Association of NK Cells with the Severity of Fibrosis in Patients with Chronic Hepatitis C
Abstract
:1. Introduction
2. Materials and Methods
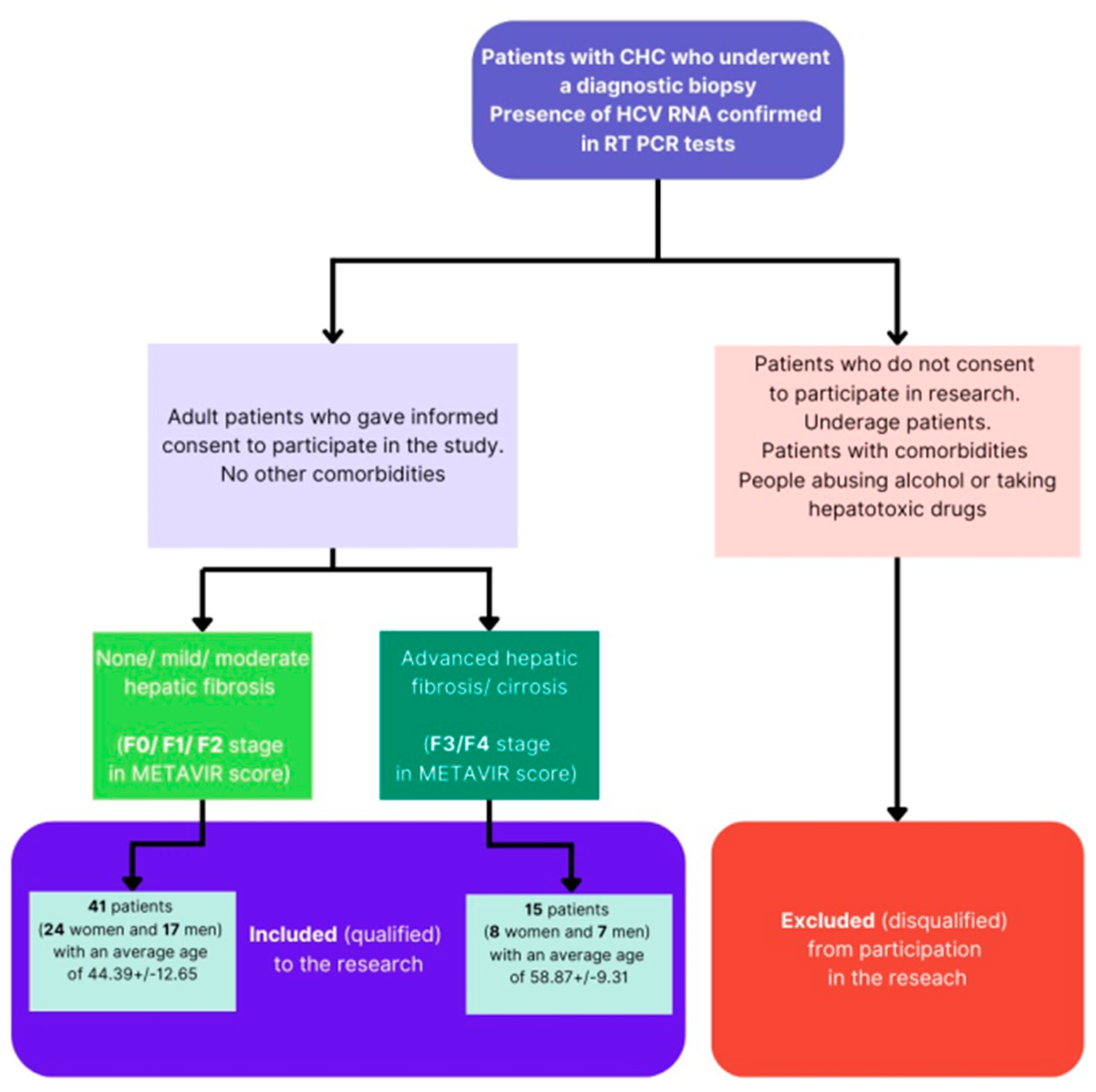
2.1. Patient Characteristics
2.2. Tissue Material
2.2.1. Histological Preparation
2.2.2. Histopathological Evaluation
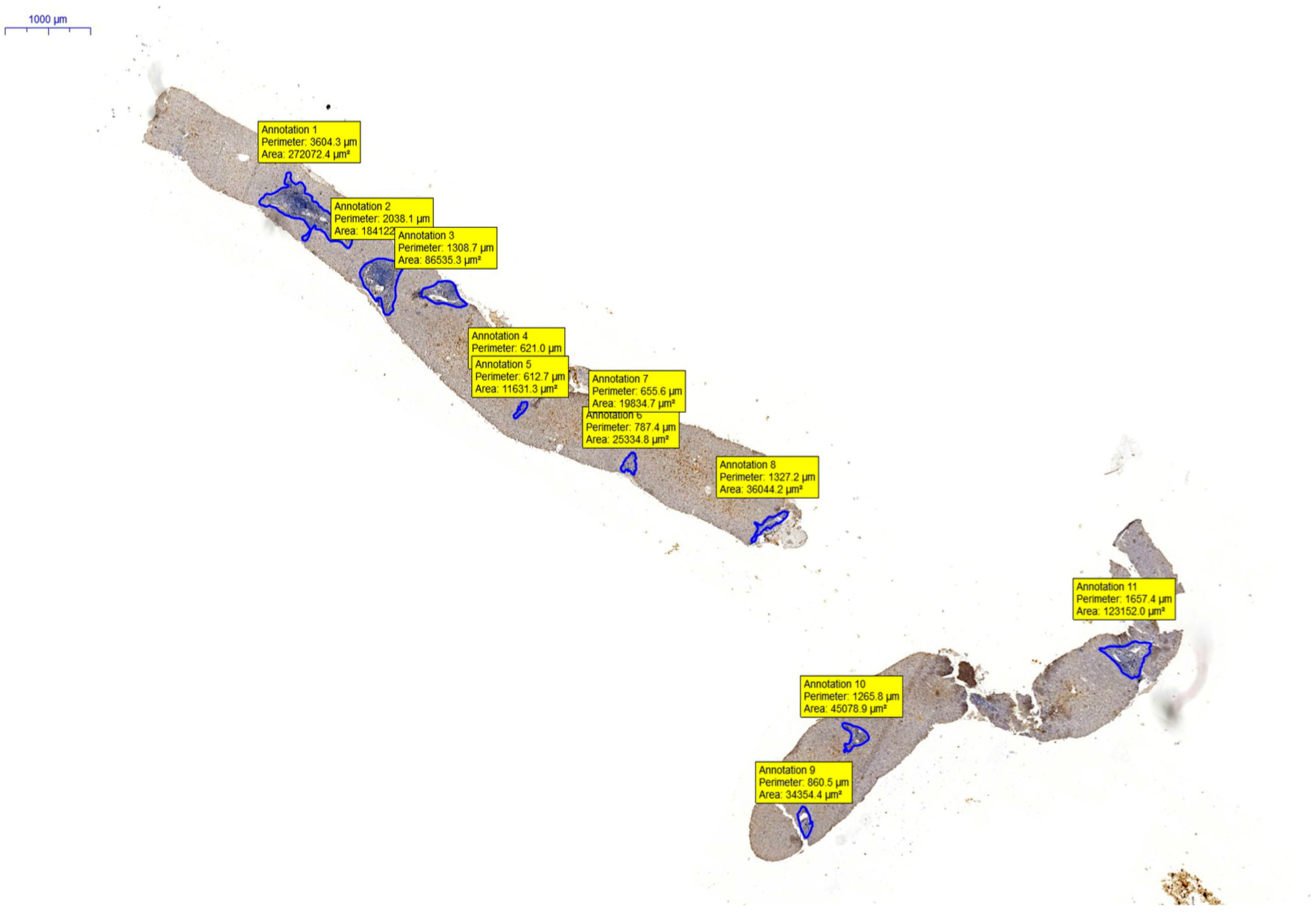
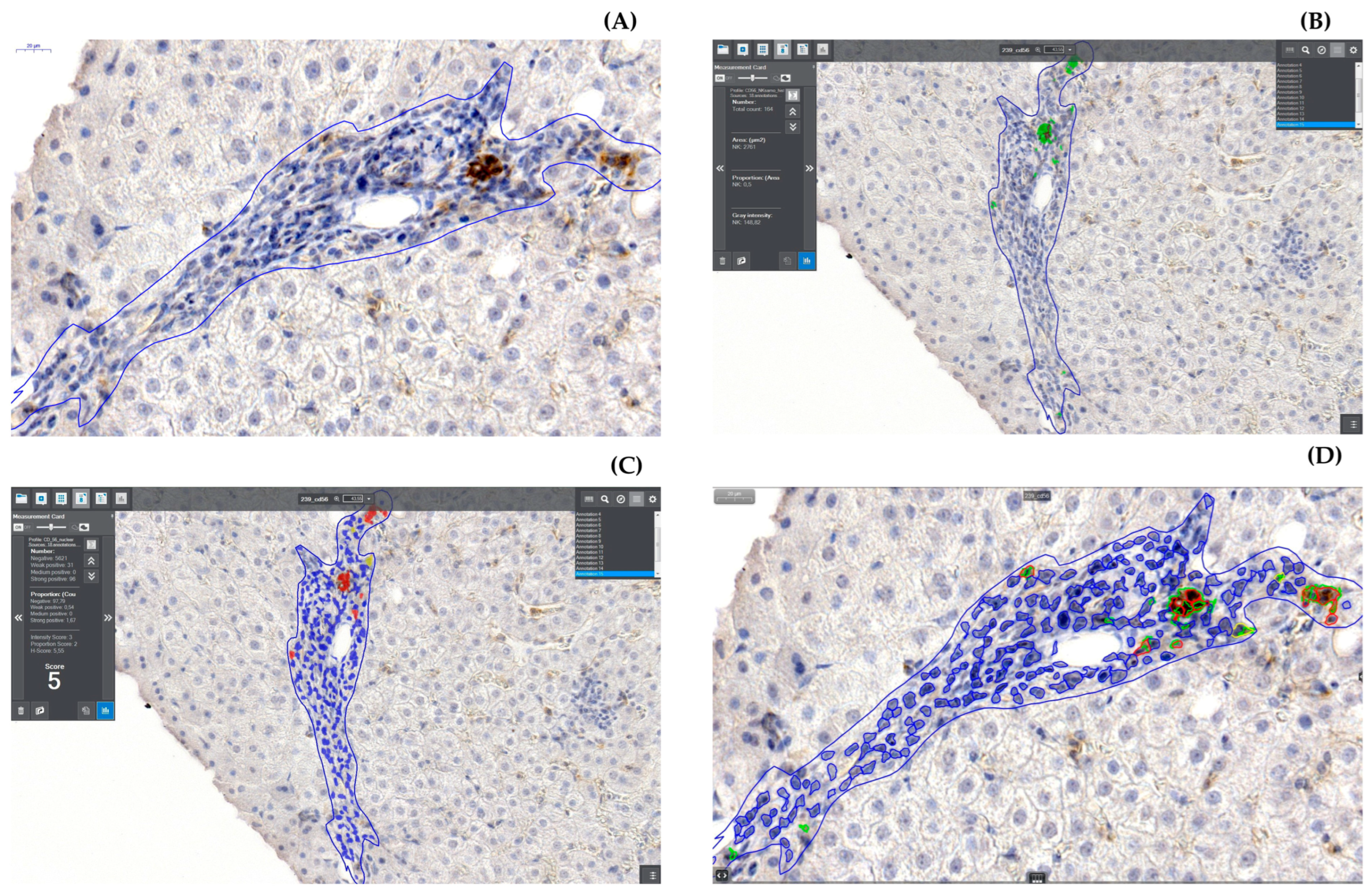
2.2.3. Digitization and Digital Analysis
2.3. Cytofluorometric Analysis
2.4. Statistical Analysis
- For unrelated quantitative variables, after checking the test assumptions (Shapiro–Wilk test, Levene test) using parametric tests (t-tests), non-parametric tests (Mann–Whitney U test) were used if the assumptions were not met.
