Identifying Optical Coherence Tomography Markers for Multiple Sclerosis Diagnosis and Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Clinical and Paraclinical Assessment
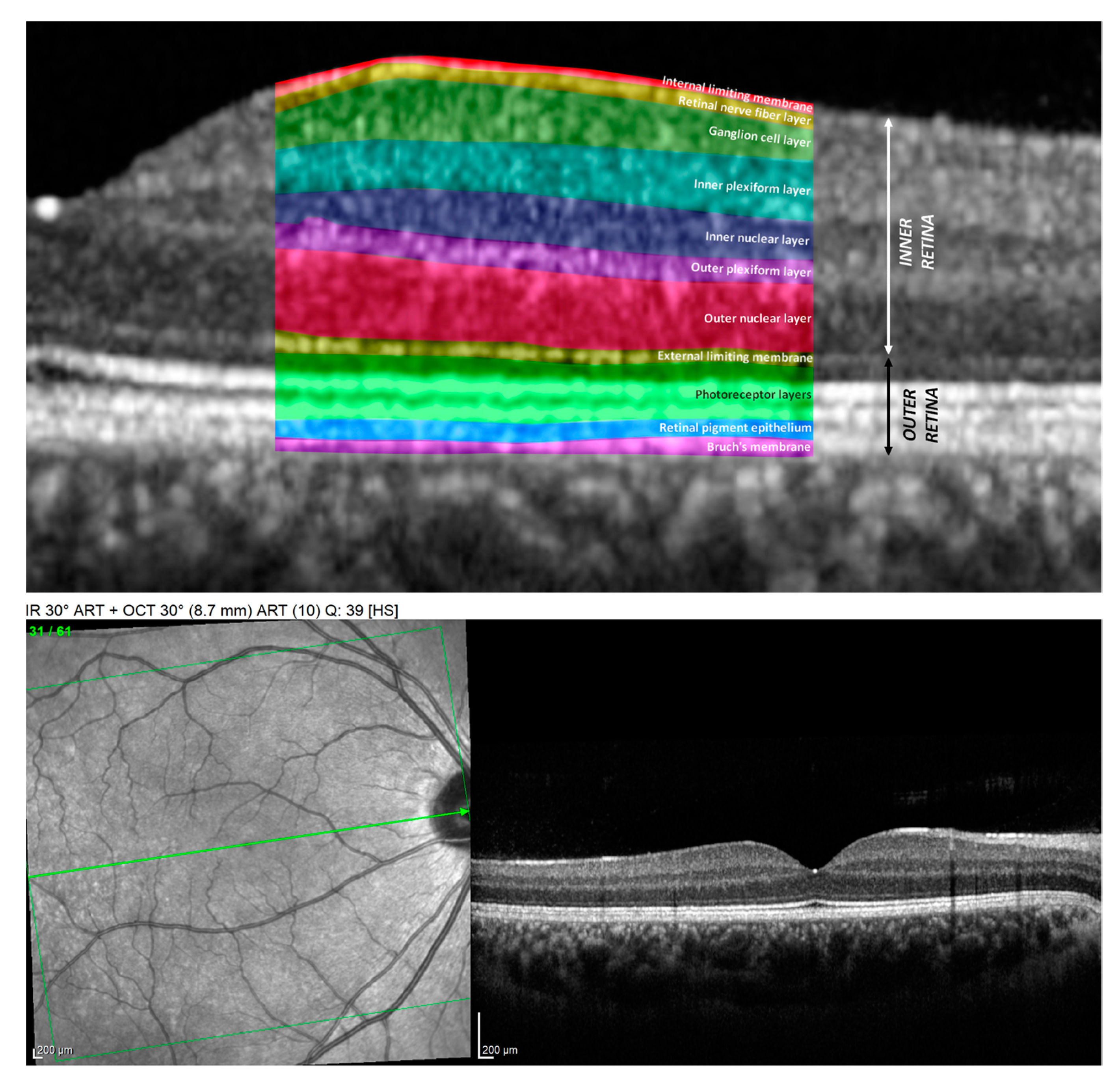
2.4. The Methodology of OCT Measurements
2.5. Statistical Analysis
3. Results
3.1. Peripapillary RNFL Changes in MS
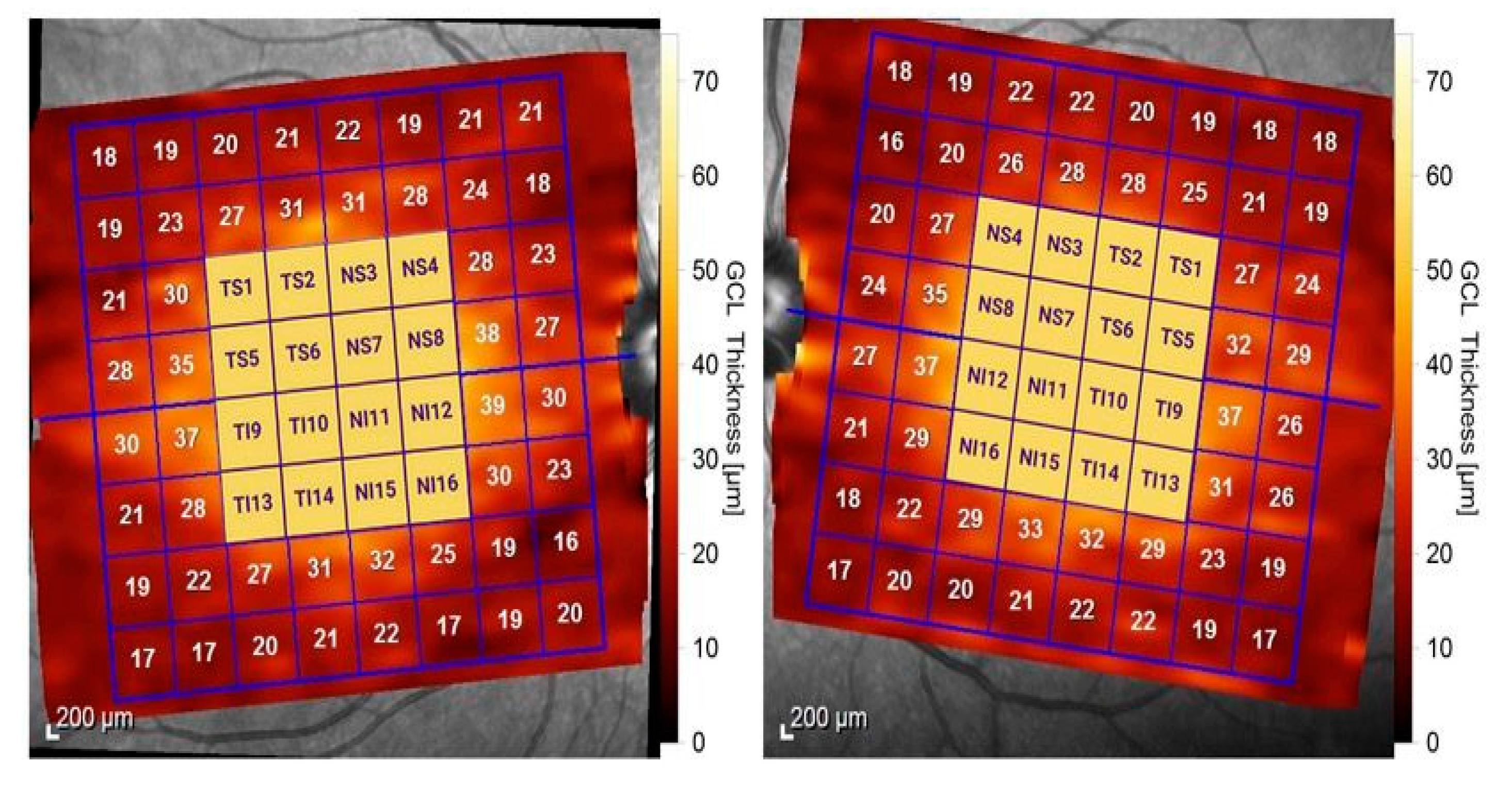
3.2. Macular Thickness Changes in MS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, A.L. Multiple Sclerosis Sourcebook: Basic Consumer Health Information about Multiple Sclerosis (MS) and Its Effects on Mobility, Vision, Bladder Function, Speech, Swallowing, and Cognition, Including Facts about Risk Factors, Causes, Diagnostic Procedures, Pain Management, Drug Treatments, and Physical and Occupational Therapies; Omnigraphics: Detroit, MI, USA, 2019; p. xiii. [Google Scholar]
- Wallin, M.T.; Culpepper, W.J.; Nichols, E.; Bhutta, Z.A.; Gebrehiwot, T.T.; Hay, S.I.; Khalil, I.A.; Krohn, K.J.; Liang, X.; Naghavi, M.; et al. Global, regional, and national burden of multiple sclerosis 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 269–285. [Google Scholar] [CrossRef]
- Henstridge, C.M.; Tzioras, M.; Paolicelli, R.C. Glial Contribution to Excitatory and Inhibitory Synapse Loss in Neurodegeneration. Front. Cell Neurosci. 2019, 13, 63. [Google Scholar] [CrossRef]
- Ikuta, F.; Zimmerman, H.M. Distribution of plaques in seventy autopsy cases of multiple sclerosis in the United States. Neurology 1976, 26, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Costello, F. The Afferent Visual Pathway: Designing a Structural-Functional Paradigm of Multiple Sclerosis. ISRN Neurol. 2013, 2013, 134858. [Google Scholar] [CrossRef] [PubMed]
