Hybrid Models Based on Fusion Features of a CNN and Handcrafted Features for Accurate Histopathological Image Analysis for Diagnosing Malignant Lymphomas
Abstract
:1. Introduction
- Passing histological images of malignant lymphomas through two overlapping Gaussian and Laplacian filters.
- Applying a hybrid model between DL networks with XGBoost and DT networks based on the GVF algorithm to diagnose malignant lymphoma images effectively.
- Diagnosis of malignant lymphoma images by XGBoost and DT networks with fused features of MobileNet-VGG16, VGG16-AlexNet, and MobileNet-AlexNet based on the ACO algorithm for features selection.
- Efficient and accurate diagnosis of malignant lymphoma images by XGBoost and DT grids with fused features of MobileNet-VGG16, VGG16-AlexNet, and MobileNet-AlexNet and handcrafted features based on the ACO algorithm for features selection.
2. Related Work
3. Materials and Methods
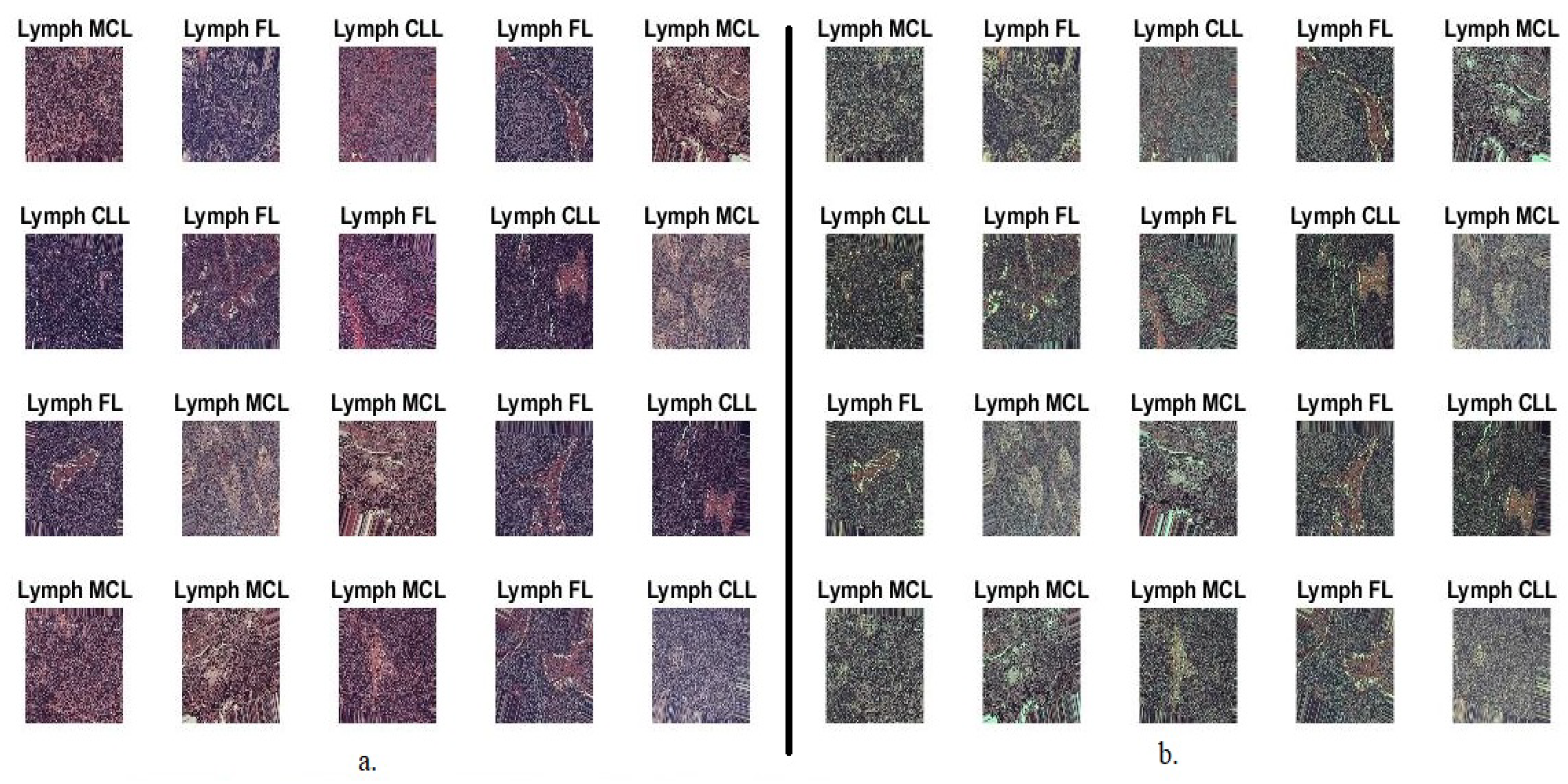
3.1. Description of the Malignant Lymphoma Dataset
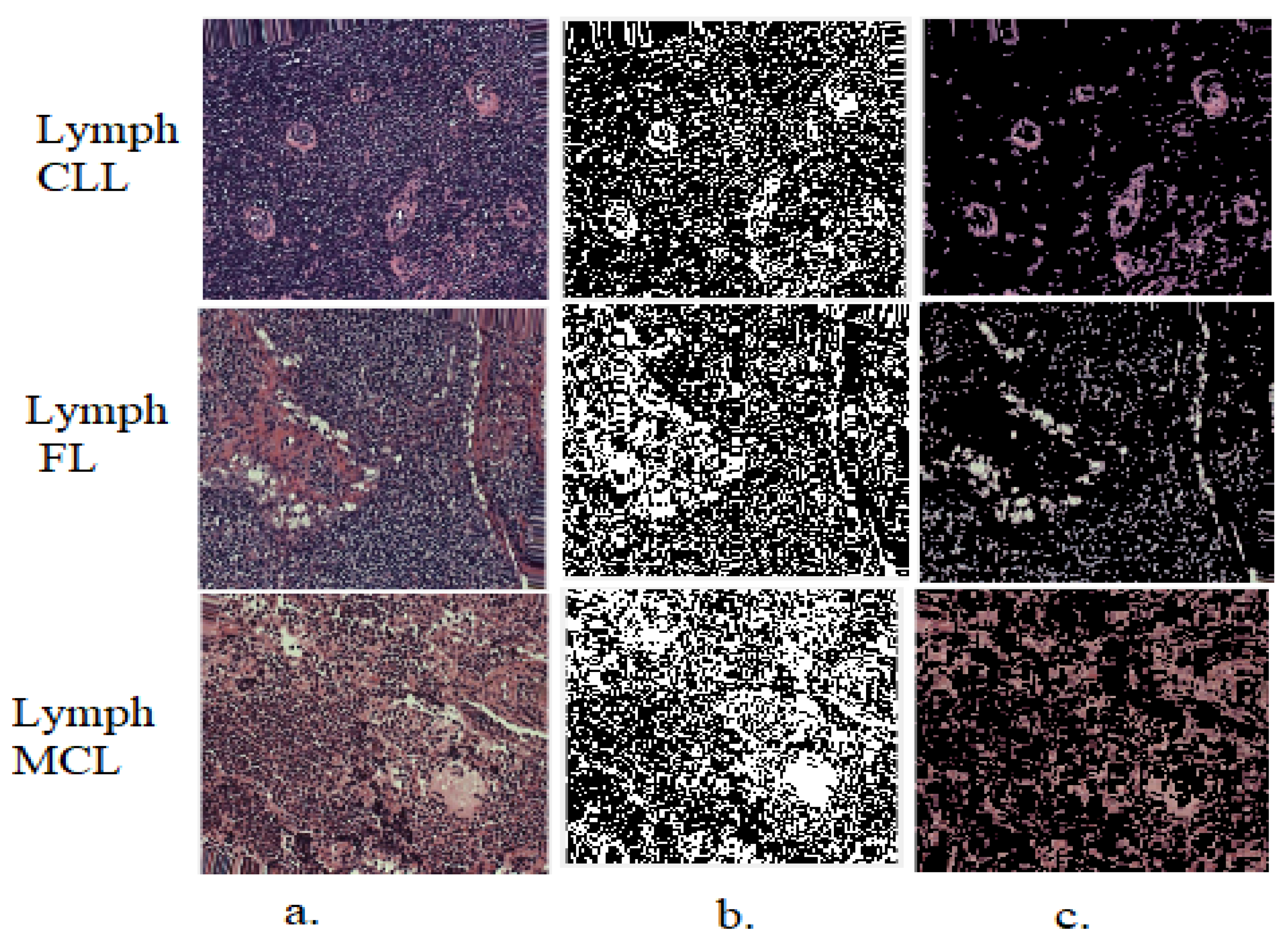
3.2. Improvement of Histopathological WSI Images
3.3. Gradient Vector Flow Algorithm
3.4. Extract Deep Feature Maps
3.5. Ant Colony Optimization Algorithm
3.6. Inductive and Deductive Phase
- High performance: XGBoost is known for its scalability and efficiency. It has been optimized to handle large datasets with millions of observations and thousands of features. It can also take advantage of parallel computing, making it suitable for high-performance computing environments.
