Cerebrospinal Fluid Anti-Neuronal Autoantibodies in COVID-19-Associated Limbic Encephalitis with Acute Cerebellar Ataxia and Myoclonus Syndrome: Case Report and Literature Review
Abstract
1. Introduction
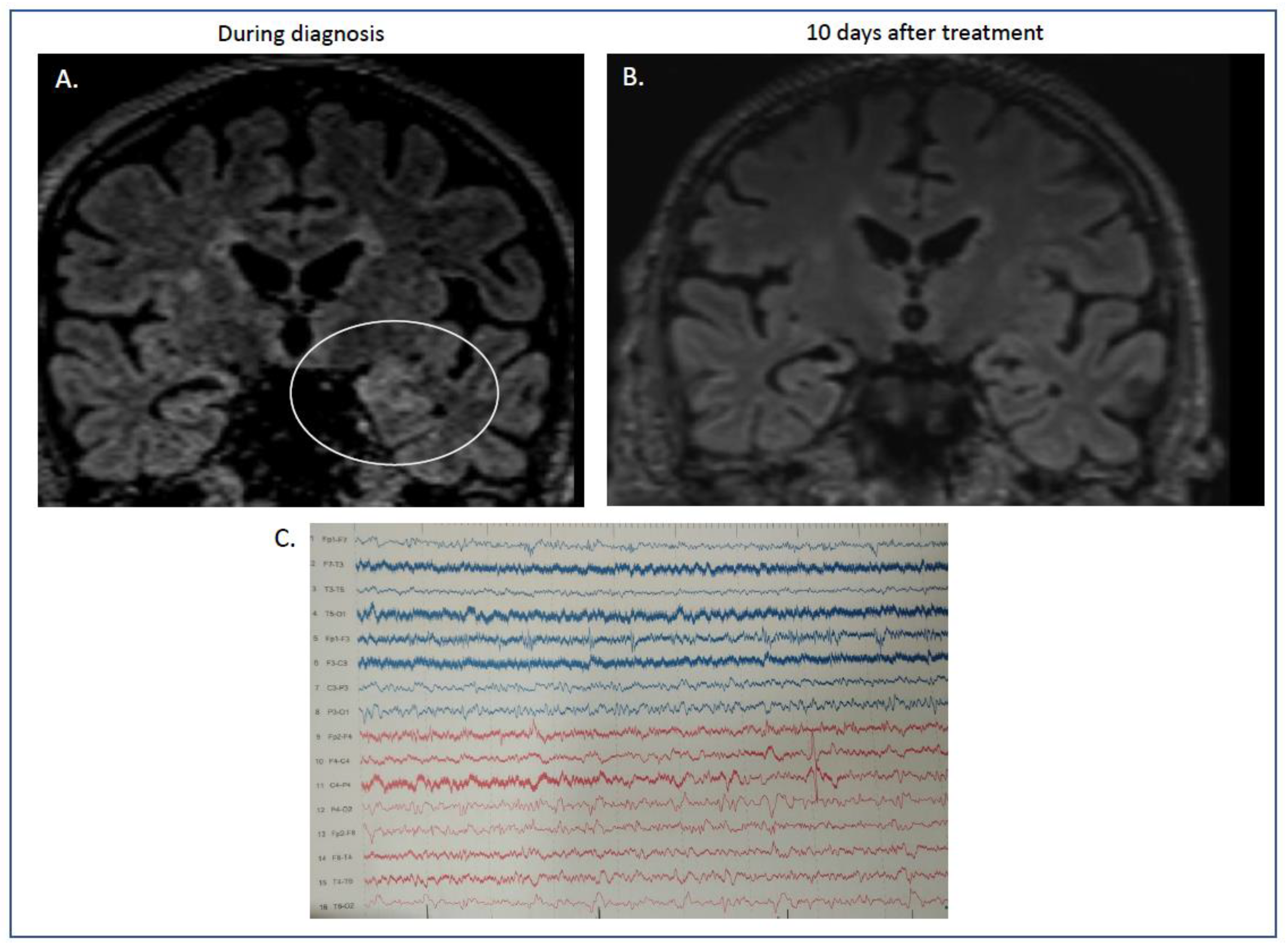
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, K.-T.; Hsu, B.-C.; Chen, D.-Y. Autoimmune and Rheumatic Manifestations Associated with COVID-19 in Adults: An Updated Systematic Review. Front. Immunol. 2021, 12, 645013. [Google Scholar] [CrossRef] [PubMed]
- Nabizadeh, F.; Balabandian, M.; Sodeifian, F.; Rezaei, N.; Rostami, M.-R.; Naser Moghadasi, A. Autoimmune encephalitis associated with COVID-19: A systematic review. Mult. Scler. Relat. Disord. 2022, 62, 103795. [Google Scholar] [CrossRef] [PubMed]
- Franke, C.; Ferse, C.; Kreye, J.; Reincke, S.M.; Sanchez-Sendin, E.; Rocco, A.; Steinbrener, M.; Angermair, S.; Treskatsch, S.; Zickler, D.; et al. High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Brain Behav. Immun. 2021, 93, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Cagnazzo, F.; Arquizan, C.; Derraz, I.; Dargazanli, C.; Lefevre, P.H.; Riquelme, C.; Gaillard, N.; Mourand, I.; Gascou, G.; Bonafe, A.; et al. Neurological manifestations of patients infected with the SARS-CoV-2: A systematic review of the literature. J. Neurol. 2021, 268, 2656–2665. [Google Scholar] [CrossRef]
- Chan, J.L.; Murphy, K.A.; Sarna, J.R. Myoclonus and Cerebellar Ataxia Associated with COVID-19: A Case Report and Systematic Review. J. Neurol. 2021, 268, 3517–3548. [Google Scholar] [CrossRef]
- Ariño, H.; Heartshorne, R.; Michael, B.D.; Nicholson, T.R.; Vincent, A.; Pollak, T.A.; Vogrig, A. Neuroimmune disorders in COVID-19. J. Neurol. 2022, 269, 2827–2839. [Google Scholar] [CrossRef]
- Parsons, T.; Banks, S.; Bae, C.; Gelber, J.; Alahmadi, H.; Tichauer, M. COVID-19-associated acute disseminated encephalomyelitis (ADEM). J. Neurol. 2020, 267, 2799–2802. [Google Scholar] [CrossRef]
