Contribution of Dynamic and Genetic Tests for Short Stature Diagnosing: A Case Report
Abstract
1. Introduction
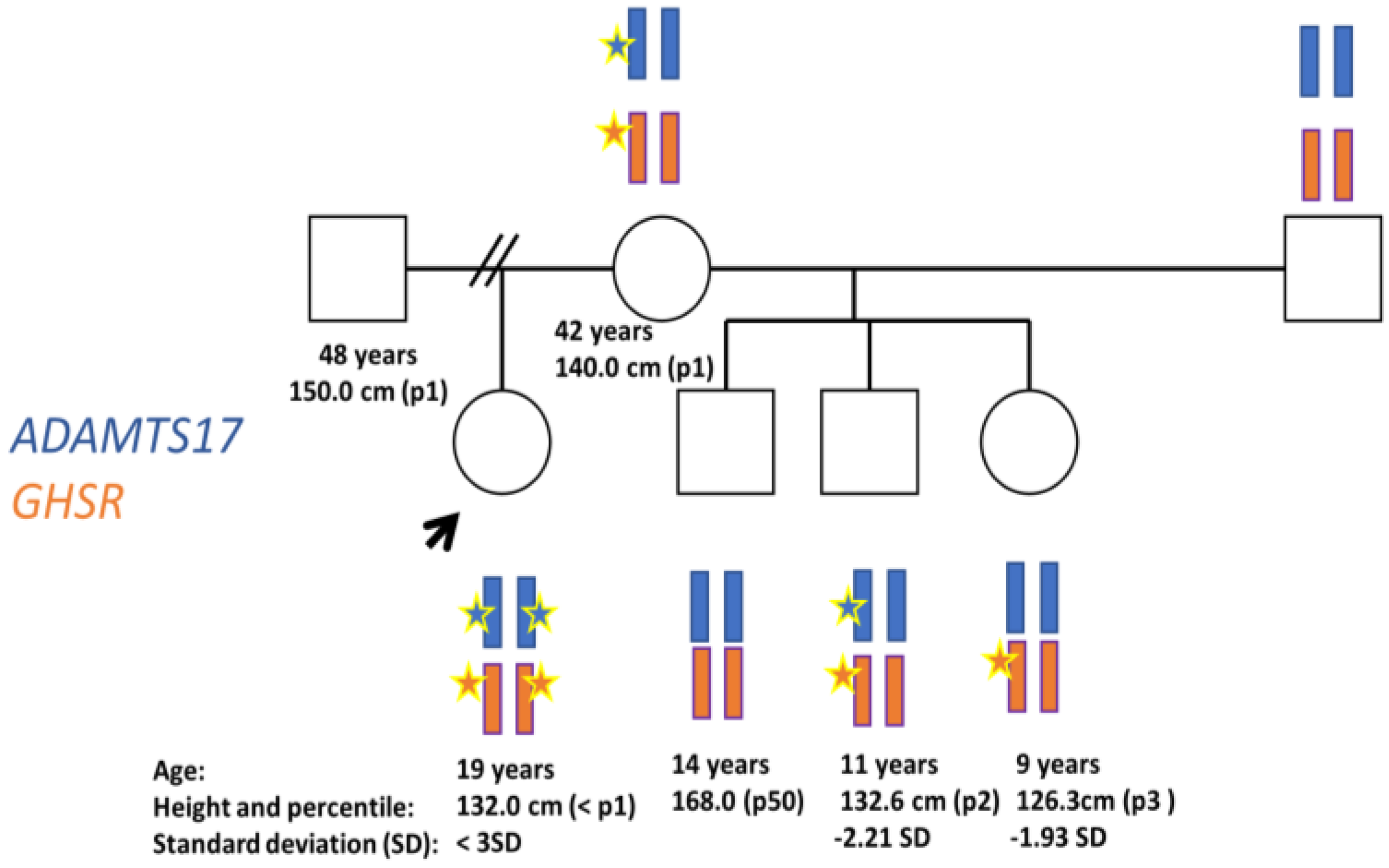
Case Presentation
2. Materials and Methods
2.1. Ethical Statement
2.2. Biochemical Assays
2.3. Genetic Tests
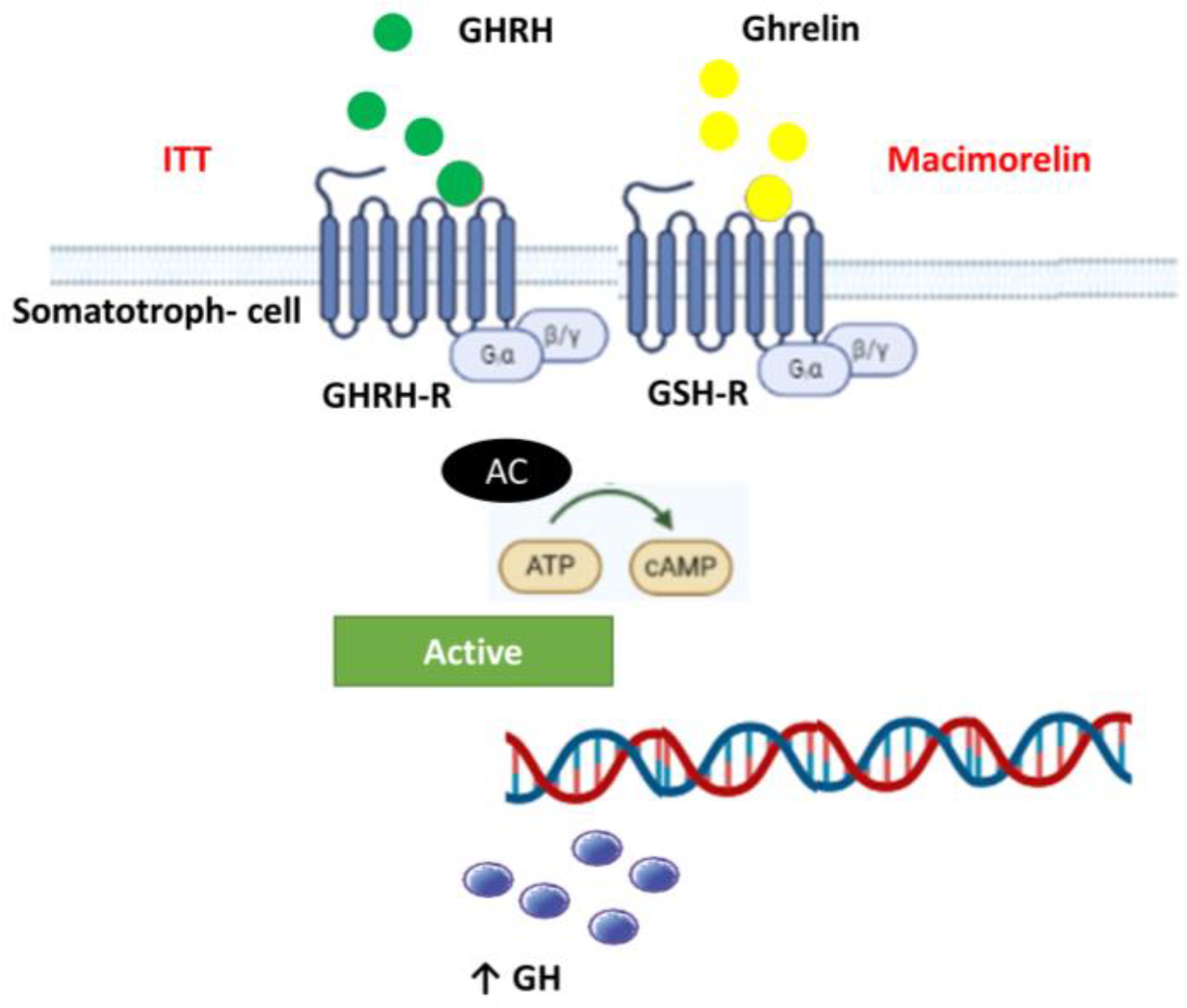
2.4. Dynamic Tests
3. Results
3.1. Baseline Phenotype
3.2. Genetics Tests
3.3. Dynamic Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cohen, P.; Rogol, A.D.; Deal, C.L.; Saenger, P.; Reiter, E.O.; Ross, J.L.; Chernausek, S.D.; Savage, M.O.; Wit, J.M. Consensus Statement on the Diagnosis and Treatment of Children with Idiopathic Short Stature: A Summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. J. Clin. Endocrinol. Metab. 2008, 93, 4210–4217. [Google Scholar] [CrossRef]
- Sisley, S.; Trujillo, M.V.; Khoury, J.; Backeljauw, P. Low Incidence of Pathology Detection and High Cost of Screening in the Evaluation of Asymptomatic Short Children. J. Pediatr. 2013, 163, 1045–1051. [Google Scholar] [CrossRef]
- Baron, J.; Sävendahl, L.; De Luca, F.; Dauber, A.; Phillip, M.; Wit, J.M.; Nilsson, O. Short and Tall Stature: A New Paradigm Emerges. Nat. Rev. Endocrinol. 2015, 11, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Molitch, M.E.; Clemmons, D.R.; Malozowski, S.; Merriam, G.R.; Vance, M.L. Endocrine Society Evaluation and Treatment of Adult Growth Hormone Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1587–1609. [Google Scholar] [CrossRef]
- Yuen, K.C.J.; Biller, B.M.K.; Radovick, S.; Carmichael, J.D.; Jasim, S.; Pantalone, K.M.; Hoffman, A.R. American association of clinical endocrinologists and american college of endocrinology guidelines for management of growth hormone deficiency in adults and patients transitioning from pediatric to adult care. Endocr. Prac. 2019, 25, 1191–1232. [Google Scholar] [CrossRef] [PubMed]