- Regularization techniques: XGBoost includes regularization techniques such as L1 and L2 regularization, which help to prevent overfitting and improve generalization.
- Feature importance: XGBoost provides a measure of feature importance, which helps in identifying the most influential features for making predictions. This can be valuable for feature selection and understanding the underlying patterns in the data.
- Flexibility: XGBoost supports a wide range of loss functions, making it adaptable to various problem types, including regression, classification, and ranking tasks.
- Interpretability: Decision trees are easy to understand and interpret. The generated tree structure allows humans to follow the decision-making process and gain insights into how the model arrives at its predictions.
- Handling non-linear relationships: Decision trees can capture non-linear relationships between features and the target variable. They can handle both continuous and categorical features without requiring extensive preprocessing.
- Feature interactions: Decision trees can capture interactions between features, which can be essential for modeling complex relationships in the data.
- Outlier robustness: Decision trees are less sensitive to outliers compared to some other machine learning methods. They partition the feature space into regions and make predictions based on the majority class within each region.
3.6.1. XGBoost Algorithm
3.6.2. Decision Tree Algorithm
- Training Complexity: The training process of XGBoost involves constructing decision trees iteratively. Each tree is built to correct the mistakes made by previous trees. The complexity of training an XGBoost model depends on the following factors:
- b.
- Prediction Complexity: Once the XGBoost model is trained, making predictions for new samples is generally fast. The complexity depends on the number of trees in the model, the maximum depth of the trees, and the number of features.
- Training Complexity: Building a decision tree involves recursively partitioning the feature space based on the selected splitting criteria, such as Gini impurity or information gain. The training complexity of a decision tree depends on:
- b.
- Prediction Complexity: Once the decision tree is constructed, making predictions for new samples is efficient. Traversing the tree and evaluating the corresponding features have a complexity proportional to the tree’s depth.
3.7. Strategy of Implementation Sequence
3.7.1. Hybrid Strategy of Machine Learning with Features of DL Models
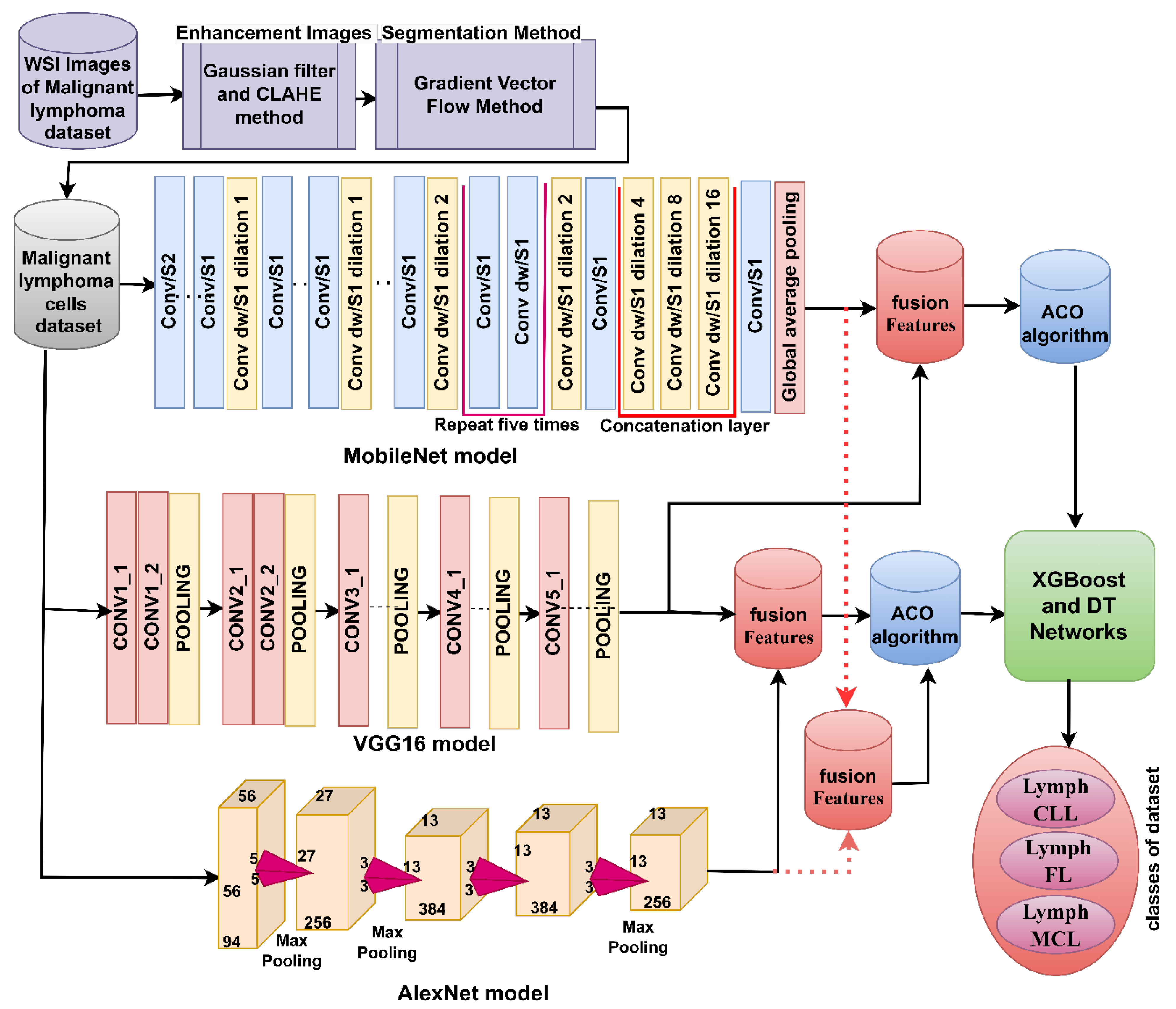
3.7.2. Hybrid Strategy of Machine Learning with Fusion Features of DL Models
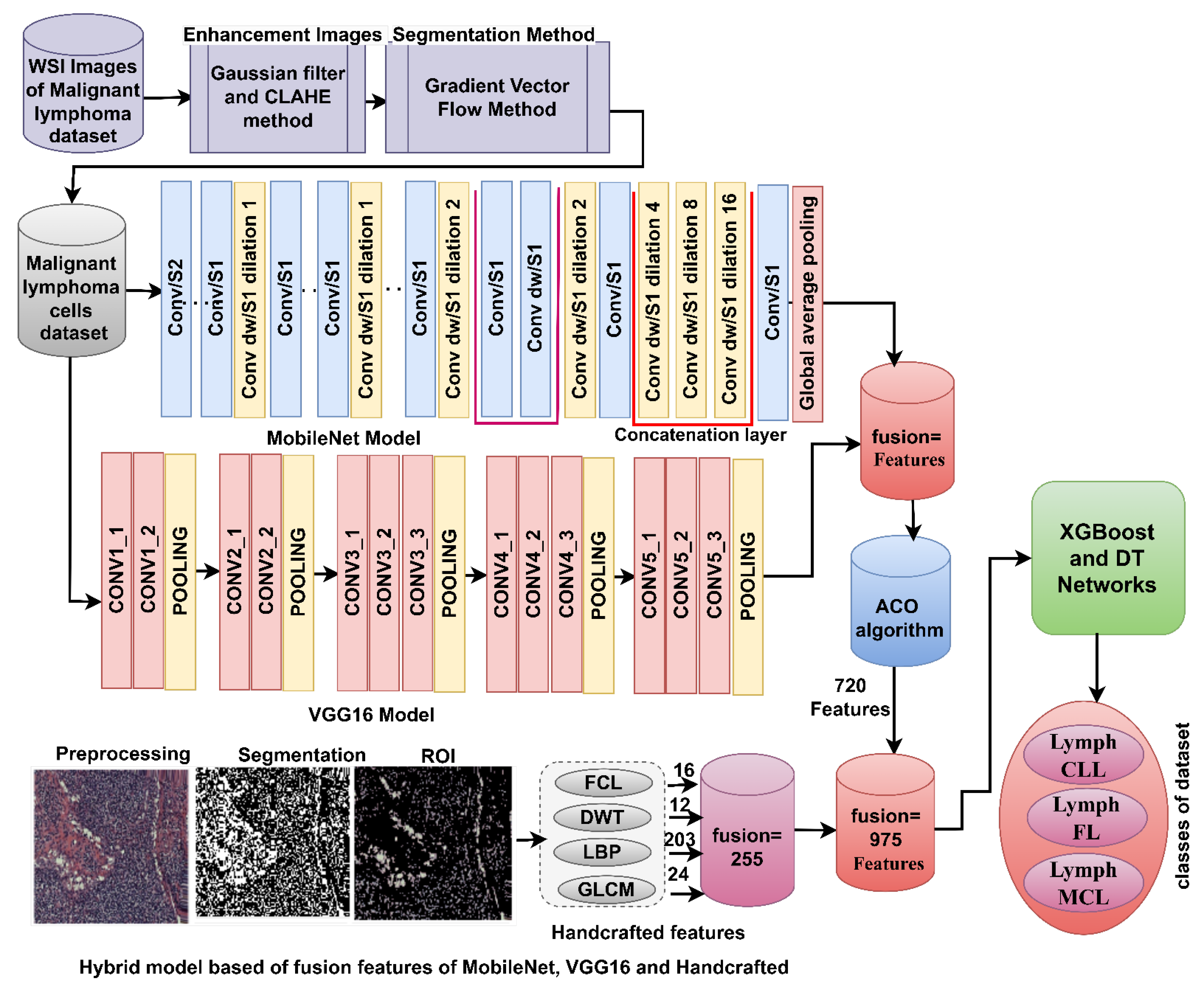
3.7.3. Hybrid Strategy of Machine Learning with Fusion Features of DL and Handcrafted
4. Results of System Performance
4.1. Evaluation Metrics
4.2. Results of Pre-trained Networks
4.3. Results of Hybrid Strategy of Machine Learning with Features of DL Models
4.4. Result of Hybrid Strategy of Machine Learning with Fusion Features of DL Models
4.5. Results of Machine Learning with Fusion Features of DL and Handcrafted
5. Discussion of Systems’ Performance
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stefaniuk, P.; Szymczyk, A.; Podhorecka, M. The neutrophil to lymphocyte and lymphocyte to monocyte ratios as new prognostic factors in hematological malignancies—A narrative review. Cancer Manag. Res. 2020, 12, 2961. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Asada, N.; Egusa, Y.; Ikeda, T.; Sakamoto, M.; Abe, M.; Maeda, Y. Transformation to diffuse large B-cell lymphoma with germinal center B-cell like subtype and discordant light chain expression in a patient with Waldenström macroglobulinemia/lymphoplasmacytic lymphoma. Int. J. Hematol. 2021, 114, 401–407. [Google Scholar] [CrossRef]
- Zmigrodzka, M.; Witkowska-Pilaszewicz, O.; Pingwara, R.; Pawlak, A.; Winnicka, A. Canine B Cell Lymphoma- and Leukemia-Derived Extracellular Vesicles Moderate Differentiation and Cytokine Production of T and B Cells In Vitro. Int. J. Mol. Sci. 2022, 23, 9831. [Google Scholar] [CrossRef] [PubMed]
- Kanas, G.; Ge, W.; Quek, R.G.; Keeven, K.; Nersesyan, K.; Arnason, J.E. Epidemiology of diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL) in the United States and Western Europe: Population-level projections for 2020–2025. Leuk. Lymphoma 2022, 63, 54–63. [Google Scholar] [CrossRef] [PubMed]
- King, R.L.; Gupta, A.; Kurtin, P.J.; Ding, W.; Call, T.G.; Rabe, K.G.; Parikh, S.A. Chronic lymphocytic leukemia (CLL) with Reed–Sternberg-like cells vs. Classic Hodgkin lymphoma transformation of CLL: Does this distinction matter? Blood Cancer J. 2022, 12, 18. [Google Scholar] [CrossRef]
- Beitinjaneh, A.; Kaufman, A.; Wang, Y.; Jain, P.; Srour, S.A.; Wang, M. Is There Still a Role for Transplant for Patients with Mantle Cell Lymphoma (MCL) in the Era of CAR-T Cell Therapy? Curr. Treat. Options Oncol. 2022, 23, 1614–1625. [Google Scholar] [CrossRef]