- Román, G.C.; Gracia, F.; Torres, A.; Palacios, A.; Gracia, K.; Harris, D. Acute Transverse Myelitis (ATM):Clinical Review of 43 Patients With COVID-19-Associated ATM and 3 Post-Vaccination ATM Serious Adverse Events With the ChAdOx1 nCoV-19 Vaccine (AZD1222). Front. Immunol. 2021, 26, 653786. [Google Scholar] [CrossRef]
- Tankisi, H.; Ochala, J. Myopathy in acute and long-term COVID-19. Clin. Neurophysiol. 2022, 134, 141–142. [Google Scholar] [CrossRef]
- Graus, F.; Titulaer, M.J.; Balu, R.; Benseler, S.; Bien, C.G.; Cellucci, T.; Cortese, I.; Dale, R.C.; Gelfand, J.M.; Geschwind, M.; et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016, 154, 391–404. [Google Scholar] [CrossRef]
- Samim, M.M.; Dhar, D.; Goyal, S.; Dey, T.; Parvin, N.; Shah, R.D.; Singh, V.; Chowdhury, S.; Lal, B.M.; Varghese, N.; et al. AI-CoV Study: Autoimmune Encephalitis Associated With COVID-19 and Its Vaccines-A Systematic Review. J. Clin. Neurol. 2022, 186, 692–710. [Google Scholar] [CrossRef]
- Mulder, J.; Feresiadou, A.; Fällmar, D.; Frithiof, R.; Virhammar, J.; Rasmusson, A.; Rostami, E.; Kumlien, E.; Cunningham, J.L. Autoimmune Encephalitis Presenting with Malignant Catatonia in a 40-Year-Old Male Patient with COVID-19. Am. J. Psychiatry 2021, 178, 485–489. [Google Scholar] [CrossRef]
- Stoian, A.; Stoian, M.; Bajko, Z.; Maier, S.; Andone, S.; Cioflinc, R.A.; Motataianu, A.; Barcutean, L.; Balasa, R. Autoimmune Encephalitis in COVID-19 Infection: Our Experience and Systematic Review of the Literature. Biomedicines 2022, 25, 774. [Google Scholar] [CrossRef]
- Grimaldi, S.; Lagarde, S.; Harlé, J.R.; Boucraut, J.; Guedj, E. Autoimmune Encephalitis Concomitant with SARS-CoV-2 Infection: Insight from 18F-FDG PET Imaging and Neuronal Autoantibodies. J. Nucl. Med. 2020, 6112, 1726–1729. [Google Scholar] [CrossRef]
- McAlpine, L.S.; Lifland, B.; Check, J.R.; Angarita, G.A.; Ngo, T.T.; Pleasure, S.J.; Wilson, M.R.; Spudich, S.S.; Farhadian, S.F.; Bartley, C.M. Remission of Subacute Psychosis in a COVID-19 Patient With an Antineuronal Autoantibody After Treatment With Intravenous Immunoglobulin. Biol. Psychiatry 2021, 15, e23–e26. [Google Scholar] [CrossRef]