- For qualitative variables, the following tests were used: Pearson’s Chi2 and Maximum Likelihood.
- The direction and strength of the association between the two quantitative variables was assessed using the regression equation and Pearson’s linear correlation coefficient or Spearman’s R nonparametric correlation.
- In all analyses, p < 0.05 was considered significant. Each subgroup of patients was analyzed separately, the groups were not intermixed.
3. Results
3.1. Analysis of Natural Killer Cell Phenotypes in Whole Blood within Groups
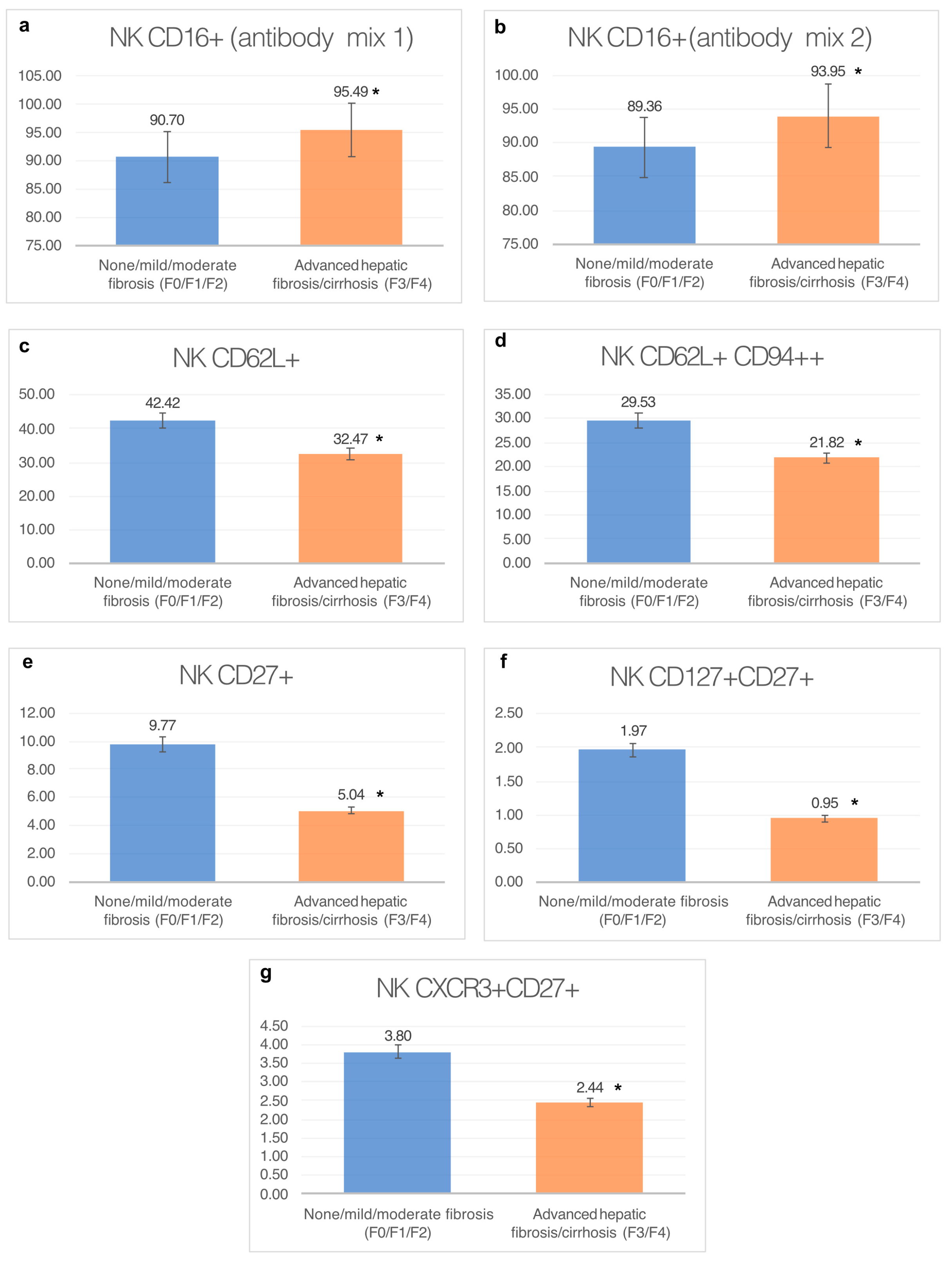
3.1.1. Percentage of CD16+ Natural Killer Cells (Antibody Mix 1)
3.1.2. Percentage of NKCD16+ Natural Killer Cells (Antibody Mix 2)
3.1.3. Percentage of CD62L+ Natural Killer Cells