- Ng, K.W.L.; Beatty, J.A.; Tse, M.P.Y.; Giuliano, A. Nasal Lymphoma with Low Mitotic Index in Three Cats Treated with Chlorambucil and Prednisolone. Vet. Sci. 2022, 9, 472. [Google Scholar] [CrossRef]
- Lisson, C.S.; Lisson, C.G.; Mezger, M.F.; Wolf, D.; Schmidt, S.A.; Thaiss, W.M.; Tausch, E.; Beer, A.J.; Stilgenbauer, S.; Beer, M.; et al. Deep Neural Networks and Machine Learning Radiomics Modelling for Prediction of Relapse in Mantle Cell Lymphoma. Cancers 2022, 14, 2008. [Google Scholar] [CrossRef]
- Wang, C.-W.; Khalil, M.-A.; Lin, Y.-J.; Lee, Y.-C.; Huang, T.-W.; Chao, T.-K. Deep Learning Using Endobronchial-Ultrasound-Guided Transbronchial Needle Aspiration Image to Improve the Overall Diagnostic Yield of Sampling Mediastinal Lymphadenopathy. Diagnostics 2022, 12, 2234. [Google Scholar] [CrossRef]
- Caldonazzi, N.; Rizzo, P.C.; Eccher, A.; Girolami, I.; Fanelli, G.N.; Naccarato, A.G.; Bonizzi, G.; Fusco, N.; d’Amati, G.; Scarpa, A.; et al. Value of Artificial Intelligence in Evaluating Lymph Node Metastases. Cancers 2023, 15, 2491. [Google Scholar] [CrossRef]
- Irshaid, L.; Bleiberg, J.; Weinberger, E.; Garritano, J.; Shallis, R.M.; Patsenker, J.; Xu, M.L. Histopathologic and machine deep learning criteria to predict lymphoma transformation in bone marrow biopsies. Arch. Pathol. Lab. Med. 2022, 146, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Hu, B.; Li, H.; Shi, W.; Tang, Y.; Yu, Y.; Li, Y. Deep learning for automatic differential diagnosis of primary central nervous system lymphoma and glioblastoma: Multi-parametric magnetic resonance imaging based convolutional neural network model. J. Magn. Reson. Imaging 2021, 54, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Savas, I. Classifying Lymphoma Subtypes using CNN and CNN LSTM Mixed Model; ResearchGate: Berlin, Germany, 2021. [Google Scholar] [CrossRef]
- Miyoshi, H.; Sato, K.; Kabeya, Y.; Yonezawa, S.; Nakano, H.; Takeuchi, Y.; Ohshima, K. Deep learning shows the capability of high-level computer-aided diagnosis in malignant lymphoma. Lab. Investig. 2020, 100, 1300–1310. [Google Scholar] [CrossRef]
- Li, D.; Bledsoe, J.R.; Zeng, Y.; Liu, W.; Hu, Y.; Bi, K.; Li, S. A deep learning diagnostic platform for diffuse large B-cell lymphoma with high accuracy across multiple hospitals. Nat. Commun. 2020, 11, 6004. [Google Scholar] [CrossRef] [PubMed]
- Syrykh, C.; Abreu, A.; Amara, N.; Siegfried, A.; Maisongrosse, V.; Frenois, F.X.; Brousset, P. Accurate diagnosis of lymphoma on whole-slide histopathology images using deep learning. NPJ Digit. Med. 2020, 3, 63. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, K.; Jiang, M.; Yang, L. Research on the classification of lymphoma pathological images based on deep residual neural network. Technol. Health Care 2021, 29, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Reena, M.R.; Ameer, P.M. A content-based image retrieval system for the diagnosis of lymphoma using blood micrographs: An incorporation of deep learning with a traditional learning approach. Comput. Biol. Med. 2022, 145, 105463. [Google Scholar] [CrossRef] [PubMed]
- Zahra, H.N.; Anshori, I.; Nadila, H.; Utami, H.M.; Chandra, J.A.; Kurniawan, M.R.; Husain, O. Classification of Lymphoma, Benign Lesions, and Carcinoma Using Convolutional Neural Network. In Proceedings of the 4th International Conference on Life Sciences and Biotechnology (ICOLIB), Jember, Indonesia, 15–16 November 2021; Atlantis Press: Jember, Indonesia, 2022; pp. 175–192. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, S.; Guo, S.; Xia, X. Lymphoma recognition based on CNN models. In Proceedings of the Second IYSF Academic Symposium on Artificial Intelligence and Computer Engineering, Xi’an, China, 8–10 October 2021; Volume 12079, pp. 602–607. [Google Scholar] [CrossRef]
- Swiderska-Chadaj, Z.; Hebeda, K.; van den Brand, M.; Litjens, G. Predicting MYC translocation in HE specimens of diffuse large B-cell lymphoma through deep learning. In Proceedings of the Medical Imaging 2020: Digital Pathology, Houston, TX, USA, 15–20 February 2020; Volume 11320, pp. 238–244. [Google Scholar] [CrossRef]
- Sheng, B.; Zhou, M.; Hu, M.; Li, Q.; Sun, L.; Wen, Y. A blood cell dataset for lymphoma classification using faster R-CNN. Biotechnol. Biotechnol. Equip. 2020, 34, 413–420. [Google Scholar] [CrossRef]
- Mohlman, J.S.; Leventhal, S.D.; Hansen, T.; Kohan, J.; Pascucci, V.; Salama, M.E. Improving augmented human intelligence to distinguish Burkitt lymphoma from diffuse large B-cell lymphoma cases. Am. J. Clin. Pathol. 2020, 153, 743–759. [Google Scholar] [CrossRef]
- Gaidano, V.; Tenace, V.; Santoro, N.; Varvello, S.; Cignetti, A.; Prato, G.; Saglio, G.; De Rosa, G.; Geuna, M. A Clinically Applicable Approach to the Classification of B-Cell Non-Hodgkin Lymphomas with Flow Cytometry and Machine Learning. Cancers 2020, 12, 1684. [Google Scholar] [CrossRef]
- Farinha, F.; Ioannidis, N. Artifact Removal and FOXP3+ Biomarker Segmentation for Follicular Lymphomas.
