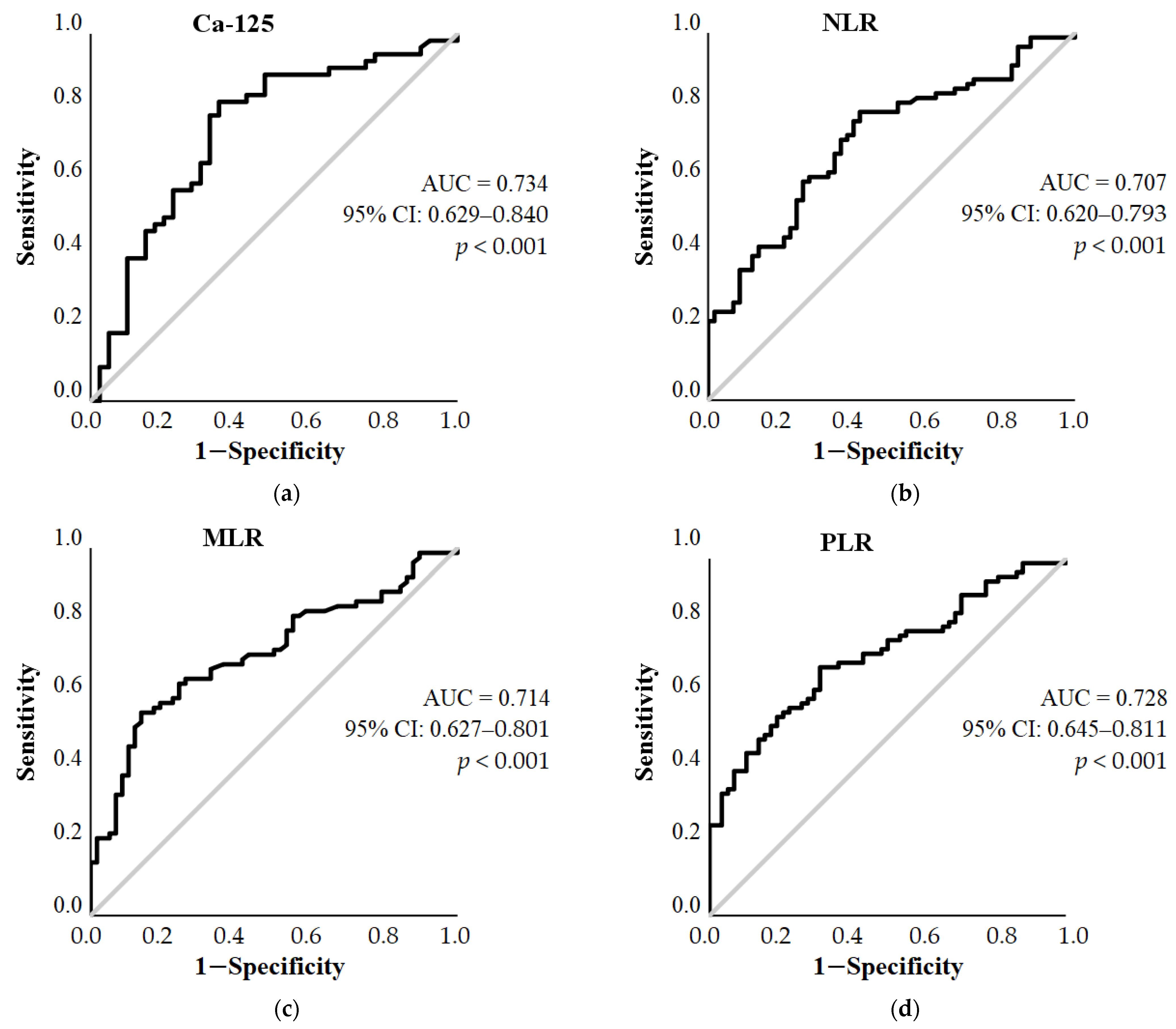
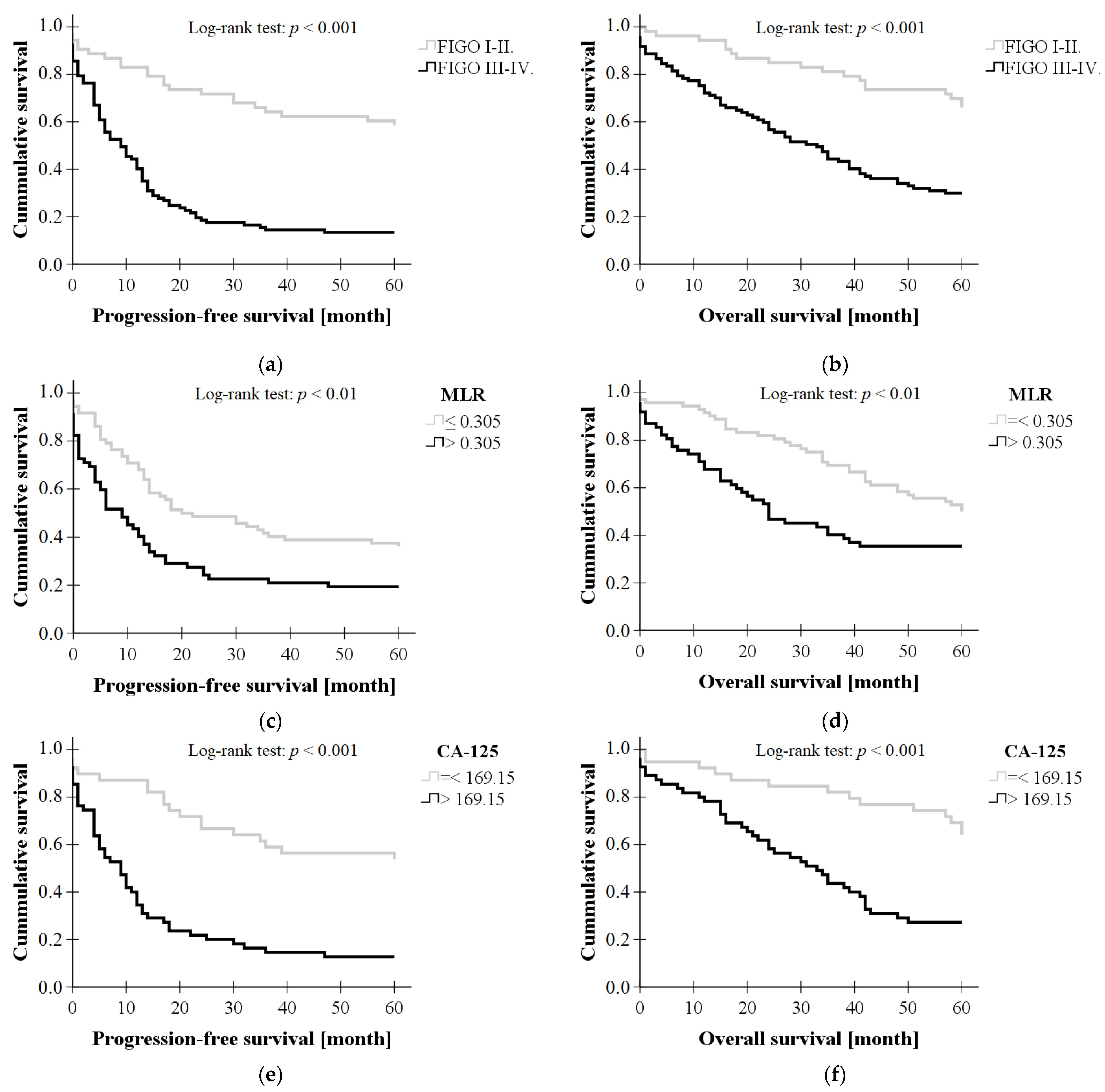
3. Results
3.1. Clinical Data of Subjects
3.2. ROC Curve Analysis
3.3. Binary Logistic Regression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, E.G.; Knight, J.; Radolec, M.; Buckanovich, R.J.; Edwards, R.P.; Vlad, A.M. Immunotherapy Advances for Epithelial Ovarian Cancer. Cancers 2020, 12, 3733. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, L.; Guntupalli, S.R. Treatment of Epithelial Ovarian Cancer. BMJ 2020, 371, m3773. [Google Scholar] [CrossRef]
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial Ovarian Cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef]
- Schorge, J.O.; Bregar, A.J.; Durfee, J.; Berkowitz, R.S. Meigs to Modern Times: The Evolution of Debulking Surgery in Advanced Ovarian Cancer. Gynecol. Oncol. 2018, 149, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Kurnit, K.C.; Fleming, G.F.; Lengyel, E. Updates and New Options in Advanced Epithelial Ovarian Cancer Treatment. Obstet. Gynecol. 2021, 137, 108. [Google Scholar] [CrossRef]
- Song, Y.J. Prediction of Optimal Debulking Surgery in Ovarian Cancer. Gland. Surg. 2021, 10, 1173–1181. [Google Scholar] [CrossRef]
- Kobal, B.; Noventa, M.; Cvjeticanin, B.; Barbic, M.; Meglic, L.; Herzog, M.; Bordi, G.; Vitagliano, A.; Saccardi, C.; Skof, E. Primary Debulking Surgery versus Primary Neoadjuvant Chemotherapy for High Grade Advanced Stage Ovarian Cancer: Comparison of Survivals. Radiol. Oncol. 2018, 52, 307–319. [Google Scholar] [CrossRef]
- Feng, L.; Liao, S.; Li, L. Preoperative Serum Levels of HE4 and CA125 Predict Primary Optimal Cytoreduction in Advanced Epithelial Ovarian Cancer: A Preliminary Model Study. J. Ovarian. Res. 2020, 13, 17. [Google Scholar] [CrossRef]