3.1.4. Percentage of CD62L+ CD94++ Cells
3.1.5. Percentage of CD27+ Cells
3.1.6. Percentage of CD127+ and CD27+ Natural Killer Cells
3.1.7. Percentage of CXCR3+ CD27+ Cells
3.2. Analysis of Intrahepatic NK Cells in Groups
3.3. Analysis of the Correlation between the Percentage of NK Cells in Whole Peripheral Blood and the Liver
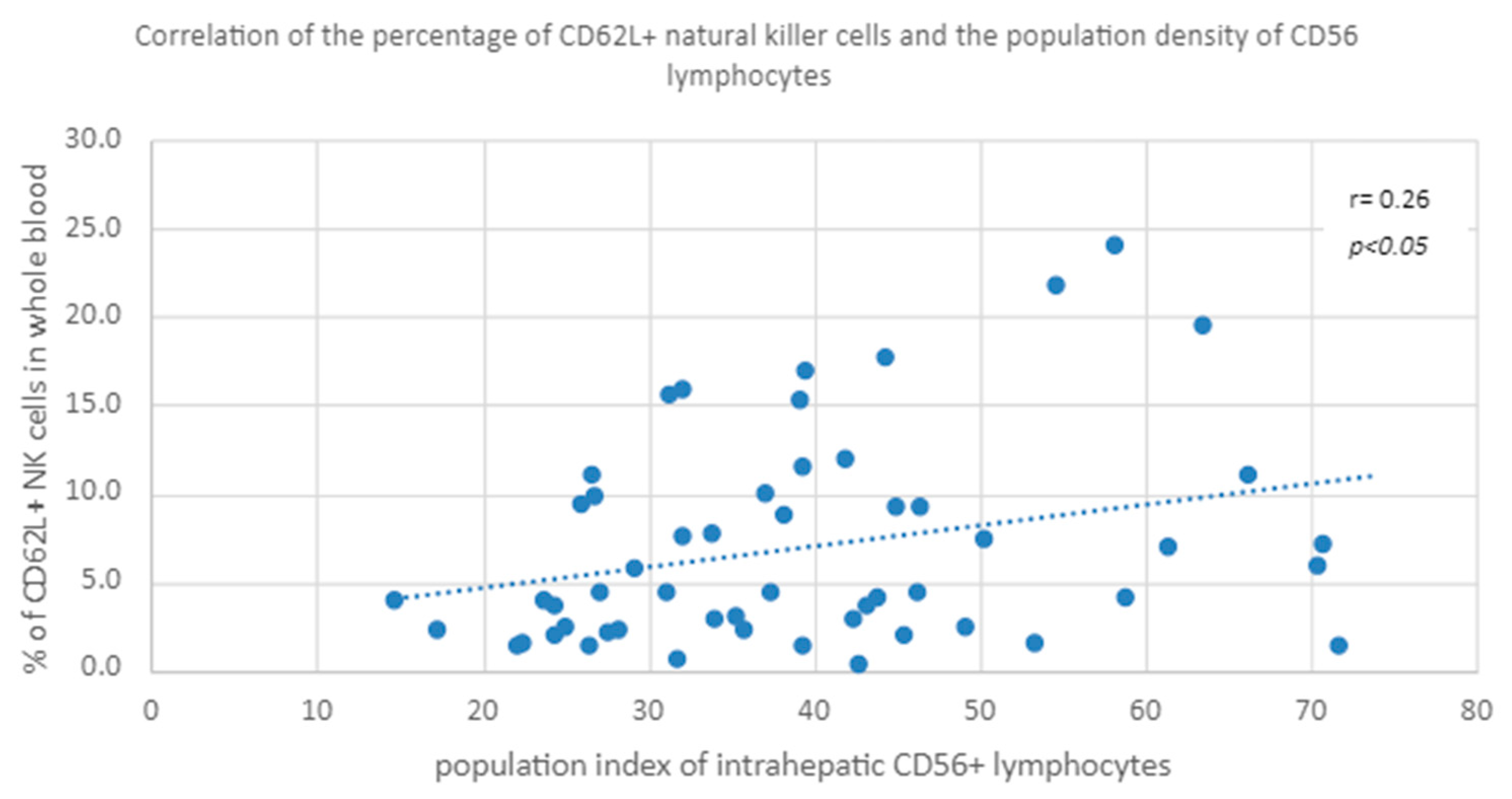
3.3.1. Peripheral Blood CD62L+ NK Percentage vs. NK Cell Population Density in the Liver
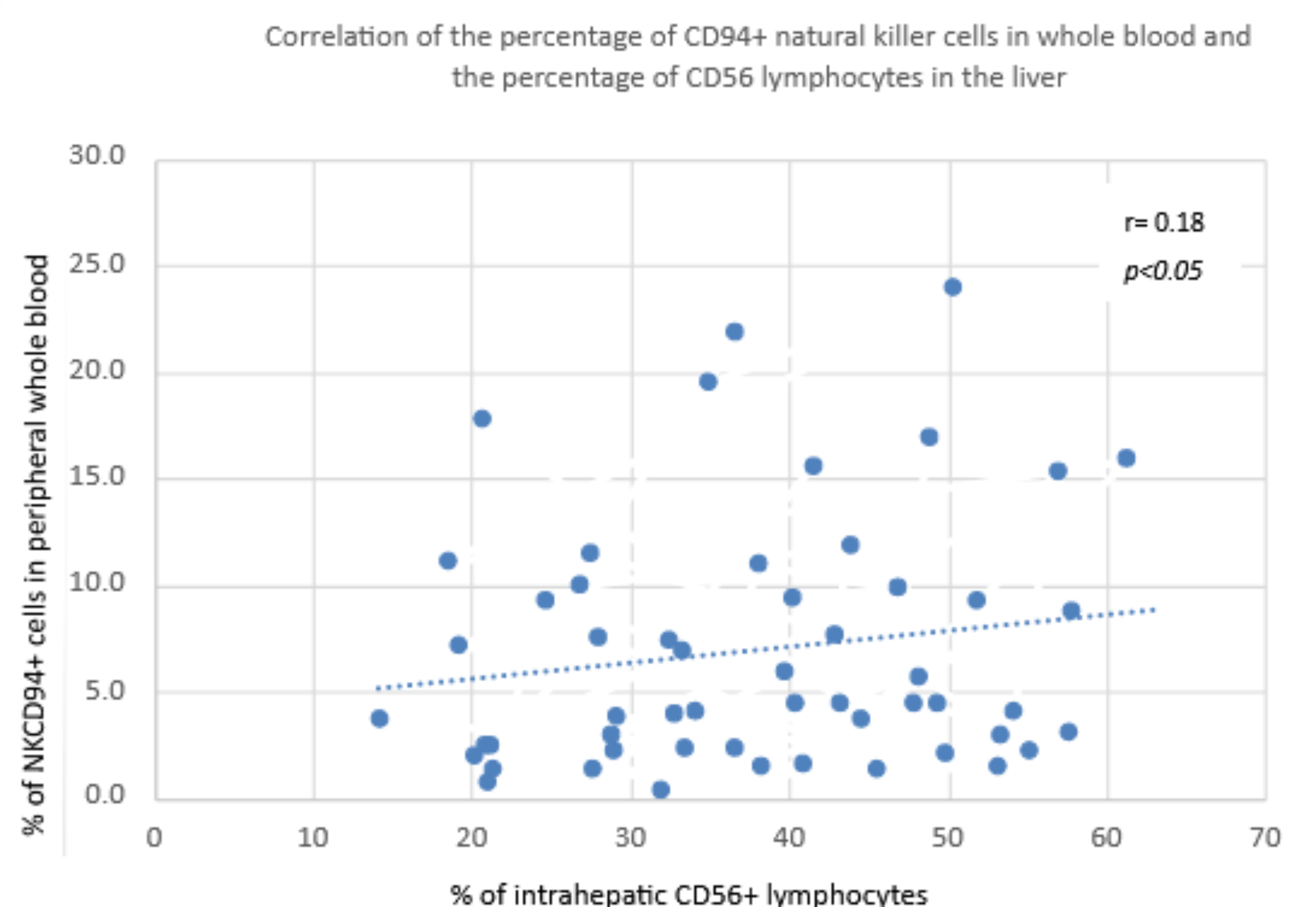
3.3.2. Percentage of CD94+ NK Cells in Peripheral Blood vs. Percentage of CD56 NK Cells in the Liver
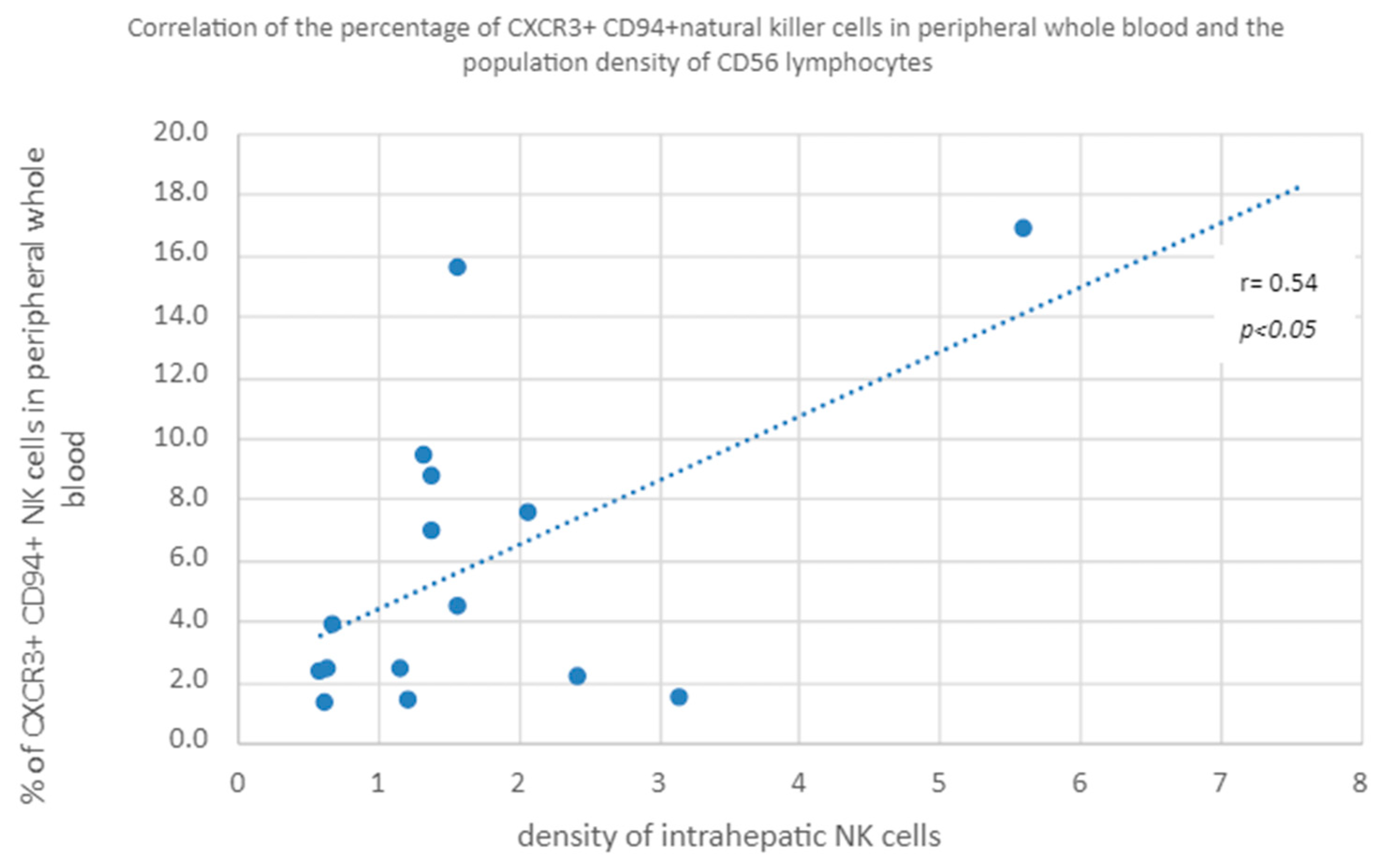
3.3.3. Percentage of CXCR3+ and CD94+ Natural Killer Cells in Peripheral Whole Blood vs. Population Density of CD56 Lymphocytes in the Liver