- Zijtregtop, E.A.M.; Winterswijk, L.A.; Beishuizen, T.P.A.; Zwaan, C.M.; Nievelstein, R.A.J.; Meyer-Wentrup, F.A.G.; Beishuizen, A. Machine Learning Logistic Regression Model for Early Decision Making in Referral of Children with Cervical Lymphadenopathy Suspected of Lymphoma. Cancers 2023, 15, 1178. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zheng, B.; Li, Y.; Li, Y.; Ma, X. Computer-aided diagnostic models to classify lymph node metastasis and lymphoma involvement in enlarged cervical lymph nodes using PET/CT. Med. Phys. 2023, 50, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, N.; Takagi, Y.; Masuda, H.; Miyoshi, H.; Kohno, K.; Nagaishi, M.; Takeuchi, I. Case-based similar image retrieval for weakly annotated large histopathological images of malignant lymphoma using deep metric learning. Med. Image Anal. 2023, 85, 102752. [Google Scholar] [CrossRef] [PubMed]
- Multi Cancer Dataset|Kaggle. Available online: https://www.kaggle.com/datasets/obulisainaren/multi-cancer (accessed on 8 May 2022).
- Shankar, K.; Dutta, A.K.; Kumar, S.; Joshi, G.P.; Doo, I.C. Chaotic Sparrow Search Algorithm with Deep Transfer Learning Enabled Breast Cancer Classification on Histopathological Images. Cancers 2022, 14, 2770. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.A.; Senan, E.M.; Shatnawi, H.S.A.; Alkhraisha, Z.M.; Al-Azzam, M.M.A. Multi-Models of Analyzing Dermoscopy Images for Early Detection of Multi-Class Skin Lesions Based on Fused Features. Processes 2023, 11, 910. [Google Scholar] [CrossRef]
- Chang, J.; Gao, X.; Yang, Y.; Wang, N. Object-Oriented Building Contour Optimization Methodology for Image Classification Results via Generalized Gradient Vector Flow Snake Model. Remote Sens. 2021, 13, 2406. [Google Scholar] [CrossRef]
- Olayah, F.; Senan, E.M.; Ahmed, I.A.; Awaji, B. AI Techniques of Dermoscopy Image Analysis for the Early Detection of Skin Lesions Based on Combined CNN Features. Diagnostics 2023, 13, 1314. [Google Scholar] [CrossRef]
- Ahmed, I.A.; Senan, E.M.; Shatnawi, H.S.A.; Alkhraisha, Z.M.; Al-Azzam, M.M.A. Multi-Techniques for Analyzing X-ray Images for Early Detection and Differentiation of Pneumonia and Tuberculosis Based on Hybrid Features. Diagnostics 2023, 13, 814. [Google Scholar] [CrossRef]
- Ansari, S.; Navin, A.H.; Babazadeh Sangar, A.; Vaez Gharamaleki, J.; Danishvar, S. Acute Leukemia Diagnosis Based on Images of Lymphocytes and Monocytes Using Type-II Fuzzy Deep Network. Electronics 2023, 12, 1116. [Google Scholar] [CrossRef]
- Al-Mekhlafi, Z.G.; Senan, E.M.; Mohammed, B.A.; Alazmi, M.; Alayba, A.M.; Alreshidi, A.; Alshahrani, M. Diagnosis of Histopathological Images to Distinguish Types of Malignant Lymphomas Using Hybrid Techniques Based on Fusion Features. Electronics 2022, 11, 2865. [Google Scholar] [CrossRef]
- Ansari, S.; Navin, A.H.; Sangar, A.B.; Gharamaleki, J.V.; Danishvar, S. A Customized Efficient Deep Learning Model for the Diagnosis of Acute Leukemia Cells Based on Lymphocyte and Monocyte Images. Electronics 2023, 12, 322. [Google Scholar] [CrossRef]
- Mohammed, B.A.; Senan, E.M.; Alshammari, T.S.; Alreshidi, A.; Alayba, A.M.; Alazmi, M.; Alsagri, A.N. Hybrid Techniques of Analyzing MRI Images for Early Diagnosis of Brain Tumours Based on Hybrid Features. Processes 2023, 11, 212. [Google Scholar] [CrossRef]