- Yuen, K.C.J.; Llahana, S.; Miller, B.S. Adult Growth Hormone Deficiency: Clinical Advances and Approaches to Improve Adherence. Expert. Rev. Endocrinol. Metab. 2019, 14, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M.; Swerdloff, R.; Wang, C.; Kyle, M.; Kipnes, M.; Biller, B.M.K.; Cook, D.; Yuen, K.C.J.; Bonert, V.; Dobs, A.; et al. Macimorelin (AEZS-130)-Stimulated Growth Hormone (GH) Test: Validation of a Novel Oral Stimulation Test for the Diagnosis of Adult GH Deficiency. J. Clin. Endocrinol. Metab. 2013, 98, 2422–2429. [Google Scholar] [CrossRef]
- Garcia, J.M.; Biller, B.M.K.; Korbonits, M.; Popovic, V.; Luger, A.; Strasburger, C.J.; Chanson, P.; Medic-Stojanoska, M.; Schopohl, J.; Zakrzewska, A.; et al. Macimorelin as a Diagnostic Test for Adult GH Deficiency. J. Clin. Endocrinol. Metab. 2018, 103, 3083–3093. [Google Scholar] [CrossRef]
- Csákváry, V.; Ammer, N.; Bagci, E.B.; Bolshova, O.V.; Damholt, B.B.; Katanic, D.; Mikhailova, E.; Muzsnai, Á.; Raduk, D.; Senatorova, G.; et al. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Macimorelin in Children with Suspected Growth Hormone Deficiency: An Open-Label, Group Comparison, Dose-Escalation Trial. Horm. Res. Paediatr. 2021, 94, 239–250. [Google Scholar] [CrossRef]
- Karoulias, S.Z.; Taye, N.; Stanley, S.; Hubmacher, D. The ADAMTS/Fibrillin Connection: Insights into the Biological Functions of ADAMTS10 and ADAMTS17 and Their Respective Sister Proteases. Biomolecules 2020, 10, 596. [Google Scholar] [CrossRef]
- Khan, A.O.; Aldahmesh, M.A.; Al-Ghadeer, H.; Mohamed, J.Y.; Alkuraya, F.S. Familial Spherophakia with Short Stature Caused by a Novel Homozygous ADAMTS17 Mutation. Ophthalmic Genet. 2012, 33, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, P.; Zheng, H.; Smith, R.G. Ghrelin Stimulation of Growth Hormone Release and Appetite Is Mediated through the Growth Hormone Secretagogue Receptor. Proc. Natl. Acad. Sci. USA 2004, 101, 4679–4684. [Google Scholar] [CrossRef]
- Jelenkovic, A.; Sund, R.; Yokoyama, Y.; Latvala, A.; Sugawara, M.; Tanaka, M.; Matsumoto, S.; Freitas, D.L.; Maia, J.A.; Knafo-Noam, A.; et al. Genetic and Environmental Influences on Human Height from Infancy through Adulthood at Different Levels of Parental Education. Sci. Rep. 2020, 10, 7974. [Google Scholar] [CrossRef]