- Mulder, J.; Lindqvist, I.; Rasmusson, A.J.; Husén, E.; Rönnelid, J.; Kumlien, E.; Rostami, E.; Virhammar, J.; Cunningham, J.L. Indirect immunofluorescence for detecting anti-neuronal autoimmunity in CSF after COVID-19—Possibilities and pitfalls. Brain. Behav. Immun. 2021, 94, 473–474. [Google Scholar] [CrossRef]
- Tsouris, Z.; Provatas, A.; Bakirtzis, C.; Aloizou, A.M.; Siokas, V.; Tsimourtou, V.; Grigoriadis, N.; Hadjigeorgiou, G.M.; Dardiotis, E. Anti-MOG Positive Bilateral Optic Neuritis and Brainstem Encephalitis Secondary to COVID-19 Infection: A Case Report. Neurol. Int. 2022, 30, 991–996. [Google Scholar] [CrossRef]
- Payus, A.O.; Jeffree, M.S.; Ohn, M.H.; Tan, H.J.; Ibrahim, A.; Chia, Y.K.; Raymond, A.A. Immune-mediated neurological syndrome in SARS-CoV-2 infection: A review of literature on autoimmune encephalitis in COVID-19. Neurol. Sci. 2022, 43, 1533–1547. [Google Scholar] [CrossRef]
- Yapici-Eser, H.; Koroglu, Y.-E.; Oztop-Cakmak, O.; Keskin, O.; Gursoy, A.; Gursoy-Ozdemir, Y. Neuropsychiatric Symptoms of COVID-19 Explained by SARS-CoV-2 Proteins’ Mimicry of Human Protein Interactions. Front. Hum. Neurosci. 2021, 23, 656313. [Google Scholar] [CrossRef]
- Vasilevska, V.; Guest, P.C.; Bernstein, H.-G.; Schroeter, M.L.; Geis, C.; Steiner, J. Molecular mimicry of NMDA receptors may contribute to neuropsychiatric symptoms in severe COVID-19 cases. J. Neuroinflammation 2021, 18, 245. [Google Scholar] [CrossRef]
- Scoppettuolo, P.; Borrelli, S.; Naeije, G. Neurological involvement in SARS-CoV-2 infection: A clinical systematic review. Brain. Behav. Immun. Health. 2020, 5, 100094. [Google Scholar] [CrossRef] [PubMed]