- Ahmed, I.A.; Senan, E.M.; Rassem, T.H.; Ali, M.A.H.; Shatnawi, H.S.A.; Alwazer, S.M.; Alshahrani, M. Eye Tracking-Based Diagnosis and Early Detection of Autism Spectrum Disorder Using Machine Learning and Deep Learning Techniques. Electronics 2022, 11, 530. [Google Scholar] [CrossRef]
- Mohammed, B.A.; Senan, E.M.; Al-Mekhlafi, Z.G.; Alazmi, M.; Alayba, A.M.; Alanazi, A.A.; Alreshidi, A.; Alshahrani, M. Hybrid Techniques for Diagnosis with WSIs for Early Detection of Cervical Cancer Based on Fusion Features. Appl. Sci. 2022, 12, 8836. [Google Scholar] [CrossRef]
- Li, S.; Wei, Y.; Liu, X.; Zhu, H.; Yu, Z. A New Fast Ant Colony Optimization Algorithm: The Saltatory Evolution Ant Colony Optimization Algorithm. Mathematics 2022, 10, 925. [Google Scholar] [CrossRef]
- Paleczek, A.; Grochala, D.; Rydosz, A. Artificial Breath Classification Using XGBoost Algorithm for Diabetes Detection. Sensors 2021, 21, 4187. [Google Scholar] [CrossRef]
- Zhao, L.; Lee, S.; Jeong, S.-P. Decision Tree Application to Classification Problems with Boosting Algorithm. Electronics 2021, 10, 1903. [Google Scholar] [CrossRef]
- Fati, S.M.; Senan, E.M.; Javed, Y. Early Diagnosis of Oral Squamous Cell Carcinoma Based on Histopathological Images Using Deep and Hybrid Learning Approaches. Diagnostics 2022, 12, 1899. [Google Scholar] [CrossRef]
- Berganzo-Besga, I.; Orengo, H.A.; Lumbreras, F.; Carrero-Pazos, M.; Fonte, J.; Vilas-Estévez, B. Hybrid MSRM-Based Deep Learning and Multitemporal Sentinel 2-Based Machine Learning Algorithm Detects Near 10k Archaeological Tumuli in North-Western Iberia. Remote Sens. 2021, 13, 4181. [Google Scholar] [CrossRef]
- Khan, I.U.; Afzal, S.; Lee, J.W. Human Activity Recognition via Hybrid Deep Learning Based Model. Sensors 2022, 22, 323. [Google Scholar] [CrossRef]
- Alkinani, M.H.; Khan, W.Z.; Arshad, Q.; Raza, M. HSDDD: A Hybrid Scheme for the Detection of Distracted Driving through Fusion of Deep Learning and Handcrafted Features. Sensors 2022, 22, 1864. [Google Scholar] [CrossRef] [PubMed]

| Approach | Strengths | Weaknesses |
|---|---|---|
| Irshad et al. [11] | Uses morphological parameters and features extracted from images to improve the accuracy of CNNs. | Requires good methods to extract features. |
| Xia et al. [12] | Uses a novel CNN architecture to improve the accuracy of lymphoma image classification. | Requires a large dataset of lymphoma images to train the CNN. |
| Savas et al. [13] | Uses a CNN-LSTM hybrid model to improve the accuracy of lymphoma image classification. | Requires a large dataset of lymphoma images to train the CNN-LSTM model. |
| Miyoshi et al. [14] | Uses deep learning networks to achieve high accuracy in the classification of lymphoma images with different magnification factors. | Requires a large dataset of lymphoma images with different magnification factors to train the deep learning networks. |
| Li et al. [15] | Uses a novel GOTDP-MP-CNNs network architecture to improve the accuracy of lymphoma image classification. | Requires a large dataset of lymphoma images to train the GOTDP-MP-CNNs network. |