- Pfäffle, R.; Knüpfer, M.; Göbert, M.; Vogel, M.; Gausche, R.; Beger, C.; Keller, E.; Körner, A.; Thome, U.; Kiess, W. Growth Patterns of Children with Short Stature in Adulthood According to Auxological Status and Maturity at Birth. J. Clin. Endocrinol. Metab. 2022, 107, 3320–3327. [Google Scholar] [CrossRef] [PubMed]
- Lifshitz, F. Nutrition and Growth. J. Clin. Res. Pediatr. Endocrinol. 2009, 1, 157–163. [Google Scholar] [CrossRef]
- Yengo, L.; Vedantam, S.; Marouli, E.; Sidorenko, J.; Bartell, E.; Sakaue, S.; Graff, M.; Eliasen, A.U.; Jiang, Y.; Raghavan, S.; et al. A Saturated Map of Common Genetic Variants Associated with Human Height. Nature 2022, 610, 704–712. [Google Scholar] [CrossRef]
- Durand, C.; Rappold, G.A. Height Matters—From Monogenic Disorders to Normal Variation. Nat. Rev. Endocrinol. 2013, 9, 171–177. [Google Scholar] [CrossRef]
- Kelwick, R.; Desanlis, I.; Wheeler, G.N.; Edwards, D.R. The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin Motifs) Family. Genome Biol. 2015, 16, 113. [Google Scholar] [CrossRef] [PubMed]
- Hassani, M.; Taghizadeh, S.; Farahzad Broujeni, A.; Habibi, M.; Banitalebi, S.; Kasiri, M.; Sadeghi, A.; Nozari, A. A Novel Missense Mutation in the TGF-β-Binding Protein-Like Domain 3 of FBN1 Causes Weill-Marchesani Syndrome with Intellectual Disability. Adv. Biomed. Res. 2023, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.H.; Bhat, V.; Shetty, J.S.; Kumar, A. Whole Exome Sequencing Identifies a Novel Splice-Site Mutation in ADAMTS17 in an Indian Family with Weill-Marchesani Syndrome. Mol. Vis. 2014, 20, 790–796. [Google Scholar]
- Morales, J.; Al-Sharif, L.; Khalil, D.S.; Shinwari, J.M.A.; Bavi, P.; Al-Mahrouqi, R.A.; Al-Rajhi, A.; Alkuraya, F.S.; Meyer, B.F.; Al Tassan, N. Homozygous Mutations in ADAMTS10 and ADAMTS17 Cause Lenticular Myopia, Ectopia Lentis, Glaucoma, Spherophakia, and Short Stature. Am. J. Hum. Genet. 2009, 85, 558–568. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, J.; Cheng, C.M.; Kopchick, J.J.; Bondy, C.A. Evidence Supporting Dual, IGF-I-Independent and IGF-I-Dependent, Roles for GH in Promoting Longitudinal Bone Growth. J. Endocrinol. 