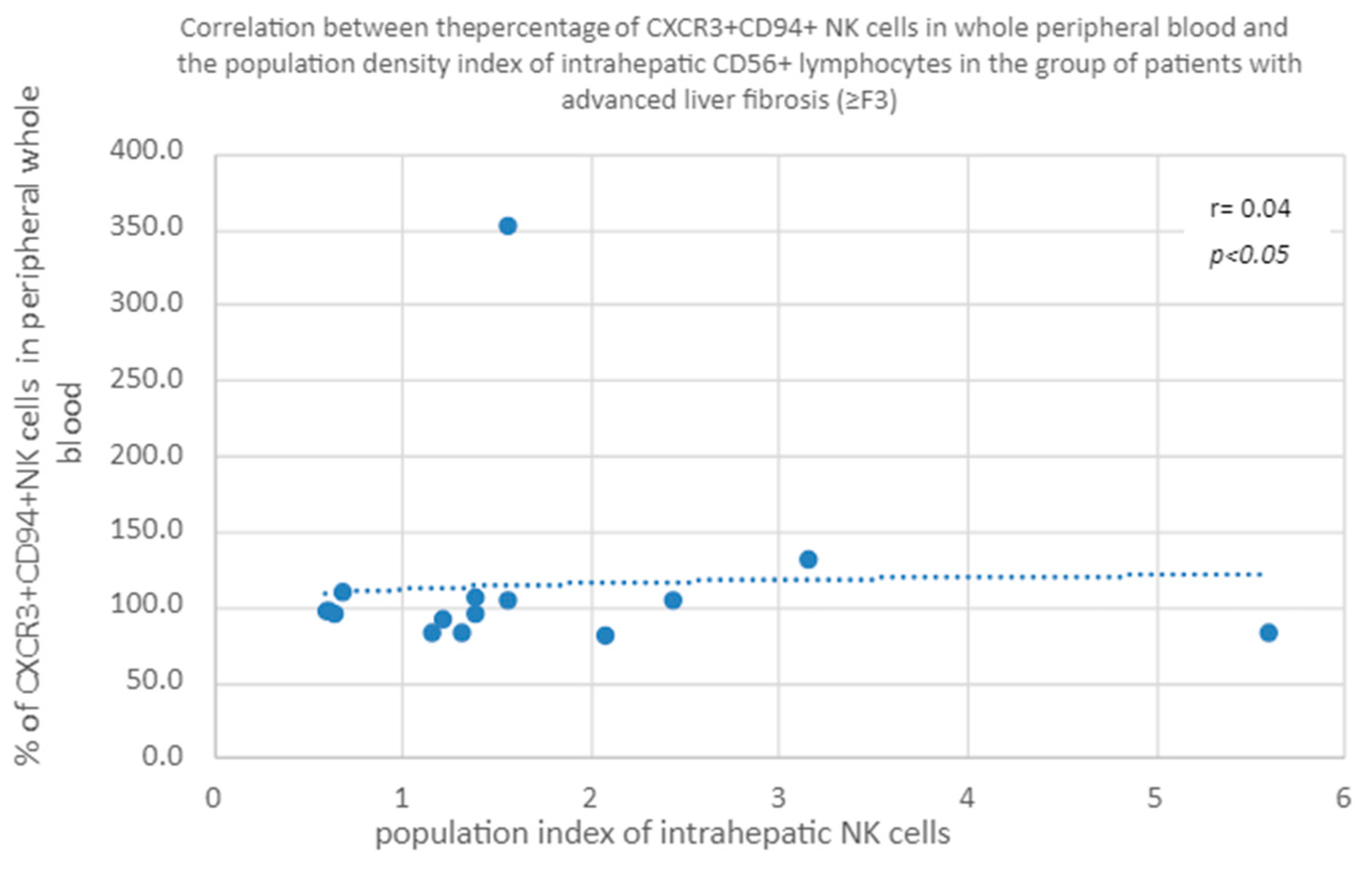
3.3.4. Correlation of CXCR3+ CD94+ NK Percentage in Whole Peripheral Blood a Percentage of Intrahepatic CD56+ Lymphocytes in the Group of Patients with Advanced Liver Fibrosis/Cirrhosis (≥F3)
3.3.5. Correlation of the Percentage of CXCR3+CD94+ in Peripheral Blood and the Population Index of Intrahepatic CD56+ Lymphocytes in the Group of Patients with Advanced Liver Fibrosis (≥F3)
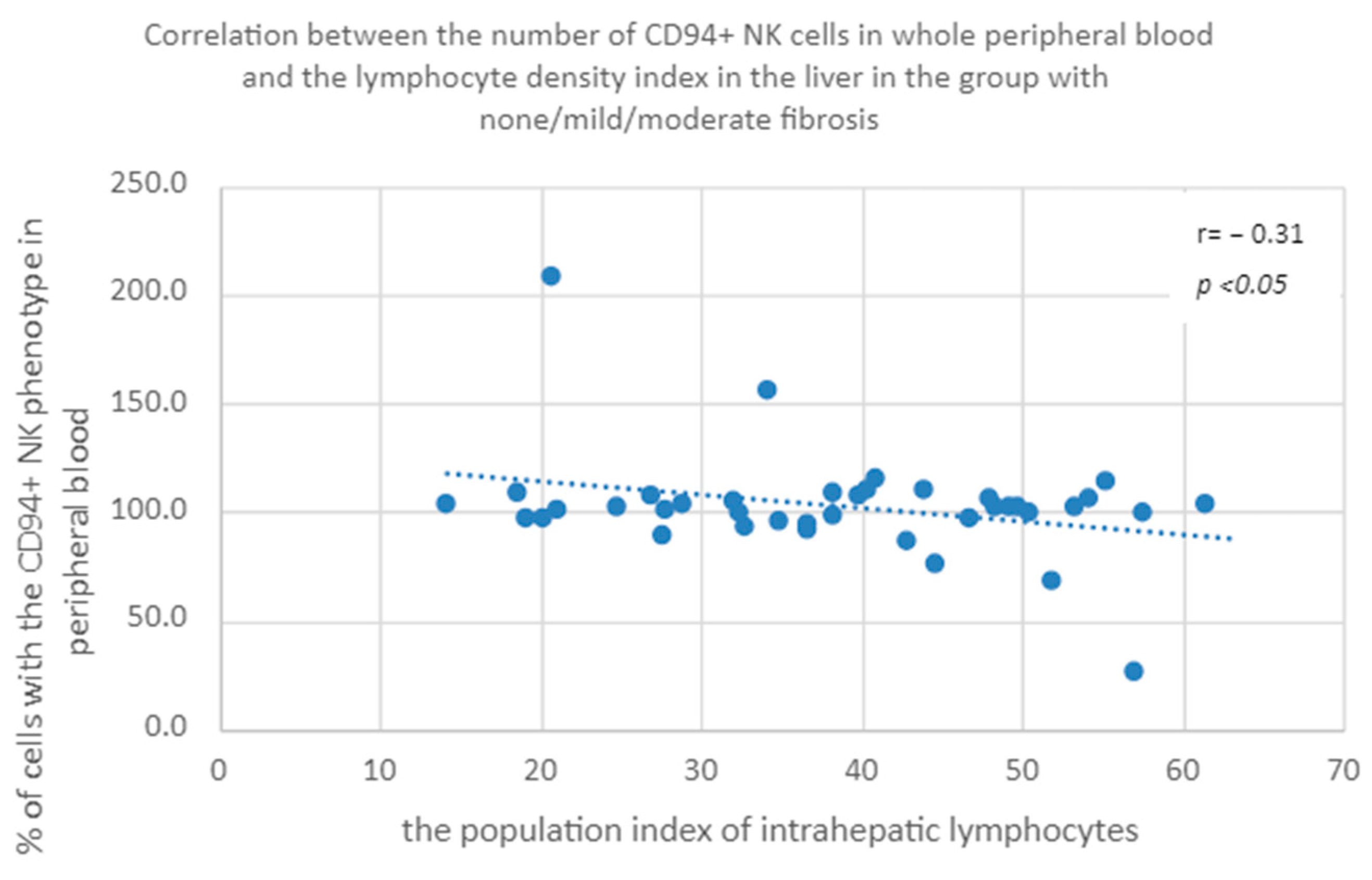
3.3.6. Percentage of CD94+ Natural Killer Cells in Peripheral Whole Blood a Population Index of Intrahepatic Lymphocytes in the Group of Patients with None/Mild/Moderate Fibrosis
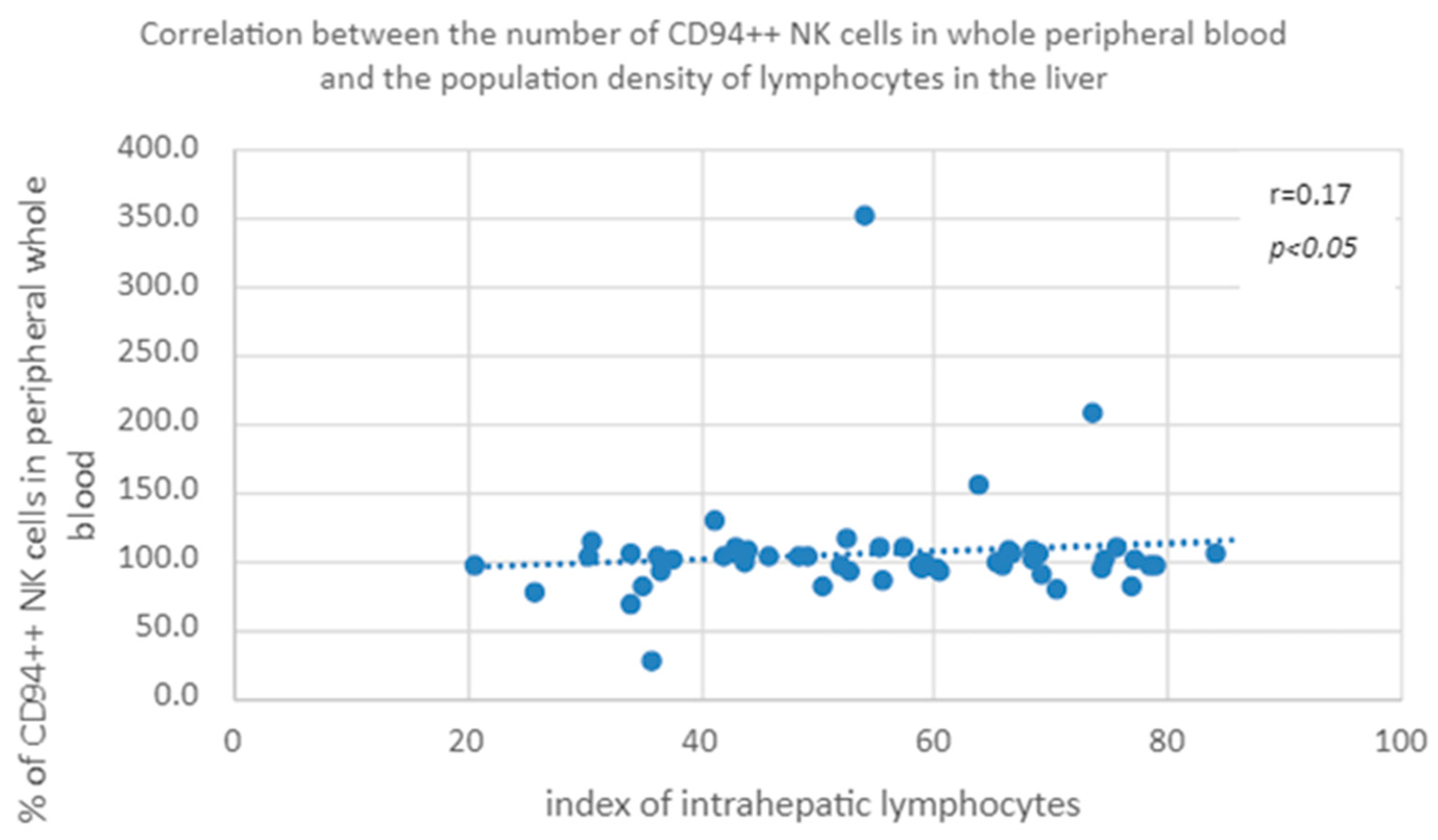
3.3.7. Correlation of the Percentage of Cells with the CD94++ NK Phenotype in Peripheral Blood and the Population Index of Intrahepatic Lymphocytes in the Group of Patients with None/Mild/Moderate Fibrosis
4. Discussion
5. Conclusions
- Patients with CHC with advanced liver fibrosis ≥ F3 have a higher percentage of the total population of CD56+ CD16+ NK cells in peripheral blood compared to patients with mild or moderate fibrosis.
- There is a higher percentage of NK cells with CD62L+, CD94+, CD27+, CD127+ and CXCR3+ phenotypes in the peripheral blood of patients with mild or moderate fibrosis in relation to patients with advanced liver fibrosis or cirrhosis. This indicates a lower availability of functionally active NK cells in the peripheral blood of patients with advanced fibrosis.