| Syrykh et al. [16] | Uses Bayesian neural networks (BNNs) to improve the accuracy of lymphoma image classification. | Requires a large dataset of lymphoma images to train the BNNs. |
| Zhang et al. [17] | Uses a backpropagation (BP) network and genetic algorithm (GA) to improve the accuracy of lymphoma image classification. | Requires a large dataset of lymphoma images to train the BP-GA network. |
| Reena et al. [18] | Uses a feature-based image retrieval method to improve the accuracy of lymphoma image classification. | Requires a large dataset of lymphoma images to train the feature-based image retrieval method. |
| Zahra et al. [19] | Uses a novel method to discover the best deep learning methods for diagnosing lymphoma images. | Requires a large dataset of lymphoma images to train the deep learning methods. |
| Zhang et al. [20] | Uses a CNN model to achieve high accuracy in the classification of lymphoma images. | Requires a large dataset of lymphoma images to train the CNN model. |
| Swiderska et al. [21] | Uses a CNN model to automate the transfusion of B lymph nodes. | Requires a large dataset of B lymph node images to train the CNN model. |
| Sheng et al. [22] | Uses an R-CNN network to identify lymphocytes from lymphoma images. | Requires a large dataset of lymphoma images to train the R-CNN network. |
| Mohlman et al. [23] | Uses a CNN to distinguish between Burkitt lymphoma and diffuse large B-cell lymphoma (DLBCL). | Requires a large dataset of Burkitt lymphoma and DLBCL images to train the CNN. |
| Gaidano et al. [24] | Develops machine learning networks to diagnose DLBCL images collected from several sources. | Requires a large dataset of DLBCL images collected from several sources to train the machine learning networks. |
| Francisco et al. [25] | Develops a framework for extracting lymphoma biomarkers to determine their value compared to the rest of the nucleus. | Requires a large dataset of lymphoma images to train the framework. |
| Proposed method | Fuses deep learning models with color, texture, and shape features Extracts radiomics features from traditional methods. | Does not require expert input from doctors or a large dataset of lymphoma images to train the hybrid technique. |
| Models | Classes | AUC % | Accuracy % | Precision % | Sensitivity % | Specificity % |
|---|---|---|---|---|---|---|
| MobileNet | Lymph CLL | 95.1 | 94.2 | 93.2 | 94.2 | 97.2 |
| Lymph FL | 93.4 | 92.3 | 93.2 | 92.3 | 96.8 | |
| Lymph MCL | 92.4 | 91.7 | 91.8 | 91.9 | 95.8 | |
| Average ratio | 93.63 | 92.70 | 92.73 | 92.80 | 96.60 | |
| VGG16 | Lymph CLL | 94.3 | 93 | 93.7 | 93.2 | 96.7 |
| Lymph FL | 91.5 | 93.3 | 91.7 | 92.8 | 96.5 | |
| Lymph MCL | 92.6 | 90.7 | 91.7 | 91.2 | 96.2 | |
| Average ratio | 92.80 | 92.30 | 92.37 | 92.40 | 96.47 | |
| AlexNet | Lymph CLL | 92.9 | 92.8 | 94 | 93.2 | 96.8 |
| Lymph FL | 93.1 | 92.3 | 91.4 | 91.7 | 96.2 | |
| Lymph MCL | 91.5 | 90.3 | 90.2 | 90.6 | 94.8 | |
| Average ratio | 92.50 | 91.90 | 91.87 | 91.83 | 95.93 |
| Models | Classes | AUC % | Accuracy % | Precision % | Sensitivity % | Specificity % |
|---|---|---|---|---|---|---|
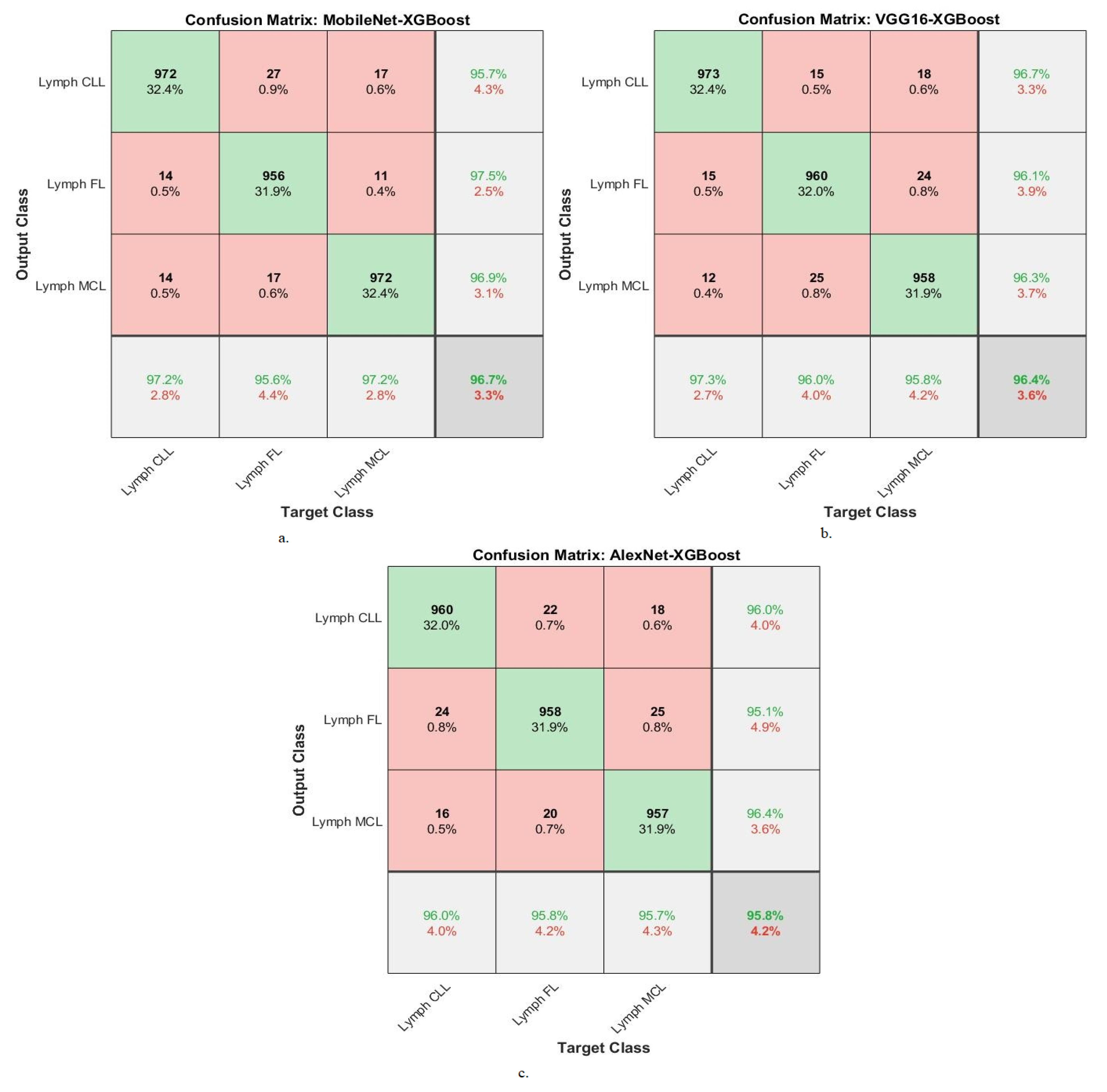