- Monti, G.; Giovannini, G.; Marudi, A.; Bedin, R.; Melegari, A.; Simone, A.M.; Santangelo, M.; Pignatti, A.; Bertellini, E.; Trenti, T.; et al. Anti-NMDA receptor encephalitis presenting as new onset refractory status epilepticus in COVID-19. Seizure 2020, 81, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Asan, L.; Klebe, S.; Kleinschnitz, C.; Stettner, M.; Köhrmann, M. Anti-GFAP-antibody positive postinfectious acute cerebellar ataxia and myoclonus after COVID-19: A case report. Ther. Adv. Neurol. Disord. 2021, 8, 17562864211062824. [Google Scholar] [CrossRef] [PubMed]
- Foucard, C.; San-Galli, A.; Tarrano, C.; Chaumont, H.; Lannuzel, A.; Roze, E. Acute cerebellar ataxia and myoclonus with or without opsoclonus: A para-infectious syndrome associated with COVID-19. Eur. J. Neurol. 2021, 28, 3533–3536. [Google Scholar] [CrossRef]
- Nelson, J.L.; Blume, G.M.; Bansal, S.K.; Kaufman, J.R.; Woods, F.R.; Zhang, X.; Kattah, J.C. Postinfectious SARS-CoV-2 Opsoclonus-Myoclonus-Ataxia Syndrome. J. Neuroophthalmol. 2022, 1, 251–255. [Google Scholar] [CrossRef]
- Álvarez Bravo, G.; Sánchez Cirera, L.; Angerri Nadal, M.; Ramió I Torrentà, L. Clinical heterogeneity in patients with myoclonus associated to COVID-19. Neurol. Sci. 2022, 43, 1587–1592. [Google Scholar] [CrossRef]
- Osawa, K.; Sugiyama, A.; Uzawa, A.; Hirano, S.; Yamamoto, T.; Nezu, M.; Araki, N.; Kano, H.; Kuwabara, S. Temporal Changes in Brain Perfusion in a Patient with Myoclonus and Ataxia Syndrome Associated with COVID-19. Intern. Med. 2022, 1, 1071–1076. [Google Scholar] [CrossRef]
- Ben Mohamed, D.; Zouari, R.; Ketata, J.; Nabli, F.; Blel, S.; Ben Sassi, S. Myoclonus status revealing COVID-19 infection. Seizure 2023, 104, 12–14. [Google Scholar] [CrossRef]
- Oh, S.Y.; Kim, J.S.; Dieterich, M. Update on opsoclonus-myoclonus syndrome in adults. J. Neurol. 2019, 266, 1541–1548. [Google Scholar] [CrossRef]
- Kfoury Baz, E.M.; Khatib, M.F.; Mahfouz, R.A.; Jamaleddine, G.W. Deterioration of gas exchange in patients with severe thrombotic thrombocytopenic purpura with respiratory failure during therapeutic plasma exchange. J. Clin. Apher. 2001, 16, 143–147. [Google Scholar] [CrossRef]