- Efendi, H. Clinically Isolated Syndromes: Clinical Characteristics, Differential Diagnosis, and Management. Noro Psikiyatr. Arsivi 2016, 52, S1–S11. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Cordon, B.; Vilades, E.; Orduna, E.; Satue, M.; Perez-Velilla, J.; Sebastian, B.; Polo, V.; Larrosa, J.M.; Pablo, L.E.; Garcia-Martin, E. Angiography with optical coherence tomography as a biomarker in multiple sclerosis. PLoS ONE 2020, 15, e0243236. [Google Scholar] [CrossRef] [PubMed]
- Petzold, A.; Chua, S.Y.L.; Khawaja, A.P.; Keane, P.A.; Khaw, P.T.; Reisman, C.; Dhillon, B.; Strouthidis, N.G.; Foster, P.J.; Patel, P.J.; et al. Retinal asymmetry in multiple sclerosis. Brain 2020, 144, 224–235. [Google Scholar] [CrossRef]
- Galetta, K.M.; Balcer, L.J. Measures of visual pathway structure and function in MS: Clinical usefulness and role for MS trials. Mult. Scler. Relat. Disord. 2013, 2, 172–182. [Google Scholar] [CrossRef]
- Behbehani, R.; Ahmed, S.; Al-Hashel, J.; Rousseff, R.T.; Alroughani, R. Sensitivity of visual evoked potentials and spectral domain optical coherence tomography in early relapsing remitting multiple sclerosis. Mult. Scler. Relat. Disord. 2017, 12, 15–19. [Google Scholar] [CrossRef]
- Costello, F. Vision Disturbances in Multiple Sclerosis. Semin. Neurol. 2016, 36, 185–195. [Google Scholar] [CrossRef]
- Ciapă, M.A.; Șalaru, D.L.; Stătescu, C.; Sascău, R.A.; Bogdănici, C.M. Optic Neuritis in Multiple Sclerosis—A Review of Molecular Mechanisms Involved in the Degenerative Process. Curr. Issues Mol. Biol. 2022, 44, 3959–3979. [Google Scholar] [CrossRef] [PubMed]
- Wicki, C.A.; Hanson, J.V.; Schippling, S. Optical coherence tomography as a means to characterize visual pathway involvement in multiple sclerosis. Curr. Opin. Neurol. 2018, 31, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Petzold, A.; Balcer, L.J.; Calabresi, P.A.; Costello, F.; Frohman, T.C.; Frohman, E.M.; Martinez-Lapiscina, E.H.; Green, A.J.; Kardon, R.; Outteryck, O.; et al. Retinal layer segmentation in multiple sclerosis: A systematic review and meta-analysis. Lancet Neurol. 2017, 16, 797–812. [Google Scholar] [CrossRef]