| MobileNet-XGBoost | Lymph CLL | 96.8 | 97.2 | 95.7 | 97.2 | 97.8 |
| Lymph FL | 97.2 | 95.6 | 97.5 | 96.1 | 98.7 | |
| Lymph MCL | 98.2 | 97.2 | 96.9 | 97.3 | 98.2 | |
| Average ratio | 97.40 | 96.70 | 96.70 | 96.87 | 98.23 | |
| VGG16-XGBoost | Lymph CLL | 95.9 | 97.3 | 96.7 | 96.9 | 98.2 |
| Lymph FL | 96.1 | 96 | 96.1 | 96.2 | 97.7 | |
| Lymph MCL | 96.7 | 95.8 | 96.3 | 95.8 | 97.6 | |
| Average ratio | 96.23 | 96.40 | 96.37 | 96.30 | 97.83 | |
| AlexNet-XGBoost | Lymph CLL | 97.3 | 96 | 96 | 95.6 | 98.2 |
| Lymph FL | 96.4 | 95.8 | 95.1 | 96.2 | 97.8 | |
| Lymph MCL | 96.1 | 95.7 | 96.4 | 95.8 | 97.6 | |
| Average ratio | 96.60 | 95.80 | 95.83 | 95.87 | 97.87 |
| Models | Classes | AUC % | Accuracy % | Precision % | Sensitivity % | Specificity % |
|---|---|---|---|---|---|---|
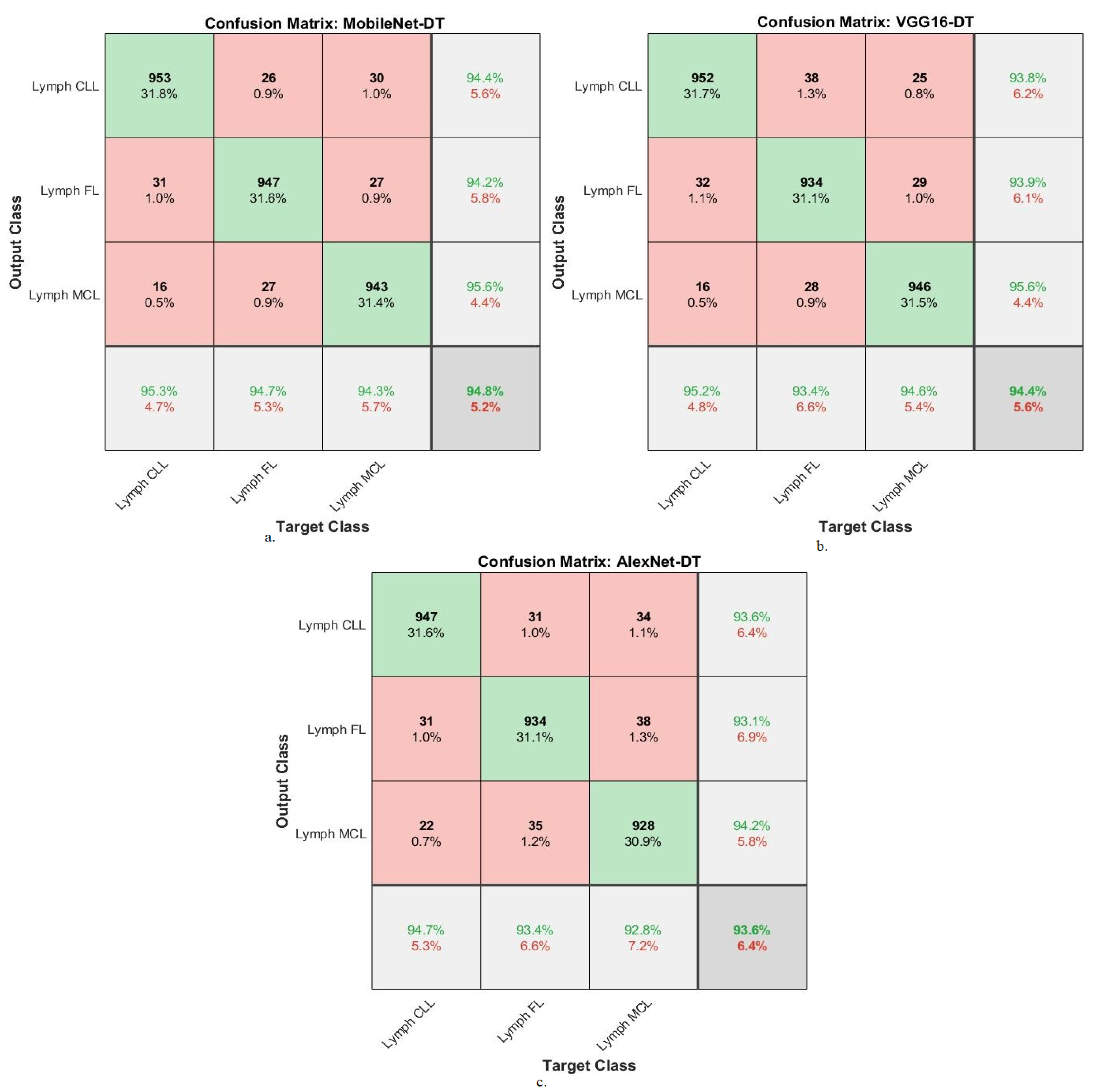
| MobileNet-DT | Lymph CLL | 95.1 | 95.3 | 94.4 | 95.1 | 97.1 |
| Lymph FL | 95.9 | 94.7 | 94.2 | 95.4 | 96.8 | |
| Lymph MCL | 96.5 | 94.3 | 95.6 | 94.3 | 98.2 | |
| Average ratio | 95.83 | 94.80 | 94.73 | 94.93 | 97.37 | |
| VGG16-DT | Lymph CLL | 96.8 | 95.2 | 93.8 | 95.2 | 96.8 |
| Lymph FL | 94.7 | 93.24 | 93.9 | 93.1 | 97.1 | |
| Lymph MCL | 95.8 | 94.6 | 95.6 | 94.8 | 97.6 | |
| Average ratio | 95.77 | 94.40 | 94.43 | 94.37 | 97.17 | |
| AlexNet-DT | Lymph CLL | 96.1 | 94.7 | 93.6 | 94.8 | 97.4 |
| Lymph FL | 95.8 | 93.4 | 93.1 | 93.2 | 96.8 | |
| Lymph MCL | 94.7 | 92.8 | 94.2 | 92.7 | 97.1 | |
| Average ratio | 95.53 | 93.80 | 93.63 | 93.57 | 97.10 |
| Models | Classes | AUC % | Accuracy % | Precision % | Sensitivity % | Specificity % |
|---|---|---|---|---|---|---|
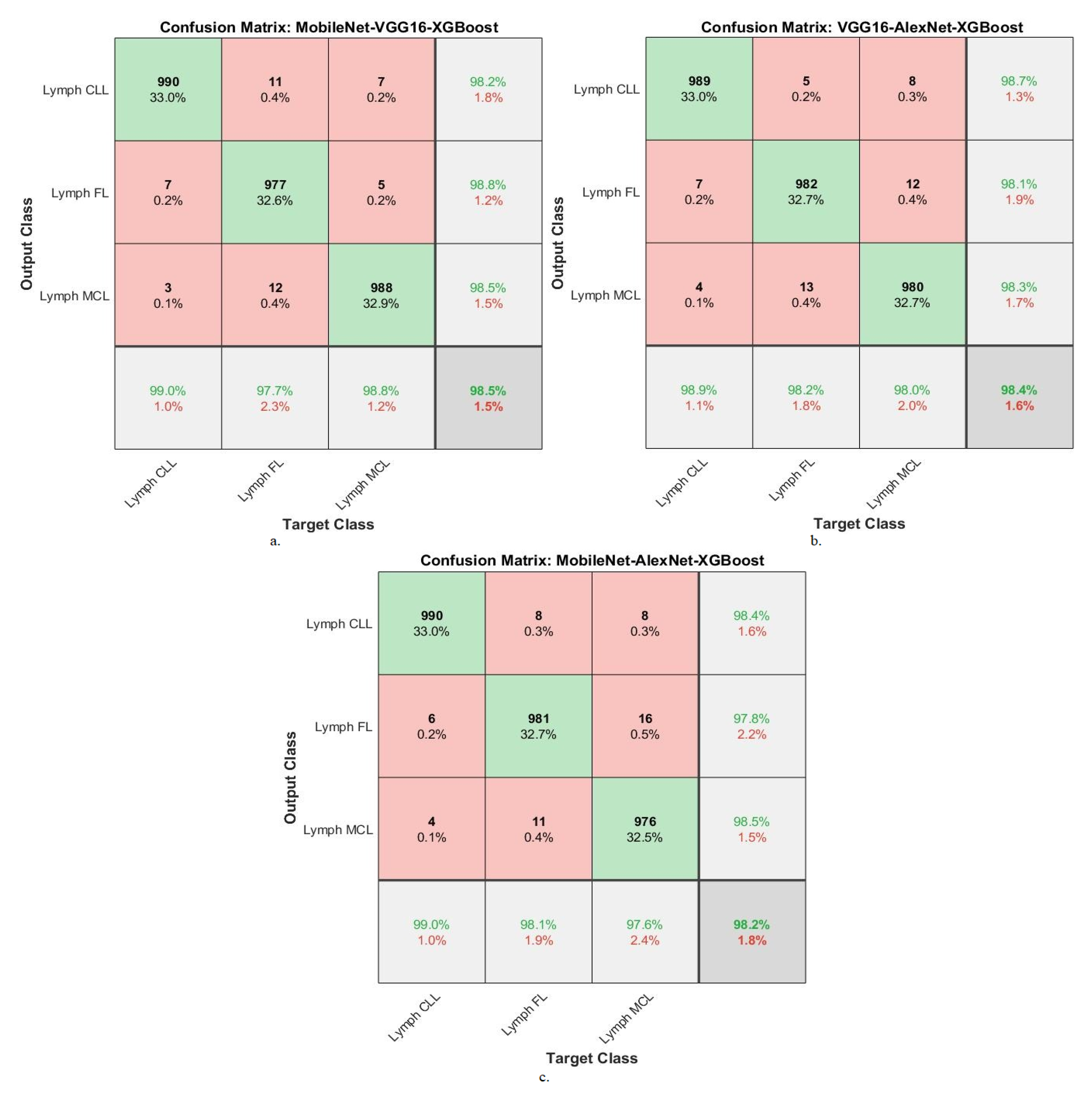
| MobileNet-VGG16-XGBoost | Lymph CLL | 98.7 | 99 | 98.2 | 98.8 | 99.1 |
| Lymph FL | 97.9 | 97.7 | 98.8 | 97.9 | 98.7 | |
| Lymph MCL | 98.2 | 98.8 | 98.5 | 98.5 | 98.6 | |
| Average ratio | 98.27 | 98.50 | 98.50 | 98.40 | 98.80 | |
| VGG16-AlexNet-XGBoost | Lymph CLL | 98.6 | 98.9 | 98.7 | 99.1 | 98.6 |
| Lymph FL | 98.5 | 98.2 | 98.1 | 98.2 | 99.2 | |
| Lymph MCL | 97.8 | 98 | 98.3 | 97.9 | 98.8 | |
| Average ratio | 98.30 | 98.40 | 98.37 | 98.40 | 98.87 | |
| MobileNet-AlexNet-XGBoost | Lymph CLL | 98.7 | 99 | 98.4 | 98.7 | 98.9 |
| Lymph FL | 97.6 | 98.1 | 97.8 | 97.9 | 99.2 | |
| Lymph MCL | 97.1 | 97.6 | 98.5 | 98.2 | 98.7 | |
| Average ratio | 97.80 | 98.20 | 98.23 | 98.27 | 98.93 |
| Models | Classes | AUC % | Accuracy % | Precision % | Sensitivity % | Specificity % |
|---|---|---|---|---|---|---|
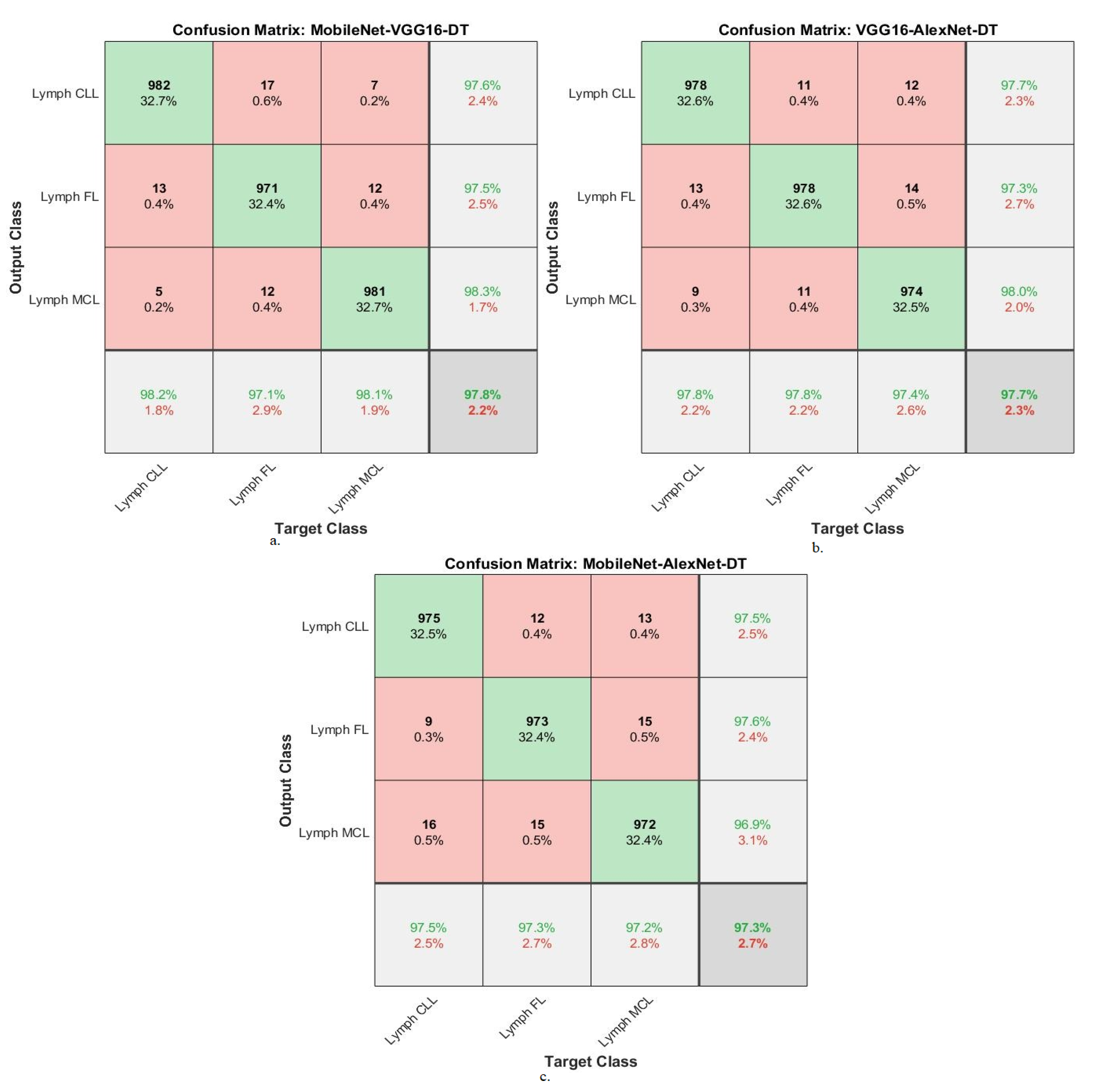
| MobileNet-VGG16-DT | Lymph CLL | 98.1 | 98.2 | 97.6 | 98.2 | 98.7 |
| Lymph FL | 97.9 | 97.1 | 97.5 | 97.1 | 99.1 | |
| Lymph MCL | 98.3 | 98.1 | 98.3 | 98.3 | 98.6 | |
| Average ratio | 98.10 | 97.80 | 97.80 | 97.87 | 98.80 | |
| VGG16-AlexNet-DT | Lymph CLL | 98.3 | 97.8 | 97.7 | 98.2 | 98.9 |
| Lymph FL | 98.6 | 97.8 | 97.3 | 98.1 | 99.3 | |
| Lymph MCL | 97.5 | 97.4 | 98 | 96.8 | 98.7 | |
| Average ratio | 98.13 | 97.70 | 97.67 | 97.70 | 98.97 | |
| MobileNet-AlexNet-DT | Lymph CLL | 98.8 | 97.5 | 97.5 | 97.2 | 98.5 |
| Lymph FL | 98.3 | 97.3 | 97.6 | 96.8 | 99.1 | |
| Lymph MCL | 98.1 | 97.2 | 96.9 | 96.5 | 97.8 | |
| Average ratio | 98.40 | 97.30 | 97.33 | 96.83 | 98.47 |