- Bristow, R.E.; Duska, L.R.; Lambrou, N.C.; Fishman, E.K.; O’Neill, M.J.; Trimble, E.L.; Montz, F.J. A Model for Predicting Surgical Outcome in Patients with Advanced Ovarian Carcinoma Using Computed Tomography. Cancer 2000, 89, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Janco, J.M.T.; Glaser, G.; Kim, B.; McGree, M.E.; Weaver, A.L.; Cliby, W.A.; Dowdy, S.C.; Bakkum-Gamez, J.N. Development of a Prediction Model for Residual Disease in Newly Diagnosed Advanced Ovarian Cancer. Gynecol. Oncol. 2015, 138, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Suidan, R.S.; Ramirez, P.T.; Sarasohn, D.M.; Teitcher, J.B.; Iyer, R.B.; Zhou, Q.; Iasonos, A.; Denesopolis, J.; Zivanovic, O.; Long Roche, K.C.; et al. A Multicenter Assessment of the Ability of Preoperative Computed Tomography Scan and CA-125 to Predict Gross Residual Disease at Primary Debulking for Advanced Epithelial Ovarian Cancer. Gynecol. Oncol. 2017, 145, 27–31. [Google Scholar] [CrossRef]
- Chang, S.-J.; Bristow, R.E.; Chi, D.S.; Cliby, W.A. Role of Aggressive Surgical Cytoreduction in Advanced Ovarian Cancer. J. Gynecol. Oncol. 2015, 26, 336–342. [Google Scholar] [CrossRef] [PubMed]
- el Bairi, K.; al Jarroudi, O.; Afqir, S. Inexpensive Systemic Inflammatory Biomarkers in Ovarian Cancer: An Umbrella Systematic Review of 17 Prognostic Meta-Analyses. Front. Oncol. 2021, 11, 694821. [Google Scholar] [CrossRef]
- Lu, C.; Zhou, L.; Ouyang, J.; Yang, H. Prognostic Value of Lymphocyte-to-Monocyte Ratio in Ovarian Cancer. Medicine 2019, 98, e15876. [Google Scholar] [CrossRef]
- Gong, J.; Jiang, H.; Shu, C.; Hu, M.; Huang, Y.; Liu, Q.; Li, R. Prognostic Value of Lymphocyte-to-Monocyte Ratio in Ovarian Cancer: A Meta-Analysis. J. Ovarian. Res. 2019, 12, 51. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, X.; Lu, J.; Xue, J.; Liu, P.; Mao, H. Prognostic Roles of Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio in Ovarian Cancer: A Meta-Analysis of Retrospective Studies. Arch. Gynecol. Obstet. 2018, 297, 849–857. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, S.; Liu, Y.; Zhai, L.; Sun, X. Prognostic Value of Systemic Inflammatory Markers in Ovarian Cancer: A PRISMA-Compliant Meta-Analysis and Systematic Review. BMC Cancer 2018, 18, 443. [Google Scholar] [CrossRef]