- User Manual Software, Version 6.5, Spectralis Glaucoma Module Premium Edition; Heidelberg Engineering GmbH: Heidelberg, Germany, 2016; 78.
- Zhang, C.; Tatham, A.J.; Weinreb, R.N.; Zangwill, L.M.; Yang, Z.; Zhang, J.Z.; Medeiros, F.A. Relationship between ganglion cell layer thickness and estimated retinal ganglion cell counts in the glaucomatous macula. Ophthalmology 2014, 121, 2371–2379. [Google Scholar] [CrossRef]
- Curcio, C.A.; Allen, K.A. Topography of ganglion cells in human retina. J. Comp. Neurol. 1990, 300, 5–25. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.P.D.; Altmann, D.R.; Trip, A.S.; Kallis, C.; Jones, S.J.; Schlottmann, P.G.; Garway-Heath, D.F.; Plant, G.T.; Miller, D.H. A serial study of retinal changes following optic neuritis with sample size estimates for acute neuroprotection trials. Brain 2010, 133, 2592–2602. [Google Scholar] [CrossRef]
- Klistorner, A.; Arvind, H.; Garrick, R.; Graham, S.; Paine, M.; Yiannikas, C. Interrelationship of optical coherence tomography and multifocal visual-evoked potentials after optic neuritis. Investig. Opthalmol. Vis. Sci. 2010, 51, 2770–2777. [Google Scholar] [CrossRef]
- Mehmood, A.; Ali, W.; Song, S.; Din, Z.U.; Guo, R.; Shah, W.; Ilahi, I.; Yin, B.; Yan, H.; Zhang, L.; et al. Optical coherence tomography monitoring and diagnosing retinal changes in multiple sclerosis. Brain Behav. 2021, 11, e2302. [Google Scholar] [CrossRef]
- Saidha, S.; Sotirchos, E.S.; Ibrahim, M.A.; Crainiceanu, C.M.; Gelfand, J.M.; Sepah, Y.J.; Ratchford, J.N.; Oh, J.; Seigo, M.A.; Newsome, S.D.; et al. Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: A retrospective study. Lancet Neurol. 2012, 11, 963–972. [Google Scholar] [CrossRef]