- Amrutiya, V.; Patel, R.; Baghal, M.; Patel, B.; Waykole, T.; Patel, H.; Govil, S.; Lo, A. Transfusion-related acute lung injury in a COVID-19-positive convalescent plasma recipient: A case report. J. Int. Med. Res. 2021, 49, 3000605211032814. [Google Scholar] [CrossRef]
- Kagawa, H.; Tsujino, K.; Yamamoto, Y.; Iwai, A.; Hara, R.; Matsuki, T.; Fukushima, K.; Oshitani, Y.; Yoshimura, K.; Miki, M.; et al. Acute lung injury after plasma exchange in a patient with anti-MDA5 antibody-positive, rapidly progressive, interstitial lung disease: A case report. Respir. Med. Case Rep. 2020, 1, 101016. [Google Scholar] [CrossRef]
| Immunological and COVID-Related Infection Parameters Tested | |||
|---|---|---|---|
| Antibody Testing for Extracellular Synaptic Antigens | |||
| Testing | Method | Biological Sample | Result |
| Anti-CASPR2 | CBA | serum | negative |
| Anti-LGI1 | CBA | serum | negative |
| Anti-NMDAR | CBA | CSF | negative |
| Anti-AMPAR1,2 | CBA | serum | negative |
| Anti-GABAbR | CBA | serum | negative |
| Anti-DPPX | CBA | serum | negative |
| Anti-mGluR5 | CBA | serum | negative |
| Anti-NMDAR | CBA | serum | negative |
| Anti-Glycin Receptor | CBA | serum | negative |
| Anti-AQP4 | CBA | serum | negative |
| Anti-MOG | CBA | serum | negative |
| Anti-GAD65 | Elisa | serum | negative |
| Anti-GFAP | CBA | serum | negative |
| Panel for intracellular antibodies | |||
| Amphiphysin, CV2/V2/CRMP5, Hu, PNMA2, Recoverin, Ri, SOX-1, Yo, Zic4, Tr, GAD, Titin | Immunoblotting | serum | negative |
| Infection by SARS-CoV-2 (COVID-19) | |||
| SARS-CoV-2 (COVID-19) RNA | RT-PCR | CSF | negative |
| SARS-CoV-2 (COVID-19) RNA | RT-PCR | TBS | positive |
| SARS-CoV-2 (COVID-19) IgM and IgG antibodies | Elisa | serum | positive |
| CSF analysis | |||
| Cell count (reference control value: <5/µL) | counting (Fuchs–Rosenthal chamber) | CSF | 1 |
| Glucose (reference range: 50 to 80 mg/dL) | biochemical analyzer | CSF | 80 |
| Protein (reference range: 15 to 60 mg/dL) | biochemical analyzer | CSF | 61 |