- Miao, Y.; Yan, Q.; Li, S.; Li, B.; Feng, Y. Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio Are Predictive of Chemotherapeutic Response and Prognosis in Epithelial Ovarian Cancer Patients Treated with Platinum-Based Chemotherapy. Cancer Biomark. 2016, 17, 33–40. [Google Scholar] [CrossRef]
- Gu, Y.; Qin, M.; Jin, Y.; Zuo, J.; Li, N.; Bian, C.; Zhang, Y.; Li, R.; Wu, Y.; Wang, C.; et al. A Prediction Model for Optimal Primary Debulking Surgery Based on Preoperative Computed Tomography Scans and Clinical Factors in Patients with Advanced Ovarian Cancer: A Multicenter Retrospective Cohort Study. Front. Oncol. 2021, 10, 611617. [Google Scholar] [CrossRef] [PubMed]
- Piedimonte, S.; Erdman, L.; So, D.; Bernardini, M.Q.; Ferguson, S.E.; Laframboise, S.; Bouchard Fortier, G.; Cybulska, P.; May, T.; Hogen, L. Using a Machine Learning Algorithm to Predict Outcome of Primary Cytoreductive Surgery in Advanced Ovarian Cancer. J. Surg. Oncol. 2023, 127, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Laios, A.; Kalampokis, E.; Johnson, R.; Thangavelu, A.; Tarabanis, C.; Nugent, D.; De Jong, D. Explainable Artificial Intelligence for Prediction of Complete Surgical Cytoreduction in Advanced-Stage Epithelial Ovarian Cancer. J. Pers. Med. 2022, 12, 607. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Laoui, D.; Van Overmeire, E.; Movahedi, K.; Van den Bossche, J.; Schouppe, E.; Mommer, C.; Nikolaou, A.; Morias, Y.; De Baetselier, P.; Van Ginderachter, J.A. Mononuclear Phagocyte Heterogeneity in Cancer: Different Subsets and Activation States Reaching out at the Tumor Site. Immunobiology 2011, 216, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.M.; Barry, S.C.; Ricciardelli, C.; Oehler, M.K. Epithelial Ovarian Cancer and the Immune System: Biology, Interactions, Challenges and Potential Advances for Immunotherapy. J. Clin. Med. 2020, 9, 2967. [Google Scholar] [CrossRef]