- Rzepiński, Ł.; Kucharczuk, J.; Maciejek, Z.; Grzybowski, A.; Parisi, V. Spectral-Domain Optical Coherence Tomography Assessment in Treatment-Naïve Patients with Clinically Isolated Syndrome and Different Multiple Sclerosis Types: Findings and Relationship with the Disability Status. J. Clin. Med. 2021, 10, 2892. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, P.; Ringelstein, M.; Müller, A.; Keser, N.; Dietlein, T.; Lappas, A.; Foerster, A.; Hartung, H.; Aktas, O.; Methner, A. Degeneration of retinal layers in multiple sclerosis subtypes quantified by optical coherence tomography. Mult. Scler. J. 2012, 18, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Syc-Mazurek, S.; Saidha, S.; Newsome, S.D.; Ratchford, J.N.; Levy, M.; Ford, E.; Crainiceanu, C.M.; Durbin, M.K.; Oakley, J.D.; Meyer, S.A.; et al. Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain 2011, 135, 521–533. [Google Scholar] [CrossRef]
- Talman, L.S.; Bisker, E.R.; Sackel, D.J.; Long, D.A., Jr.; Galetta, K.M.; Ratchford, J.N.; Lile, D.J.; Farrell, S.K.; Loguidice, M.J.; Remington, G.; et al. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann. Neurol. 2010, 67, 749–760. [Google Scholar] [PubMed]
- Walter, S.D.; Ishikawa, H.; Galetta, K.M.; Sakai, R.E.; Feller, D.J.; Henderson, S.B.; Wilson, J.A.; Maguire, M.G.; Galetta, S.L.; Frohman, E.; et al. Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology 2012, 119, 1250–1257. [Google Scholar] [CrossRef]
- Martucci, A.; Landi, D.; Cesareo, M.; Di Carlo, E.; Di Mauro, G.; Sorge, R.P.; Albanese, M.; Nicoletti, C.G.; Mataluni, G.; Mercuri, N.B.; et al. Complex Rearrangement of the Entire Retinal Posterior Pole in Patients with Relapsing Remitting Multiple Sclerosis. J. Clin. Med. 2021, 10, 4693. [Google Scholar] [CrossRef]