- In patients with CHC, there is a positive correlation between the percentage of NK cells with CD62L+ and CD62L+ CD94+ phenotypes in peripheral blood and the population index of intrahepatic NK cells, regardless of the extent of fibrosis.
- The percentage of NK cells with the CXCR3+ CD94+ phenotype in the peripheral blood was correlated with the population index and the percentage of NK cells in the liver, with the correlation increasing depending on the degree of fibrosis.
- In patients with CHC with advanced liver fibrosis, there is intrahepatic accumulation of functionally impaired natural killer cells, thus limiting their influence on further progression of fibrosis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brunner, N.; Bruggmann, P. Trends of the Global Hepatitis C Disease Burden: Strategies to Achieve Elimination. J. Prev. Med. Public Health 2021, 54, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Modin, L.; Arshad, A.; Wilkes, B.; Benselin, J.; Lloyd, C.; Irving, W.L.; Kelly, D.A. Epidemiology and natural history of hepatitis C virus infection among children and young people. J. Hepatol. 2018, 70, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Raciborski, F.; Gujski, M.; Kłak, A.; Gierczyński, J. HCV w Polsce; Raport Instytutu Ochrony Zdrowia: Warszawa, Poland, 2015. [Google Scholar]
- Fujita, T.; Narumiya, S. Roles of hepatic stellate cells in liver inflammation: A new perspective. Inflamm. Regen. 2016, 36, 1. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M. Histological Grading and Staging of Chronic Hepatitis. Clin. Liver Dis. 2021, 17, 222–226. [Google Scholar] [CrossRef]
- Goodman, Z.D. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J. Hepatol. 2007, 47, 598–607. [Google Scholar] [CrossRef]
- Bedossa, P.; Poynard, T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 1996, 24, 289–293. [Google Scholar] [CrossRef]
- Scheuer, P.J. Classification of chronic viral hepatitis: A need for reassessment. J. Hepatol. 1991, 13, 372–374. [Google Scholar] [CrossRef]
- Rinaldi, L.; Nascimbeni, F.; Giordano, M.; Masetti, C.; Guerrera, B.; Amelia, A.; Fascione, M.C.; Ballestri, S.; Romagnoli, D.; Zampino, R.; et al. Clinical features and natural history of cryptogenic cirrhosis compared to hepatitis C virus-related cirrhosis. World J. Gastroenterol. 2017, 23, 1458–1468. [Google Scholar] [CrossRef]
- Khatun, M.; Ray, R.B. Mechanisms Underlying Hepatitis C Virus-Associated Hepatic Fibrosis. Cells 2019, 8, 1249. [Google Scholar] [CrossRef]
- Gabriel, A.; Mazur, B.; Kukla, M.; Źiółkowski, A.; Szczygieł, B.; Tomaszek, K. Hepatocyte steatosis in HCV patients promotes fibrosis by enhancing TGF-beta liver expression. Hepatol Res. 2008, 38, 141–146. [Google Scholar]
- Rios, D.A.; Casciato, P.C.; Caldirola, M.S.; Gaillard, M.I.; Giadans, C.; Ameigeiras, B.; De Matteo, E.N.; Preciado, M.V.; Valva, P. Chronic Hepatitis C Pathogenesis: Immune Response in the Liver Microenvironment and Peripheral Compartment. Front. Cell. Infect. Microbiol. 2021, 11, 712105. [Google Scholar] [CrossRef] [PubMed]
- Poznanski, S.M.; Ashkar, A.A. What Defines NK Cell Functional Fate: Phenotype or Metabolism? Front. Immunol. 2019, 10, 1414. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Courtney, A.N.; Jena, B.; Heczey, A.; Liu, D.; Marinova, E.; Guo, L.; Xu, X.; Torikai, H.; Mo, Q.; et al. CD62L+ NKT cells have prolonged persistence and antitumor activity in vivo. J. Clin. Investig. 2016, 126, 2341–2355. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Mao, H.C.; Wei, M.; Hughes, T.; Zhang, J.; Park, I.-K.; Liu, S.; McClory, S.; Marcucci, G.; Trotta, R.; et al. CD94 surface density identifies a functional intermediary between the CD56bright and CD56dim human NK-cell subsets. Blood 2010, 115, 274–281. [Google Scholar] [CrossRef]
- Arachchige, A.S.P.M. Human NK cells: From development to effector functions. J. Endotoxin Res. 2021, 27, 212–229. [Google Scholar] [CrossRef]
- Ali, A.; Canaday, L.M.; Feldman, H.A.; Cevik, H.; Moran, M.T.; Rajaram, S.; Lakes, N.; Tuazon, J.A.; Seelamneni, H.; Krishnamurthy, D.; et al. Natural killer cell immunosuppressive function requires CXCR3-dependent redistribution within lymphoid tissues. J. Clin. Investig. 2021, 131, e146686. [Google Scholar] [CrossRef]
- Lapa, D.; Garbuglia, A.R.; Capobianchi, M.R.; Del Porto, P. Hepatitis C Virus Genetic Variability, Human Immune Response, and Genome Polymorphisms: Which Is the Interplay? Cells 2019, 8, 305. [Google Scholar] [CrossRef]
- Robinson, M.W.; Harmon, C.; O’farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell. Mol. Immunol. 2016, 13, 267–276. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, J.; Wang, L.; Wu, X.; Chen, Y.; Piao, H.; Lu, L.; Jiang, W.; Xu, Y.; Feng, B.; et al. New classification of liver biopsy assessment for fibrosis in chronic hepatitis B patients before and after treatment. Hepatology 2016, 65, 1438–1450. [Google Scholar] [CrossRef]
- Tseng, C.-T.K.; Klimpel, G.R. Binding of the Hepatitis C Virus Envelope Protein E2 to CD81 Inhibits Natural Killer Cell Functions. J. Exp. Med. 2001, 195, 43–49. [Google Scholar] [CrossRef]
- Fénéant, L.; Levy, S.; Cocquerel, L. CD81 and Hepatitis C Virus (HCV) Infection. Viruses 2014, 6, 535–572. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, J.; Jiang, Y. Negative Regulation and Protective Function of Natural Killer Cells in HIV Infection: Two Sides of a Coin. Front. Immunol. 2022, 13, 842831. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Liu, L.; Sun, C. The Dynamic Role of NK Cells in Liver Cancers: Role in HCC and HBV Associated HCC and Its Therapeutic Implications. Front. Immunol. 2022, 13, 887186. [Google Scholar] [CrossRef]
- Njiomegnie, G.F.; Read, S.A.; Fewings, N.; George, J.; McKay, F.; Ahlenstiel, G. Immunomodulation of the Natural Killer Cell Phenotype and Response during HCV Infection. J. Clin. Med. 2020, 9, 1030. [Google Scholar] [CrossRef] [PubMed]
- Campos-Murguía, A.; Ruiz-Margáin, A.; A González-Regueiro, J.; Macías-Rodríguez, R.U. Clinical assessment and management of liver fibrosis in non-alcoholic fatty liver disease. World J. Gastroenterol. 2020, 26, 5919–5943. [Google Scholar] [CrossRef]
- World Health Organization, New Recommendations in the Updated WHO Guidelines for the Screening, Care and Treatment of Persons with Chronic Hepatitis C Infection; Policy Brief; World Health Organization: Geneva, Switzerland, 2016.