- Savant, S.; Sriramkumar, S.; O’Hagan, H. The Role of Inflammation and Inflammatory Mediators in the Development, Progression, Metastasis, and Chemoresistance of Epithelial Ovarian Cancer. Cancers 2018, 10, 251. [Google Scholar] [CrossRef]
- Kumarasamy, C.; Sabarimurugan, S.; Madurantakam, R.M.; Lakhotiya, K.; Samiappan, S.; Baxi, S.; Nachimuthu, R.; Gothandam, K.M.; Jayaraj, R. Prognostic Significance of Blood Inflammatory Biomarkers NLR, PLR, and LMR in Cancer—A Protocol for Systematic Review and Meta-Analysis. Medicine 2019, 98, e14834. [Google Scholar] [CrossRef]
- Eo, W.K.; Chang, H.J.; Kwon, S.H.; Koh, S.B.; Kim, Y.O.; Ji, Y., II; Kim, H.-B.; Lee, J.Y.; Suh, D.S.; Kim, K.H.; et al. The Lymphocyte-Monocyte Ratio Predicts Patient Survival and Aggressiveness of Ovarian Cancer. J. Cancer 2016, 7, 289–296. [Google Scholar] [CrossRef]
- Marcus, A.; Gowen, B.G.; Thompson, T.W.; Iannello, A.; Ardolino, M.; Deng, W.; Wang, L.; Shifrin, N.; Raulet, D.H. Recognition of Tumors by the Innate Immune System and Natural Killer Cells. Adv. Immunol. 2014, 122, 91–128. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, Q.; Lau, W.B.; Lau, B.; Xu, L.; Zhao, L.; Yang, H.; Feng, M.; Xuan, Y.; Yang, Y.; et al. Tumor Microenvironment: The Culprit for Ovarian Cancer Metastasis? Cancer Lett. 2016, 377, 174–182. [Google Scholar] [CrossRef]