2004, 180, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Peñalva, A.; Burguera, B.; Casabiell, X.; Tresguerres, J.A.; Dieguez, C.; Casanueva, F.F. Activation of Cholinergic Neurotransmission by Pyridostigmine Reverses the Inhibitory Effect of Hyperglycemia on Growth Hormone (GH) Releasing Hormone-Induced GH Secretion in Man: Does Acute Hyperglycemia Act through Hypothalamic Release of Somatostatin? Neuroendocrinology 1989, 49, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Tsushima, T.; Sakuma, M.; Irie, M. Effect of Changes in Plasma Free Fatty Acids Level on Secretion of Human Growth Hormone. Endocrinol. Jpn. 1970, 17, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Melmed, S. Insulin-like Growth Factor I Action on Rat Anterior Pituitary Cells: Suppression of Growth Hormone Secretion and Messenger Ribonucleic Acid Levels. Endocrinology 1986, 118, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin Is a Growth-Hormone-Releasing Acylated Peptide from Stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef]
- Roth, J.; Glick, S.M.; Yalow, R.S. Bersonsa, null Hypoglycemia: A Potent Stimulus to Secretion of Growth Hormone. Science 1963, 140, 987–988. [Google Scholar] [CrossRef]
- Sukkar, M.Y.; Hunter, W.M.; Passmore, R. Changes in Plasma Levels of Insulin and Growth-Hormone Levels after a Protein Meal. Lancet 1967, 2, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Kangawa, K. Ghrelin: More than Endogenous Growth Hormone Secretagogue. Ann. N. Y. Acad. Sci. 2010, 1200, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, G.; Samson, S.L.; Sun, Y. Ghrelin: Much More than a Hunger Hormone. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 619–624. [Google Scholar] [CrossRef]
- Veldhuis, J.D.; Reynolds, G.A.; Iranmanesh, A.; Bowers, C.Y. Twenty-Four Hour Continuous Ghrelin Infusion Augments Physiologically Pulsatile, Nycthemeral, and Entropic (Feedback-Regulated) Modes of Growth Hormone Secretion. J. Clin. Endocrinol. Metab. 2008, 93, 3597–3603. [Google Scholar] [CrossRef] [PubMed]