- Petz, D.; Klauck, S.; Röhl, F.-W.; Malfertheiner, P.; Roessner, A.; Röcken, C. Feasibility of histological grading and staging of chronic viral hepatitis using specimens obtained by thin-needle biopsy. Virchows Arch. 2003, 442, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.J.; Salama, M.E.; Henricks, W.H.; Pantanowitz, L. Implementation of Whole Slide Imaging for Clinical Purposes: Issues to Consider from the Perspective of Early Adopters. Arch. Pathol. Lab. Med. 2017, 141, 944–959. [Google Scholar] [CrossRef]
- Jiang, K.; Mohammad, M.K.; Dar, W.A.; Kong, J.; Farris, A.B. Quantitative assessment of liver fibrosis by digital image analysis reveals correlation with qualitative clinical fibrosis staging in liver transplant patients. PLoS ONE 2020, 15, e0239624. [Google Scholar] [CrossRef]
- Bonorino, P.; Ramzan, M.; Camous, X.; Dufeu-Duchesne, T.; Thélu, M.-A.; Sturm, N.; Dariz, A.; Guillermet, C.; Pernollet, M.; Zarski, J.-P.; et al. Fine characterization of intrahepatic NK cells expressing natural killer receptors in chronic hepatitis B and C. J. Hepatol. 2009, 51, 458–467. [Google Scholar] [CrossRef]
- Tosello-Trampont, A.; Surette, F.A.; Ewald, S.E.; Hahn, Y.S. Immunoregulatory Role of NK Cells in Tissue Inflammation and Regeneration. Front. Immunol. 2017, 8, 301. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Shen, W.; Wang, B.; Yuan, X. Crosstalk between NK cells and hepatic stellate cells in liver fibrosis (Review). Mol. Med. Rep. 2022, 25, 208. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, C.; Berger, C.T.; Kroy, D.C.; Cheney, P.C.; Ghebremichael, M.; Aneja, J.; Tomlinson, M.; Kim, A.Y.; Lauer, G.; Alter, G. Chronic HCV Infection Affects the NK Cell Phenotype in the Blood More than in the Liver. PLoS ONE 2014, 9, e105950. [Google Scholar] [CrossRef] [PubMed]
- Holder, K.A.; Stapleton, S.N.; Gallant, M.E.; Russell, R.S.; Grant, M.D. Hepatitis C Virus–Infected Cells Downregulate NKp30 and Inhibit Ex Vivo NK Cell Functions. J. Immunol. 2013, 191, 3308–3318. [Google Scholar] [CrossRef]
- Muhanna, N.; Doron, S.; Wald, O.; Horani, A.; Eid, A.; Pappo, O.; Friedman, S.L.; Safadi, R. Activation of hepatic stellate cells after phagocytosis of lymphocytes: A novel pathway of fibrogenesis. Hepatology 2008, 48, 963–977. [Google Scholar] [CrossRef] [PubMed]
- Langhans, B.; Alwan, A.W.; Krämer, B.; Glässner, A.; Lutz, P.; Strassburg, C.P.; Nattermann, J.; Spengler, U. Regulatory CD4+ T cells modulate the interaction between NK cells and hepatic stellate cells by acting on either cell type. J. Hepatol. 2015, 62, 398–404. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Tacke, F. Interleukin-33 in the pathogenesis of liver fibrosis: Alarming ILC2 and hepatic stellate cells. Cell. Mol. Immunol. 2016, 14, 143–145. [Google Scholar] [CrossRef]
- Noel, G.; Arshad, M.I.; Filliol, A.; Genet, V.; Rauch, M.; Lucas-Clerc, C.; Lehuen, A.; Girard, J.-P.; Piquet-Pellorce, C.; Samson, M. Ablation of interaction between IL-33 and ST2+ regulatory T cells increases immune cell-mediated hepatitis and activated NK cell liver infiltration. Am. J. Physiol. Liver Physiol. 2016, 311, G313–G323. [Google Scholar] [CrossRef]
- Claassen, M.A.; de Knegt, R.J.; Tilanus, H.W.; Janssen, H.L.; Boonstra, A. Abundant numbers of regulatory T cells localize to the liver of chronic hepatitis C infected patients and limit the extent of fibrosis. J. Hepatol. 2010, 52, 315–321. [Google Scholar] [CrossRef]
- Wu, K.-J.; Qian, Q.-F.; Zhou, J.-R.; Sun, D.-L.; Duan, Y.-F.; Zhu, X.; Sartorius, K.; Lu, Y.-J. Regulatory T cells (Tregs) in liver fibrosis. Cell Death Discov. 2023, 9, 53. [Google Scholar] [CrossRef]
- Jiménez-Sousa, M.A.; Berenguer, J.; Rallón, N.; Pineda-Tenor, D.; Aldamiz-Echevarria, T.; Soriano, V.; García-Álvarez, M.; Vazquez-Morón, S.; Restrepo, C.; Carrero, A.; et al. IL15 polymorphism is associated with advanced fibrosis, inflammation-related biomarkers and virological response in human immunodeficiency virus/hepatitis C virus coinfection. Liver Int. 2016, 36, 1258–1266. [Google Scholar] [CrossRef]
- Gabriel, A.; Mazur, B.; Kukla, M.; Ziółkowski, A.; Szczygieł, B.; Tomaszek, K. Związek komórek NK i subpopulacji limfocytów z postępem włóknienia w przebiegu przewlekłego zapalenia wątroby typu C. Gastroenterol. Pol. 2009, 16, 278–283. [Google Scholar]
- Peng, H.; Sun, R.; Tang, L.; Wei, H.; Tian, Z. CD62L Is Critical for Maturation and Accumulation of Murine Hepatic NK Cells in Response to Viral Infection. J. Immunol. 2013, 190, 4255–4262. [Google Scholar] [CrossRef] [PubMed]
- Bourayou, E.; Golub, R. Inflammatory-driven NK cell maturation and its impact on pathology. Front. Immunol. 2022, 13, 1061959. [Google Scholar] [CrossRef] [PubMed]
- Cichocki, F.; Grzywacz, B.; Miller, J.S. Human NK Cell Development: One Road or Many? Front. Immunol. 2019, 10, 2078. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wei, H.; Wei, H.; Gao, Y.; Xu, L.; Yin, W.; Sun, R.; Tian, Z. Blocking the Natural Killer Cell Inhibitory Receptor NKG2A Increases Activity of Human Natural Killer Cells and Clears Hepatitis B Virus Infection in Mice. Gastroenterology 2013, 144, 392–401. [Google Scholar] [CrossRef]
- Vossen, M.T.M.; Matmati, M.; Hertoghs, K.M.L.; Baars, P.A.; Gent, M.-R.; Leclercq, G.; Hamann, J.; Kuijpers, T.W.; van Lier, R.A.W. CD27 Defines Phenotypically and Functionally Different Human NK Cell Subsets. J. Immunol. 2008, 180, 3739–3745. [Google Scholar] [CrossRef]