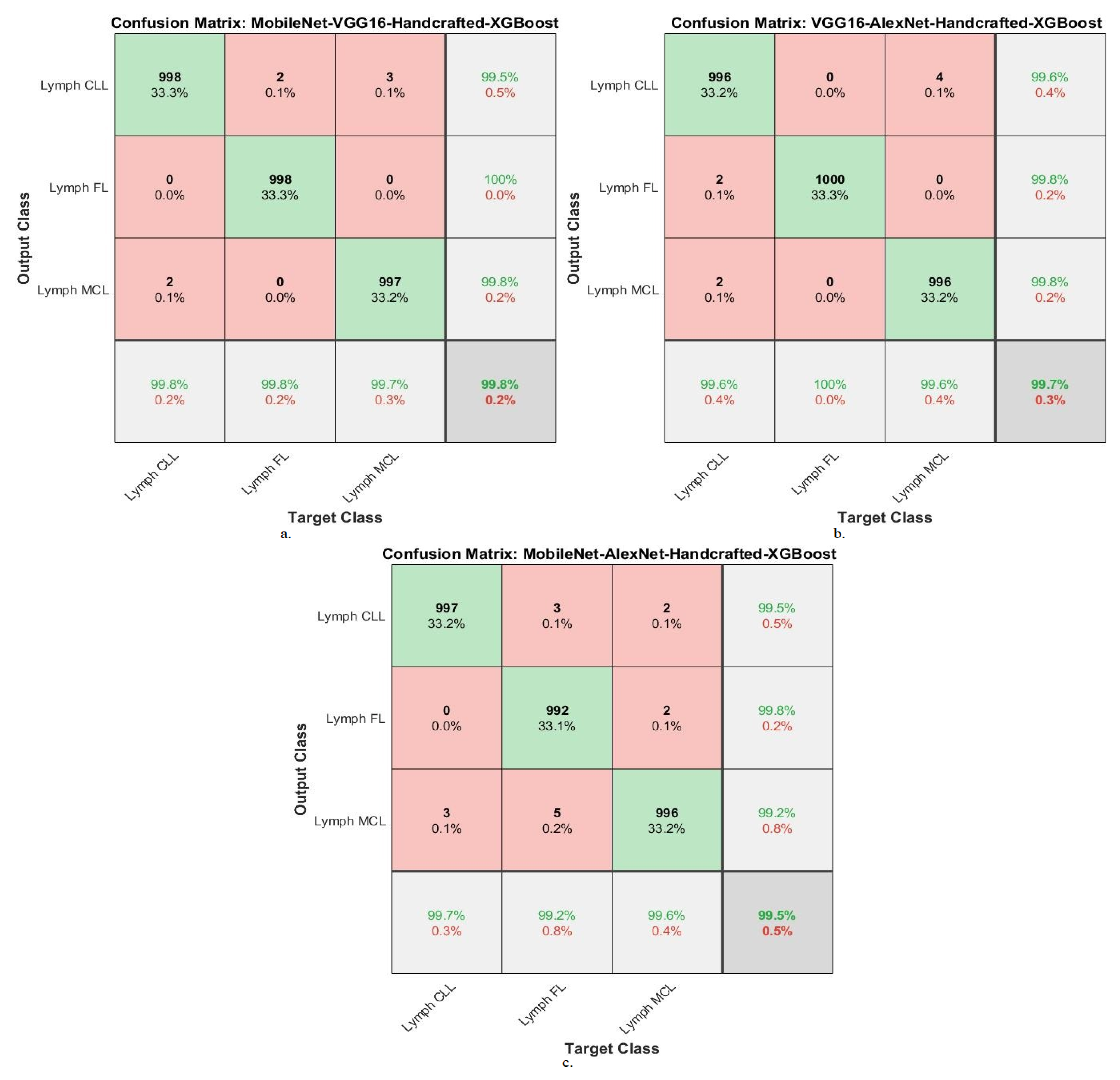
| Classifier | Fusion Features | Classes | AUC % | Accuracy % | Precision % | Sensitivity % | Specificity % |
|---|---|---|---|---|---|---|---|
| XGBoost algorithm | MobileNet-VGG16-Handcrafted | Lymph CLL | 99.2 | 99.8 | 99.5 | 99.7 | 99.9 |
| Lymph FL | 99.5 | 99.8 | 100 | 99.8 | 99.8 | ||
| Lymph MCL | 99.6 | 99.7 | 99.8 | 99.6 | 99.7 | ||
| Average ratio | 99.43 | 99.80 | 99.77 | 99.70 | 99.80 | ||
| VGG16-AlexNet-Handcrafted | Lymph CLL | 99.4 | 99.6 | 99.6 | 99.5 | 99.8 | |
| Lymph FL | 99.6 | 100 | 99.8 | 99.7 | 99.6 | ||
| Lymph MCL | 99.7 | 99.6 | 99.8 | 99.8 | 99.7 | ||
| Average ratio | 99.57 | 99.70 | 99.73 | 99.67 | 99.70 | ||
| MobileNet-AlexNet-Handcrafted | Lymph CLL | 99.6 | 99.7 | 99.5 | 99.5 | 99.8 | |
| Lymph FL | 99.1 | 99.2 | 99.8 | 99.1 | 99.7 | ||
| Lymph MCL | 99.5 | 99.6 | 99.2 | 99.7 | 99.6 | ||
| Average ratio | 99.40 | 99.50 | 99.50 | 99.43 | 99.70 |
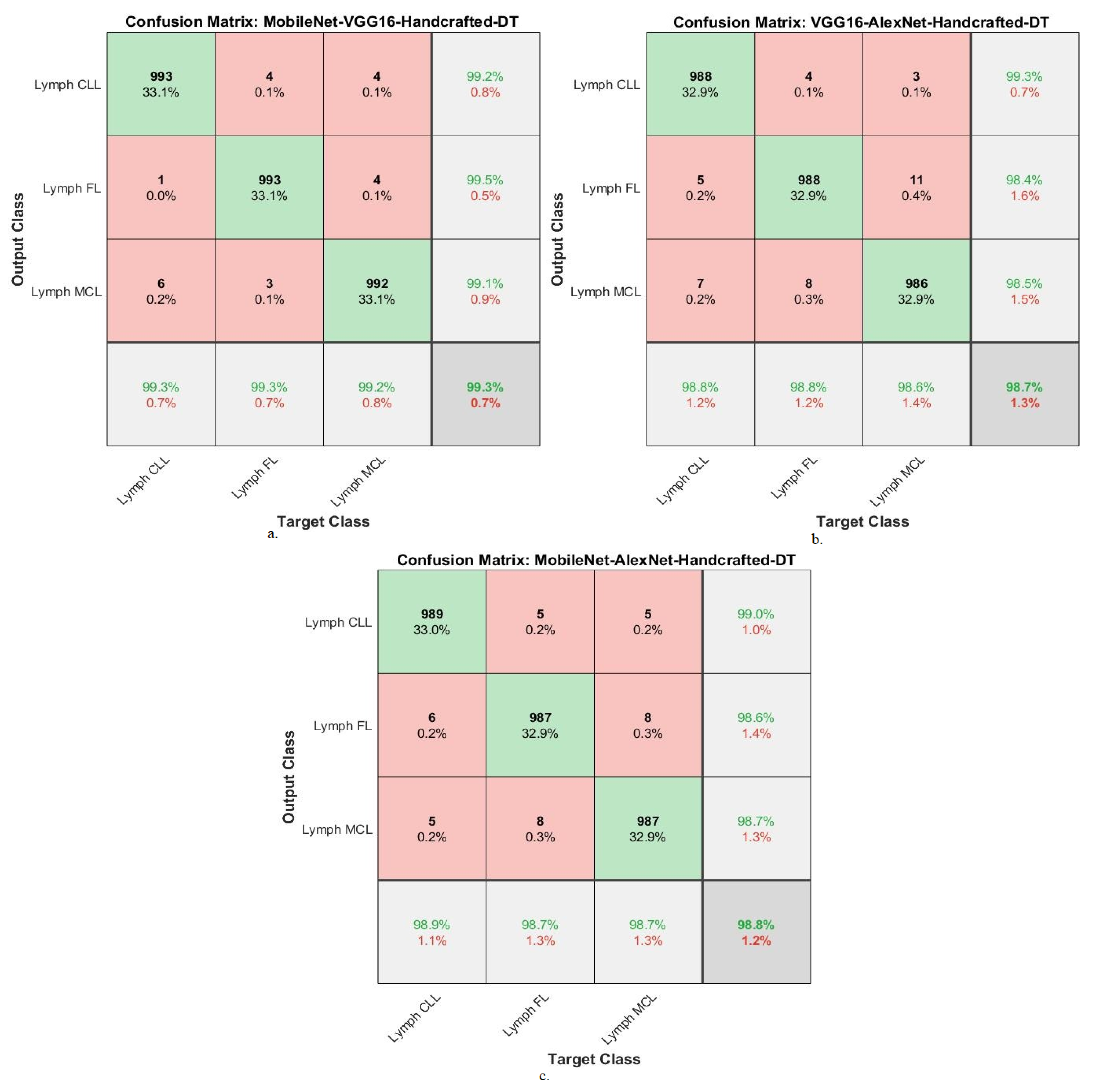
| Classifier | Fusion Features | Classes | AUC % | Accuracy % | Precision % | Sensitivity % | Specificity % |
|---|---|---|---|---|---|---|---|
| DT algorithm | MobileNet-VGG16-Handcrafted | Lymph CLL | 99.5 | 99.3 | 99.2 | 99.1 | 99.5 |
| Lymph FL | 99.4 | 99.3 | 99.5 | 99.2 | 99.8 | ||
| Lymph MCL | 99.2 | 99.2 | 99.1 | 98.8 | 99.7 | ||
| Average ratio | 99.37 | 99.30 | 99.27 | 99.03 | 99.67 | ||
| VGG16-AlexNet-Handcrafted | Lymph CLL | 98.9 | 98.8 | 99.3 | 99.2 | 99.8 | |
| Lymph FL | 98.5 | 98.8 | 98.4 | 99.1 | 99.1 | ||
| Lymph MCL | 98.2 | 98.6 | 98.5 | 98.7 | 98.7 | ||
| Average ratio | 98.53 | 98.70 | 98.73 | 99.00 | 99.20 | ||
| MobileNet-AlexNet-Handcrafted | Lymph CLL | 99.2 | 98.9 | 99 | 99.2 | 98.8 | |
| Lymph FL | 98.9 | 98.7 | 98.6 | 98.9 | 99.2 | ||
| Lymph MCL | 99 | 98.7 | 98.7 | 99.1 | 99.4 | ||
| Average ratio | 99.03 | 98.80 | 98.77 | 99.07 | 99.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamdi, M.; Senan, E.M.; Jadhav, M.E.; Olayah, F.; Awaji, B.; Alalayah, K.M. Hybrid Models Based on Fusion Features of a CNN and Handcrafted Features for Accurate Histopathological Image Analysis for Diagnosing Malignant Lymphomas. Diagnostics 2023, 13, 2258. https://doi.org/10.3390/diagnostics13132258
Hamdi M, Senan EM, Jadhav ME, Olayah F, Awaji B, Alalayah KM. Hybrid Models Based on Fusion Features of a CNN and Handcrafted Features for Accurate Histopathological Image Analysis for Diagnosing Malignant Lymphomas. Diagnostics. 2023; 13(13):2258. https://doi.org/10.3390/diagnostics13132258
Chicago/Turabian StyleHamdi, Mohammed, Ebrahim Mohammed Senan, Mukti E. Jadhav, Fekry Olayah, Bakri Awaji, and Khaled M. Alalayah. 2023. "Hybrid Models Based on Fusion Features of a CNN and Handcrafted Features for Accurate Histopathological Image Analysis for Diagnosing Malignant Lymphomas" Diagnostics 13, no. 13: 2258. https://doi.org/10.3390/diagnostics13132258
APA StyleHamdi, M., Senan, E. M., Jadhav, M. E., Olayah, F., Awaji, B., & Alalayah, K. M. (2023). Hybrid Models Based on Fusion Features of a CNN and Handcrafted Features for Accurate Histopathological Image Analysis for Diagnosing Malignant Lymphomas. Diagnostics, 13(13), 2258. https://doi.org/10.3390/diagnostics13132258