- Pantel, J.; Legendre, M.; Cabrol, S.; Hilal, L.; Hajaji, Y.; Morisset, S.; Nivot, S.; Vie-Luton, M.-P.; Grouselle, D.; de Kerdanet, M.; et al. Loss of Constitutive Activity of the Growth Hormone Secretagogue Receptor in Familial Short Stature. J. Clin. Investig. 2006, 116, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Pantel, J.; Legendre, M.; Nivot, S.; Morisset, S.; Vie-Luton, M.-P.; le Bouc, Y.; Epelbaum, J.; Amselem, S. Recessive Isolated Growth Hormone Deficiency and Mutations in the Ghrelin Receptor. J. Clin. Endocrinol. Metab. 2009, 94, 4334–4341. [Google Scholar] [CrossRef] [PubMed]
- Zizzari, P.; Halem, H.; Taylor, J.; Dong, J.Z.; Datta, R.; Culler, M.D.; Epelbaum, J.; Bluet-Pajot, M.T. Endogenous Ghrelin Regulates Episodic Growth Hormone (GH) Secretion by Amplifying GH Pulse Amplitude: Evidence from Antagonism of the GH Secretagogue-R1a Receptor. Endocrinology 2005, 146, 3836–3842. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biagetti, B.; Valenzuela, I.; Campos-Martorell, A.; Campos, B.; Hernandez, S.; Giralt, M.; Díaz-Troyano, N.; Iniesta-Serrano, E.; Yeste, D.; Simó, R. Contribution of Dynamic and Genetic Tests for Short Stature Diagnosing: A Case Report. Diagnostics 2023, 13, 2259. https://doi.org/10.3390/diagnostics13132259
Biagetti B, Valenzuela I, Campos-Martorell A, Campos B, Hernandez S, Giralt M, Díaz-Troyano N, Iniesta-Serrano E, Yeste D, Simó R. Contribution of Dynamic and Genetic Tests for Short Stature Diagnosing: A Case Report. Diagnostics. 2023; 13(13):2259. https://doi.org/10.3390/diagnostics13132259
Chicago/Turabian StyleBiagetti, Betina, Irene Valenzuela, Ariadna Campos-Martorell, Berta Campos, Sara Hernandez, Marina Giralt, Noelia Díaz-Troyano, Emilio Iniesta-Serrano, Diego Yeste, and Rafael Simó. 2023. "Contribution of Dynamic and Genetic Tests for Short Stature Diagnosing: A Case Report" Diagnostics 13, no. 13: 2259. https://doi.org/10.3390/diagnostics13132259
APA StyleBiagetti, B., Valenzuela, I., Campos-Martorell, A., Campos, B., Hernandez, S., Giralt, M., Díaz-Troyano, N., Iniesta-Serrano, E., Yeste, D., & Simó, R. (2023). Contribution of Dynamic and Genetic Tests for Short Stature Diagnosing: A Case Report. Diagnostics, 13(13), 2259. https://doi.org/10.3390/diagnostics13132259