- Ziccardi, L.; Barbano, L.; Boffa, L.; Albanese, M.; Grzybowski, A.; Centonze, D.; Parisi, V. Morphological Outer Retina Findings in Multiple Sclerosis Patients with or without Optic Neuritis. Front. Neurol. 2020, 11, 858. [Google Scholar] [CrossRef]
- Schneider, E.; Zimmermann, H.; Oberwahrenbrock, T.; Kaufhold, F.; Kadas, E.M.; Petzold, A.; Bilger, F.; Borisow, N.; Jarius, S.; Wildemann, B.; et al. Optical coherence tomography reveals distinct patterns of retinal damage in neuromyelitis optica and multiple sclerosis. PLoS ONE 2013, 8, e66151. [Google Scholar] [CrossRef]
- Balk, L.J.; Tewarie, P.; Killestein, J.; Polman, C.H.; Uitdehaag, B.; Petzold, A. Disease course heterogeneity and OCT in multiple sclerosis. Mult. Scler. J. 2014, 20, 1198–1206. [Google Scholar] [CrossRef]
- Saidha, S.; Syc, S.B.; Ibrahim, M.A.; Eckstein, C.; Warner, C.V.; Farrell, S.K.; Oakley, J.D.; Durbin, M.K.; Meyer, S.A.; Balcer, L.J.; et al. Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography. Brain 2011, 134, 518–533. [Google Scholar] [CrossRef]
- Eslami, F.; Ghiasian, M.; Khanlarzade, E.; Moradi, E. Retinal Nerve Fiber Layer Thickness and Total Macular Volume in Multiple Sclerosis Subtypes and Their Relationship with Severity of Disease, a Cross-Sectional Study. Eye Brain 2020, 12, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Sotirchos, E.S.; Caldito, N.G.; Filippatou, A.; Fitzgerald, K.C.; Murphy, O.C.; Lambe, J.; Nguyen, J.; Button, J.; Ogbuokiri, E.; Crainiceanu, C.M.; et al. Progressive Multiple Sclerosis Is Associated with Faster and Specific Retinal Layer Atrophy. Ann. Neurol. 2020, 87, 885–896. [Google Scholar] [CrossRef]