- Dymicka-Piekarska, V.; Koper-Lenkiewicz, O.M.; Zińczuk, J.; Kratz, E.; Kamińska, J. Inflammatory Cell-Associated Tumors. Not Only Macrophages (TAMs), Fibroblasts (TAFs) and Neutrophils (TANs) Can Infiltrate the Tumor Microenvironment. The Unique Role of Tumor Associated Platelets (TAPs). Cancer Immunol. Immunother. 2021, 70, 1497–1510. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, C.; Wang, L.; Zhong, M.; Tang, H.; Wang, H. Peripheral Blood Lymphocyte-to-Monocyte Ratio as a Prognostic Factor in Advanced Epithelial Ovarian Cancer: A Multicenter Retrospective Study. J. Cancer 2017, 8, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xu, J.; Lan, H. Tumor-Associated Macrophages in Tumor Metastasis: Biological Roles and Clinical Therapeutic Applications. J. Hematol. Oncol. 2019, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Abu-Shawer, O.; Abu-Shawer, M.; Hirmas, N.; Alhouri, A.; Massad, A.; Alsibai, B.; Sultan, H.; Hammo, H.; Souleiman, M.; Shebli, Y.; et al. Hematologic Markers of Distant Metastases and Poor Prognosis in Gynecological Cancers. BMC Cancer 2019, 19, 141. [Google Scholar] [CrossRef] [PubMed]
- Eo, W.; Kim, H.-B.; Lee, Y.J.; Suh, D.S.; Kim, K.H.; Kim, H. Preoperative Lymphocyte-Monocyte Ratio Is a Predictor of Suboptimal Cytoreduction in Stage III-IV Epithelial Ovarian Cancer. J. Cancer 2016, 7, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
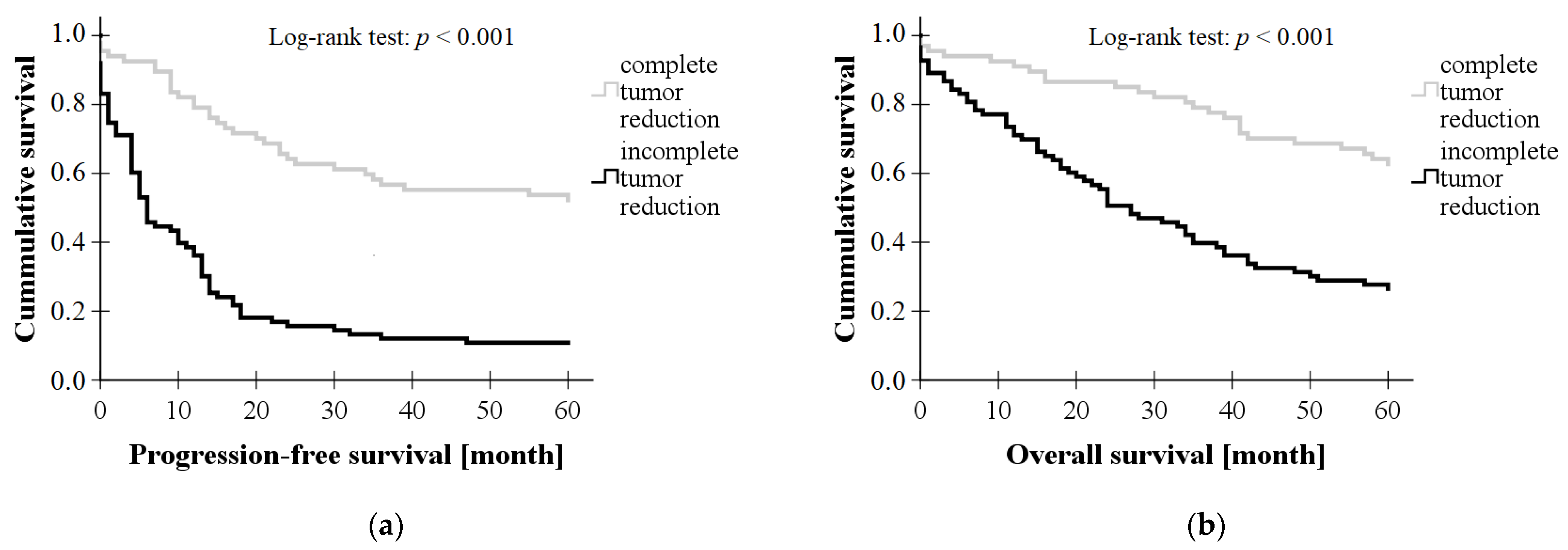


| Complete Tumor Reduction (n = 67) | Incomplete Tumor Reduction (n = 83) | p Values | ||
|---|---|---|---|---|
| FIGO stage | I II | n = 35 n = 5 n = 22 n = 5 | n = 10 n = 3 n = 50 n = 20 | |
| III | ||||
| IV | ||||
| Age [year] | 53.25 ± 11.88 | 60.22 ± 11.99 | <0.01 * | |
| BMI [kg/m2] | 25.40 ± 5.27 | 26.56 ± 4.75 | NS | |
| WBC [G/L] | 8.11 (3.99–20.43) | 8.64 (4.88–19.49) | <0.05 * | |
| Neu# [G/L] | 5.25 (2.46–15.28) | 6.21 (3.18–16.61) | <0.01 * | |
| Ly# [G/L] | 1.63 (0.77–4.20) | 1.32 (0.39–11.62) | <0.001 * | |
| Mono# [G/L] | 0.38 (0.21–1.13) | 0.49 (0.16–1.46) | <0.01 * | |
| PLT [G/L] | 314 (135.0–659.0) | 348.5 (125.0–854.0) | <0.01 * | |
| NLR | 2.802 (0.99–9.35) | 4.737 (0.377–34.56) | <0.001 * | |