- Silva, A.; Andrews, D.M.; Brooks, A.G.; Smyth, M.J.; Hayakawa, Y. Application of CD27 as a marker for distinguishing human NK cell subsets. Int. Immunol. 2008, 20, 625–630. [Google Scholar] [CrossRef]
- Ran, G.H.; Lin, Y.Q.; Tian, L.; Zhang, T.; Yan, D.M.; Yu, J.H.; Deng, Y.C. Natural killer cell homing and trafficking in tissues and tumors: From biology to application. Signal Transduct. Target. Ther. 2022, 7, 205. [Google Scholar] [CrossRef]
- De Colvenaer, V.; Taveirne, S.; Delforche, M.; De Smedt, M.; Vandekerckhove, B.; Taghon, T.; Boon, L.; Plum, J.; Leclercq, G. CD27-deficient mice show normal NK-cell differentiation but impaired function upon stimulation. Immunol. Cell Biol. 2011, 89, 803–811. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Ahmad, F.; Wedemeyer, H.; Cornberg, M.; Wiesch, J.S.Z.; van Lunzen, J.; Sarin, S.K.; Schmidt, R.E.; Meyer-Olson, D. Increased CD56bright NK cells in HIV-HCV co-infection and HCV mono-infection are associated with distinctive alterations of their phenotype. Virol. J. 2016, 13, 67. [Google Scholar] [CrossRef]
- Lima, M.; Leander, M.; Santos, M.; Santos, A.H.; Lau, C.; Queirós, M.L.; Gonçalves, M.; Fonseca, S.; Moura, J.; Teixeira, M.D.A.; et al. Chemokine Receptor Expression on Normal Blood CD56+NK-Cells Elucidates Cell Partners That Comigrate during the Innate and Adaptive Immune Responses and Identifies a Transitional NK-Cell Population. J. Immunol. Res. 2015, 2015, 839684. [Google Scholar] [CrossRef] [PubMed]
- Argirion, I.; Pfeiffer, R.M.; Lam, T.K.; O’brien, T.R.; Yu, K.; McGlynn, K.A.; Petrick, J.L.; Pinto, L.; Chen, C.-J.; Lee, M.-H.; et al. Association between immunologic markers and cirrhosis in individuals with chronic hepatitis B. Sci. Rep. 2021, 11, 21194. [Google Scholar] [CrossRef]
- Cao, S.; Liu, M.; Sehrawat, T.S.; Shah, V.H. Regulation and functional roles of chemokines in liver diseases. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 630–647. [Google Scholar] [CrossRef] [PubMed]
- Eisenhardt, M.; Glässner, A.; Krämer, B.; Körner, C.; Sibbing, B.; Kokordelis, P.; Nischalke, H.D.; Sauerbruch, T.; Spengler, U.; Nattermann, J. The CXCR3(+)CD56Bright Phenotype Characterizes a Distinct NK Cell Subset with Anti-Fibrotic Potential That Shows Dys-Regulated Activity in Hepatitis C. PLoS ONE 2012, 7, e38846. [Google Scholar] [CrossRef]
- Nel, I.; Lucar, O.; Petitdemange, C.; Béziat, V.; Lapalus, M.; Bédossa, P.; Debré, P.; Asselah, T.; Marcellin, P.; Vieillard, V. Accumulation of Intrahepatic TNF-α-Producing NKp44+ NK Cells Correlates with Liver Fibrosis and Viral Load in Chronic HCV Infection. Medicine 2016, 95, e3678. [Google Scholar] [CrossRef] [PubMed]
- Kamm, D.R.; McCommis, K.S. Hepatic stellate cells in physiology and pathology. J. Physiol. 2022, 600, 1825–1837. [Google Scholar] [CrossRef]
- Fugier, E.; Marche, H.; Thelu, M.A.; Macek Jilkova, Z.; Van Campenhout, N.; Dufeu-Duchesne, T.; Leroy, V.; Zarski, J.P.; Sturm, N.; Marche, P.N.; et al. Functions of Liver Natural Killer Cells Are Dependent on the Severity of Liver Inflammation and Fibrosis in Chronic Hepatitis C. PLoS ONE 2014, 9, e95614. [Google Scholar] [CrossRef]
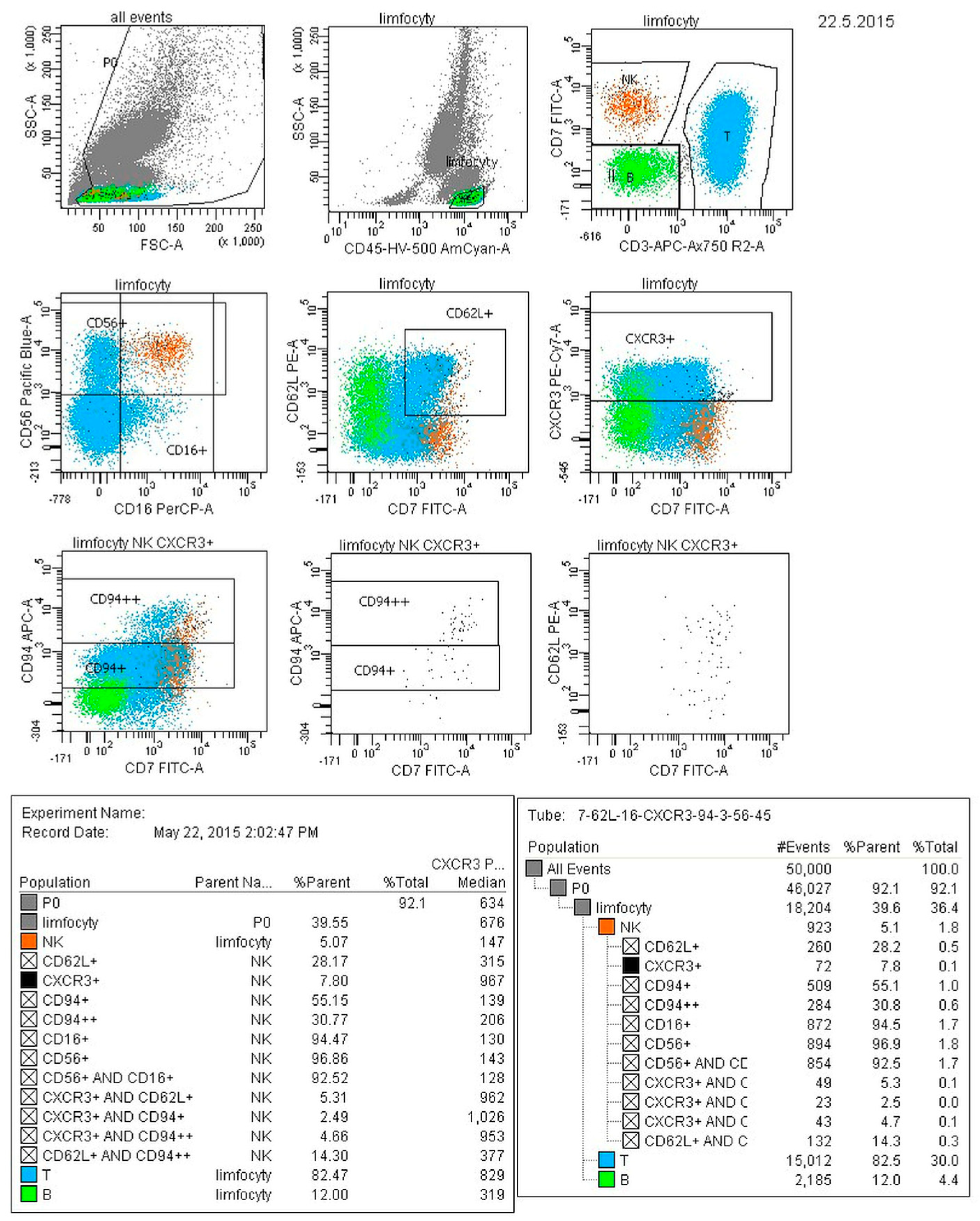

| FITC | PE | PerCP-Cy5.5 | PE-Cy7 | APC | APC-Ax750 | Pacific Blue | V500 | |
|---|---|---|---|---|---|---|---|---|
| 1 | CD7 | CD62L | CD16 | CXCR3 | CD94 | CD3 | CD56 | CD45 |
| 2 | CD7 | CD127 | CD16 | CXCR3 | CD27 | CD3 | CD56 | CD45 |
| NK CD16+ | n | Mean Value ± Standard Deviation | |
|---|---|---|---|
| None/mild/moderate fibrosis (F0/F1/F2) | antibody mix 1 | 41 | 90.70 ± 6.69 * |
| antibody mix 2 | 89.36 ± 6.84 * | ||
| Advanced hepatic fibrosis/cirrhosis (F3/F4) | antibody mix 1 | 15 | 95.49 ± 1.87 * |
| antibody mix 2 | 93.95 ± 3.56 * | ||
| Total in the group | antibody mix 1 | 56 | 91.98 ± 6.17 |
| antibody mix 2 | 90.64 ± 6.42 | ||
| Group of Patients | n | Analyzed Parameter | Mean Value ± Standard Deviation |
|---|---|---|---|
| None/mild/moderate fibrosis (F0/F1/F2) | 41 | % of intrahepatic CD56 cells | 8.12 ± 0.10 |
| population index of intrahepatic CD56 | 7.02 ± 6.12 | ||
| Advanced hepatic fibrosis/cirrhosis (F3/F4) | 15 | % of intrahepatic CD56 cells | 5.61 ± 0.05 |
| population index of intrahepatic CD56 | 5.82 ± 5.05 | ||
| Total in the group | 56 | % of intrahepatic CD56 cells | 7.45 ± 0.09 |
| population index of intrahepatic CD56 | 6.69 ± 5.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleczka, A.; Mazur, B.; Tomaszek, K.; Gabriel, A.; Dzik, R.; Kabała-Dzik, A. Association of NK Cells with the Severity of Fibrosis in Patients with Chronic Hepatitis C. Diagnostics 2023, 13, 2187. https://doi.org/10.3390/diagnostics13132187
Kleczka A, Mazur B, Tomaszek K, Gabriel A, Dzik R, Kabała-Dzik A. Association of NK Cells with the Severity of Fibrosis in Patients with Chronic Hepatitis C. Diagnostics. 2023; 13(13):2187. https://doi.org/10.3390/diagnostics13132187
Chicago/Turabian StyleKleczka, Anna, Bogdan Mazur, Krzysztof Tomaszek, Andrzej Gabriel, Radosław Dzik, and Agata Kabała-Dzik. 2023. "Association of NK Cells with the Severity of Fibrosis in Patients with Chronic Hepatitis C" Diagnostics 13, no. 13: 2187. https://doi.org/10.3390/diagnostics13132187
APA StyleKleczka, A., Mazur, B., Tomaszek, K., Gabriel, A., Dzik, R., & Kabała-Dzik, A. (2023). Association of NK Cells with the Severity of Fibrosis in Patients with Chronic Hepatitis C. Diagnostics, 13(13), 2187. https://doi.org/10.3390/diagnostics13132187