- Green, A.J.; McQuaid, S.; Hauser, S.L.; Allen, I.V.; Lyness, R. Ocular pathology in multiple sclerosis: Retinal atrophy and inflammation irrespective of disease duration. Brain 2010, 133, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, M.; Wang, C.Y.; Barnett, M.H.; Garrick, R.; Parratt, J.; Graham, S.L.; Sriram, P.; Yiannikas, C.; Klistorner, A. Inner nuclear layer thickening is inversley proportional to retinal ganglion cell loss in optic neuritis. PLoS ONE 2013, 8, e78341. [Google Scholar] [CrossRef] [PubMed]

| CTRL | CIS-NON | CIS-ON | RRMS-NON | RRMS-ON | ||
|---|---|---|---|---|---|---|
| Sex | F | 42 | 7 | 5 | 47 | 21 |
| M | 12 | 1 | 1 | 17 | 5 | |
| Mean Age | 31.85 | 30.88 | 33.83 | 37.64 | 30.35 | |
| CTRL | CIS-NON | CIS-ON | RRMS-NON | RRMS-ON | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
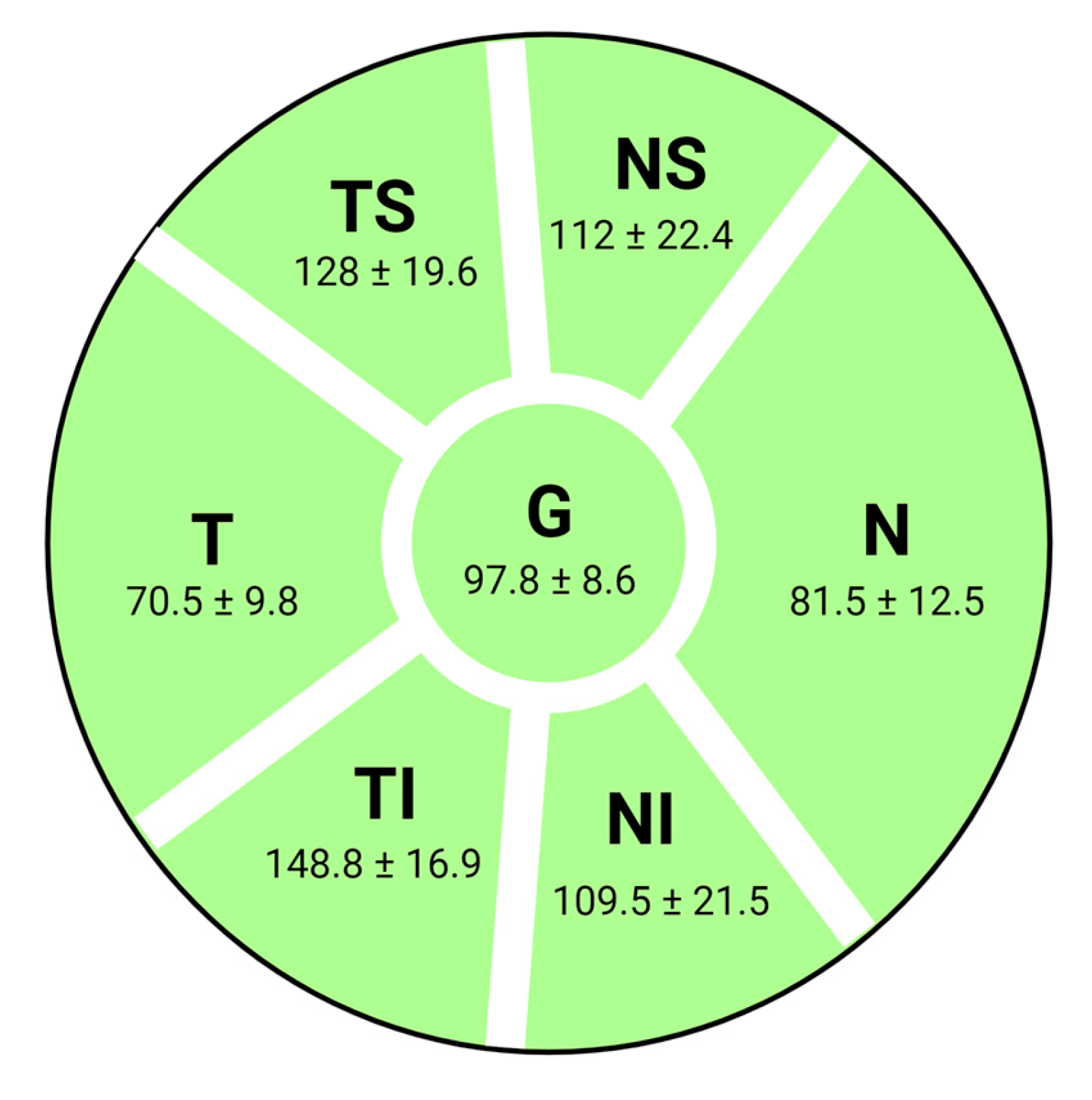
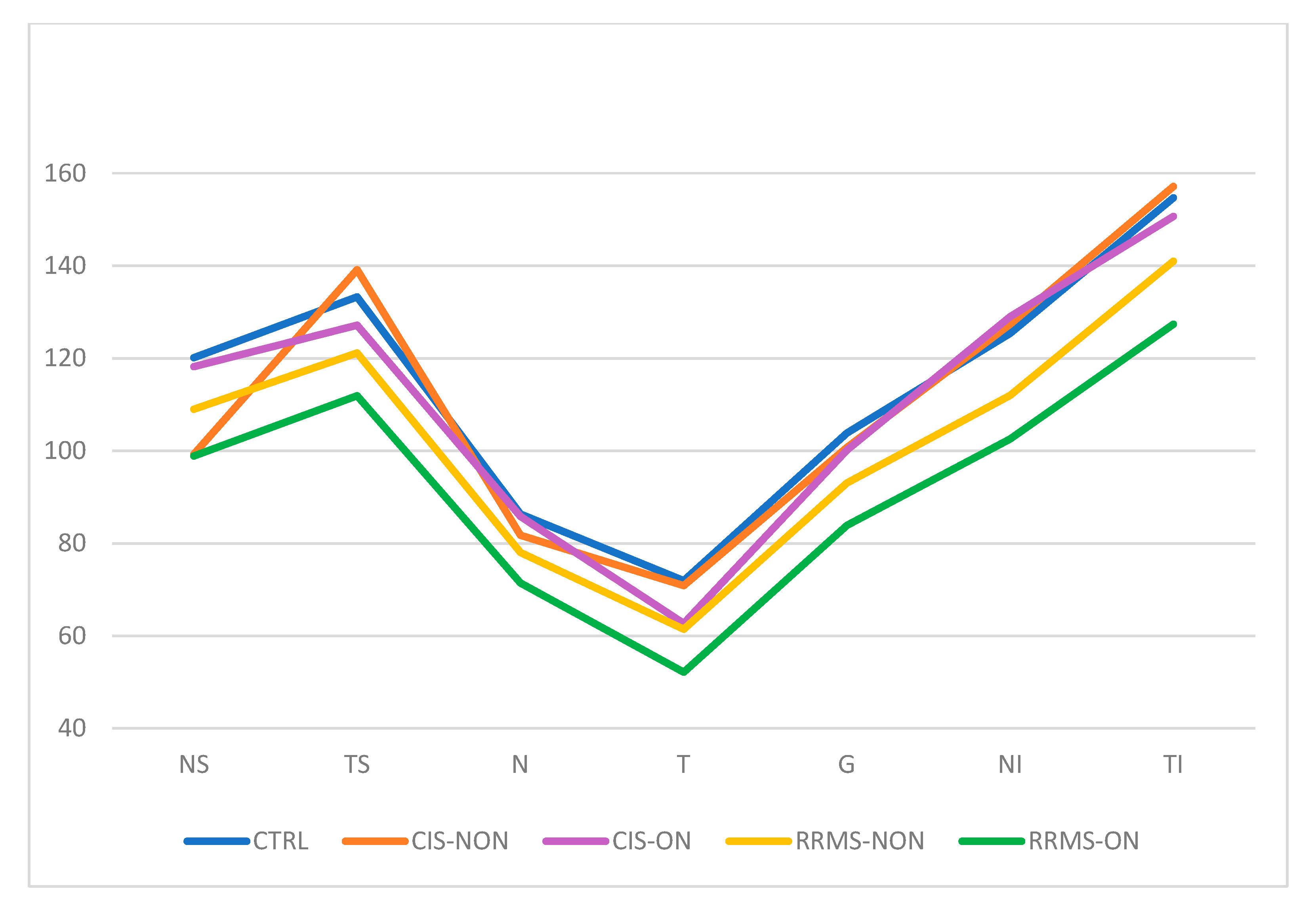
| NS | 120.11 | 24.96 | 99.25 | 24.11 | 118.17 | 24.11 | 109.00 | 23.20 | 98.88 | 18.47 |
| TS | 133.30 | 18.66 | 139.13 | 17.75 | 127.17 | 17.75 | 121.19 | 21.95 | 111.92 | 26.94 |
| N | 86.35 | 12.88 | 81.75 | 7.36 | 85.83 | 7.36 | 78.02 | 13.18 | 71.42 | 12.31 |
| T | 71.93 | 9.63 | 70.88 | 10.93 | 62.67 | 10.93 | 61.45 | 13.39 | 52.15 | 13.73 |
| G | 103.83 | 8.62 | 100.75 | 9.09 | 100.17 | 9.09 | 93.05 | 12.39 | 83.88 | 12.60 |
| NI | 125.39 | 18.49 | 127.38 | 16.15 | 129.00 | 16.15 | 111.98 | 25.29 | 102.54 | 23.17 |
| TI | 154.70 | 20.07 | 157.13 | 22.78 | 150.67 | 22.78 | 141.00 | 22.38 | 127.35 | 24.97 |
| NS | TS | N | G | T | NI | TI | |
|---|---|---|---|---|---|---|---|
| CIS-NON | −20.86 | 5.83 | −4.60 | −3.08 | −1.05 | 1.99 | 2.42 |
| CIS-ON | −1.94 | −6.13 | −0.52 | −3.67 | −9.26 | 3.61 | −4.04 |
| RRMS-NON | −11.11 * | −12.11 * | −8.34 * | −10.79 * | −10.47 * | −13.40 * | −13.70 * |
| RRMS-ON | −21.23 * | −21.37 * | −14.93 * | −19.95 * | −19.77 * | −22.85 * | −27.36 * |