| MLR | 0.232 (0.104–0.860) | 0.361 (0.076–3.318) | <0.001 * | |
| PLR | 168.133 (78.512–428.571) | 279.845 (21.084–1213.636) | <0.001 * | |
| MPV [fl] | 9.00 (7.40–12.0) | 8.70 (7.0–12.30) | NS | |
| PDW [fl] | 44.40 (9.40–62.80) | 39.90 (8.50–58.80) | <0.05 * | |
| Ca-125 [kU/L] | 81.20 (8.90–7915.0) | 565.05 (8.40–6917.0) | <0.001 * | |
| Odds Ratio | 95% Confidence Interval | p Value | |
|---|---|---|---|
| Stage (FIGO III-IV.) | 5.102 | 1.659–15.693 | <0.01 |
| MLR > 0.305 | 5.028 | 1.561–16.201 | <0.01 |
| Ca-125 > 169.15 | 4.671 | 1.540–14.167 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, A.R.; Sulina, A.; Kovács, K.S.; Lukács, L.; Török, P.; Lampé, R. Prognostic Significance of Preoperative NLR, MLR, and PLR Values in Predicting the Outcome of Primary Cytoreductive Surgery in Serous Epithelial Ovarian Cancer. Diagnostics 2023, 13, 2268. https://doi.org/10.3390/diagnostics13132268
Kovács AR, Sulina A, Kovács KS, Lukács L, Török P, Lampé R. Prognostic Significance of Preoperative NLR, MLR, and PLR Values in Predicting the Outcome of Primary Cytoreductive Surgery in Serous Epithelial Ovarian Cancer. Diagnostics. 2023; 13(13):2268. https://doi.org/10.3390/diagnostics13132268
Chicago/Turabian StyleKovács, Anna Rebeka, Anita Sulina, Kincső Sára Kovács, Luca Lukács, Péter Török, and Rudolf Lampé. 2023. "Prognostic Significance of Preoperative NLR, MLR, and PLR Values in Predicting the Outcome of Primary Cytoreductive Surgery in Serous Epithelial Ovarian Cancer" Diagnostics 13, no. 13: 2268. https://doi.org/10.3390/diagnostics13132268
APA StyleKovács, A. R., Sulina, A., Kovács, K. S., Lukács, L., Török, P., & Lampé, R. (2023). Prognostic Significance of Preoperative NLR, MLR, and PLR Values in Predicting the Outcome of Primary Cytoreductive Surgery in Serous Epithelial Ovarian Cancer. Diagnostics, 13(13), 2268. https://doi.org/10.3390/diagnostics13132268