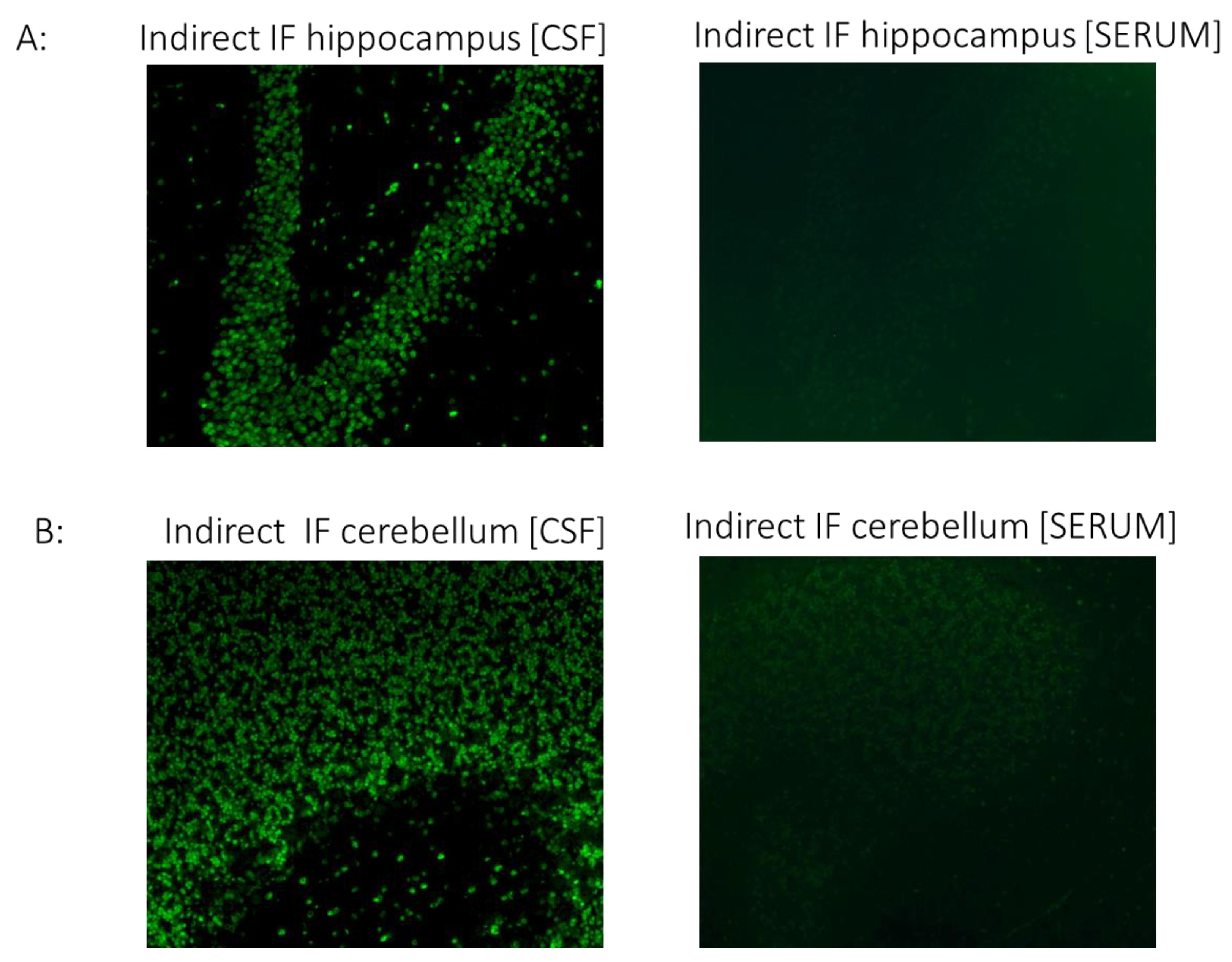
| CSF indirect immunofluorescence (IgG) | indirect immunofluorescence on unfixed murine brain sections | serum | negative |
| indirect immunofluorescence on unfixed murine brain sections | CSF | positive (granular layer of cerebellum, neuronal cells of hippocampus) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yiannopoulou, K.; Vakrakou, A.G.; Anastasiou, A.; Nikolopoulou, G.; Sourdi, A.; Tzartos, J.S.; Kilidireas, C.; Dimitrakopoulos, A. Cerebrospinal Fluid Anti-Neuronal Autoantibodies in COVID-19-Associated Limbic Encephalitis with Acute Cerebellar Ataxia and Myoclonus Syndrome: Case Report and Literature Review. Diagnostics 2023, 13, 2055. https://doi.org/10.3390/diagnostics13122055
Yiannopoulou K, Vakrakou AG, Anastasiou A, Nikolopoulou G, Sourdi A, Tzartos JS, Kilidireas C, Dimitrakopoulos A. Cerebrospinal Fluid Anti-Neuronal Autoantibodies in COVID-19-Associated Limbic Encephalitis with Acute Cerebellar Ataxia and Myoclonus Syndrome: Case Report and Literature Review. Diagnostics. 2023; 13(12):2055. https://doi.org/10.3390/diagnostics13122055
Chicago/Turabian StyleYiannopoulou, Konstantina, Aigli G. Vakrakou, Aikaterini Anastasiou, Georgia Nikolopoulou, Athina Sourdi, John S. Tzartos, Constantinos Kilidireas, and Antonios Dimitrakopoulos. 2023. "Cerebrospinal Fluid Anti-Neuronal Autoantibodies in COVID-19-Associated Limbic Encephalitis with Acute Cerebellar Ataxia and Myoclonus Syndrome: Case Report and Literature Review" Diagnostics 13, no. 12: 2055. https://doi.org/10.3390/diagnostics13122055
APA StyleYiannopoulou, K., Vakrakou, A. G., Anastasiou, A., Nikolopoulou, G., Sourdi, A., Tzartos, J. S., Kilidireas, C., & Dimitrakopoulos, A. (2023). Cerebrospinal Fluid Anti-Neuronal Autoantibodies in COVID-19-Associated Limbic Encephalitis with Acute Cerebellar Ataxia and Myoclonus Syndrome: Case Report and Literature Review. Diagnostics, 13(12), 2055. https://doi.org/10.3390/diagnostics13122055