| CTRL | CIS-NON | CIS-ON | RRMS-NON | RRMS-ON | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4 × 4 Sectors | 4 × 4 Sectors | 4 × 4 Sectors | 4 × 4 Sectors | 4 × 4 Sectors | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
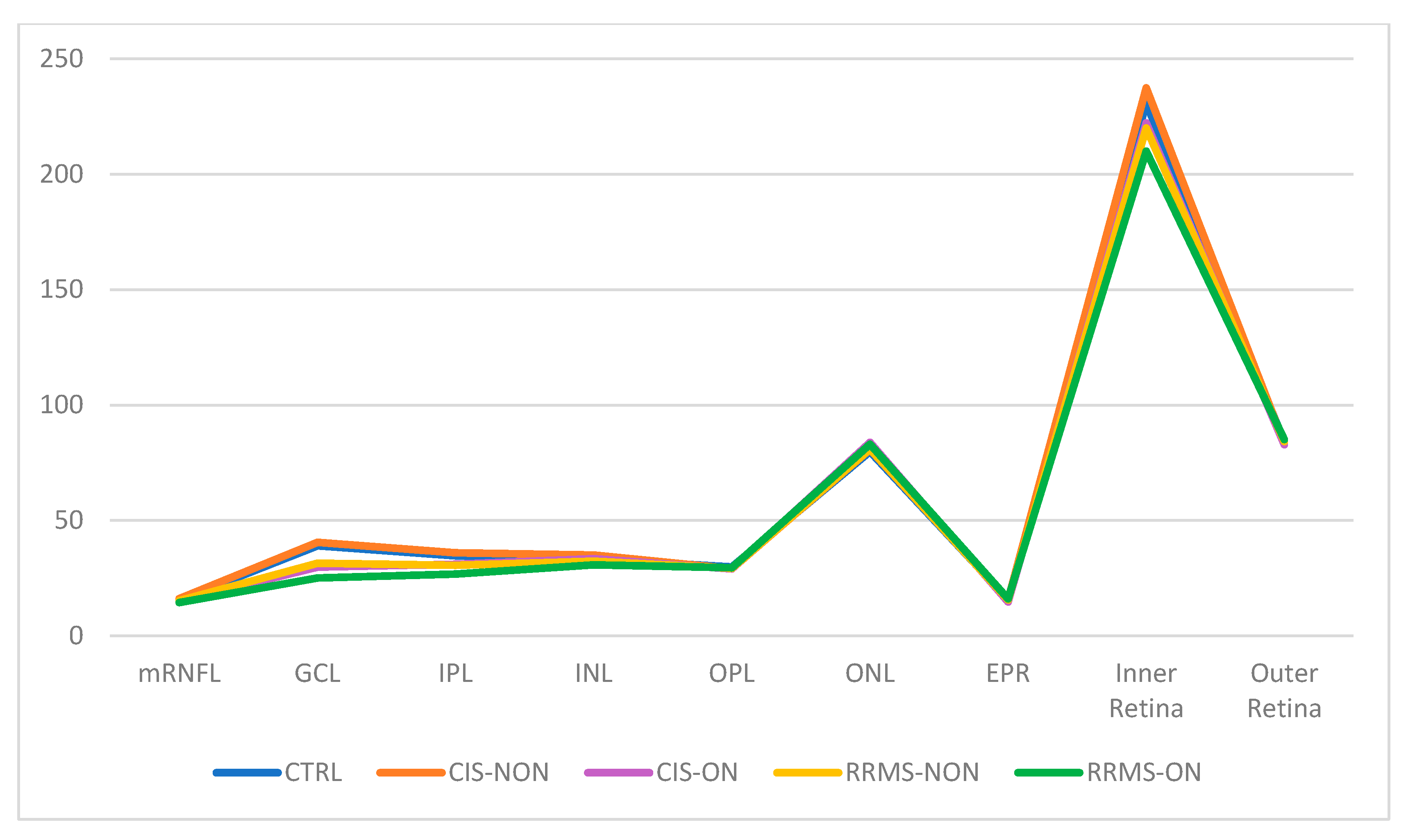
| mRNFL | 24.027 | 2.343 | 25.164 | 1.538 | 21.427 | 1.971 | 22.633 | 2.592 | 20.565 | 2.145 |
| GCL | 47.816 | 2.578 | 46.164 | 3.192 | 40.000 | 5.085 | 41.142 | 6.205 | 35.315 | 6.358 |
| IPL | 38.182 | 2.251 | 37.657 | 2.522 | 34.073 | 3.143 | 34.919 | 4.949 | 31.185 | 3.584 |
| INL | 38.371 | 2.761 | 38.711 | 2.726 | 39.990 | 1.931 | 38.403 | 2.156 | 38.505 | 2.936 |
| OPL | 29.113 | 2.068 | 28.617 | 1.926 | 29.698 | 4.017 | 29.216 | 2.281 | 29.890 | 2.492 |
| ONL | 70.149 | 6.128 | 70.602 | 6.211 | 72.709 | 5.170 | 70.422 | 6.971 | 71.608 | 6.795 |
| EPR | 13.948 | 1.102 | 14.047 | 1.095 | 13.927 | 0.749 | 14.765 | 1.792 | 13.927 | 0.749 |
| Inner Retina | 246.603 | 11.584 | 247.016 | 8.100 | 247.016 | 8.100 | 226.382 | 14.068 | 238.188 | 10.118 |
| Outer Retina | 80.408 | 6.183 | 79.438 | 1.923 | 79.438 | 1.923 | 81.253 | 1.674 | 79.219 | 3.083 |
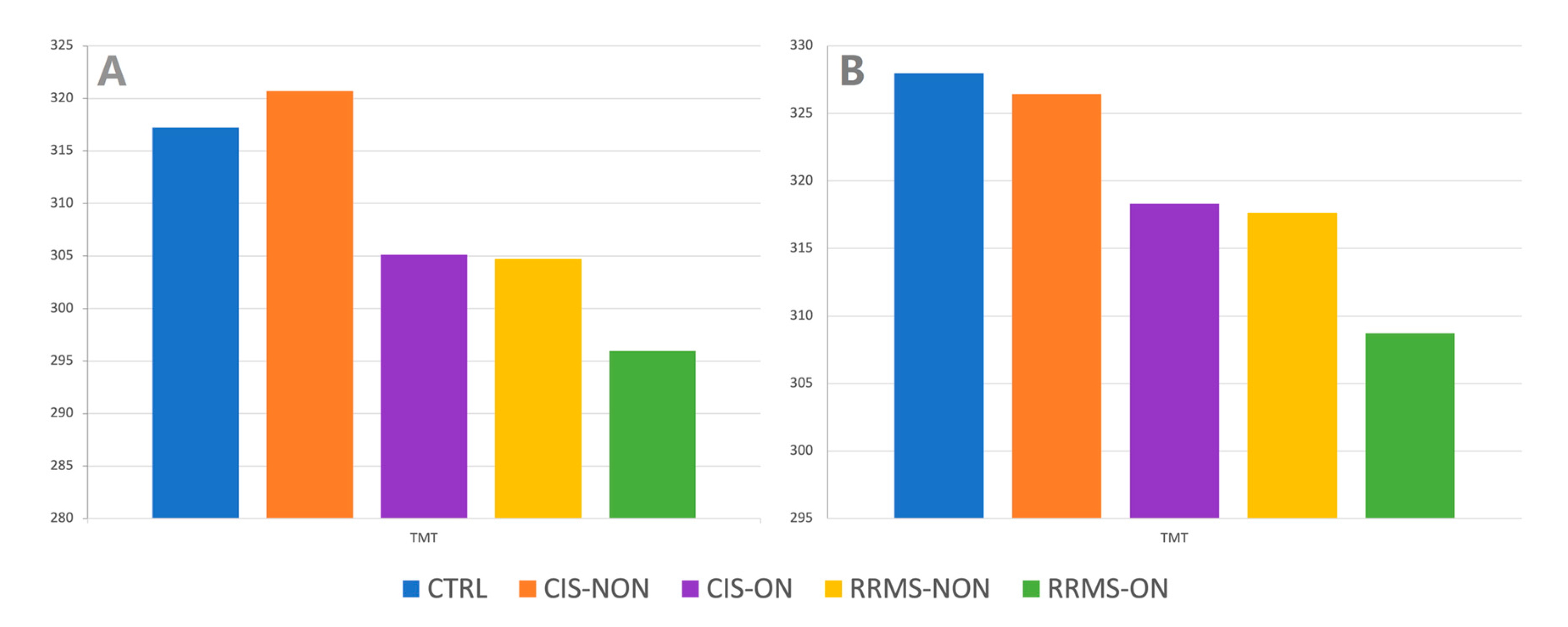
| TMT | 327.959 | 12.124 | 326.430 | 7.608 | 326.430 | 7.608 | 308.730 | 12.978 | 318.302 | 11.275 |
| CTRL | CIS-NON | CIS-ON | RRMS-NON | RRMS-ON | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 × 2 Central Grid | 2 × 2 Central Grid | 2 × 2 Central Grid | 2 × 2 Central Grid | 2 × 2 Central Grid | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| mRNFL | 15.431 | 1.115 | 16.094 | 0.743 | 15.167 | 0.785 | 15.215 | 0.903 | 14.462 | 1.254 |
| GCL | 39.000 | 4.300 | 40.500 | 3.703 | 29.792 | 6.454 | 31.492 | 6.485 | 25.163 | 6.355 |
| IPL | 34.663 | 3.422 | 35.969 | 2.750 | 31.042 | 3.610 | 30.551 | 4.039 | 26.837 | 3.752 |
| INL | 33.306 | 3.830 | 35.094 | 3.119 | 33.417 | 1.821 | 32.361 | 3.344 | 30.800 | 2.896 |
| OPL | 29.846 | 2.947 | 29.031 | 2.466 | 29.000 | 5.736 | 29.195 | 3.655 | 29.558 | 3.106 |
| ONL | 79.830 | 7.519 | 80.750 | 8.169 | 83.833 | 7.627 | 80.944 | 8.107 | 83.029 | 6.859 |
| EPR | 15.838 | 1.231 | 15.688 | 1.534 | 14.750 | 0.880 | 15.586 | 1.449 | 15.990 | 1.419 |
| Inner Retina | 230.731 | 14.363 | 237.344 | 6.463 | 222.167 | 9.497 | 219.953 | 14.056 | 209.990 | 13.025 |
| Outer Retina | 84.019 | 2.529 | 83.375 | 2.722 | 82.833 | 2.910 | 84.195 | 3.283 | 85.087 | 1.512 |
| TMT | 317.234 | 14.780 | 305.125 | 7.325 | 295.960 | 11.667 | 295.960 | 14.940 | 295.960 | 13.994 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cujba, L.; Stan, C.; Samoila, O.; Drugan, T.; Benedec, A.; Nicula, C. Identifying Optical Coherence Tomography Markers for Multiple Sclerosis Diagnosis and Management. Diagnostics 2023, 13, 2077. https://doi.org/10.3390/diagnostics13122077
Cujba L, Stan C, Samoila O, Drugan T, Benedec A, Nicula C. Identifying Optical Coherence Tomography Markers for Multiple Sclerosis Diagnosis and Management. Diagnostics. 2023; 13(12):2077. https://doi.org/10.3390/diagnostics13122077
Chicago/Turabian StyleCujba, Larisa, Cristina Stan, Ovidiu Samoila, Tudor Drugan, Ancuta Benedec (Cutas), and Cristina Nicula. 2023. "Identifying Optical Coherence Tomography Markers for Multiple Sclerosis Diagnosis and Management" Diagnostics 13, no. 12: 2077. https://doi.org/10.3390/diagnostics13122077
APA StyleCujba, L., Stan, C., Samoila, O., Drugan, T., Benedec, A., & Nicula, C. (2023). Identifying Optical Coherence Tomography Markers for Multiple Sclerosis Diagnosis and Management. Diagnostics, 13(12), 2077. https://doi.org/10.3390/diagnostics13122077







