Abstract
Although circulating tumour DNA (ctDNA)-based next-generation sequencing (NGS) is a less invasive method for assessing ESR1 mutations that are essential mechanisms of endocrine therapy resistance in patients with oestrogen receptor-positive breast cancer, adequate amounts of DNA are required to assess polyclonal ESR1 mutations. By combining a peptide nucleic acid and locked nucleic acid polymerase chain reaction (PNA-LNA PCR) clamping assay, we have developed a novel detection system to screen for polyclonal ESR1 mutations in ctDNA. A validation assay was prospectively performed on clinical samples and compared with the NGS results. The PNA-LNA PCR clamp assay was validated using six and four blood samples in which ESR1 mutations were detected by NGS and no mutations were detected, respectively. The PNA-LNA assay results were comparable with those of NGS. We prospectively assessed the concordance between the PNA-LNA PCR clamp method and NGS. Using the PNA-LNA PCR clamp method, ESR1 mutations were detected in 5 out of 18 samples, including those in which mutations were not detected by NGS due to small amounts of ctDNA. The PNA-LNA PCR clamping method is a highly sensitive and minimally invasive assay for polyclonal ESR1 mutation detection in the ctDNA of patients with breast cancer.
1. Introduction
Oestrogen receptor alpha (ERα), encoded by the ESR1 gene, is a member of the nuclear hormone receptor superfamily and is expressed in approximately 70% of breast cancer cases [1]. Patients with metastatic oestrogen receptor (ER)-positive breast cancer are treated with a variety of endocrine therapies until they eventually develop endocrine resistance or visceral crisis [2]. Although ESR1 mutations are rare in primary breast cancers, they are frequently reported in patients with recurrent breast cancer who have previously received endocrine therapy [3,4,5]. Furthermore, ESR1 mutations have emerged as an essential mechanism of resistance to endocrine therapy and as predictive biomarkers for resistance to therapy and potential therapeutic targets.
With the improvements to next-generation sequencing (NGS) technology, genomic analysis of blood samples from cancer patients has been conducted in various studies [6], and the detection of ESR1 mutations in circulating tumour DNA (ctDNA) in patients with breast cancer has been reported [7,8,9,10,11]. In addition, the presence of ESR1 mutations has been reported to impact the efficacy of subsequent treatment modalities [7]. The most frequently occurring ESR1 mutations are in the ligand-binding domain (LBD) of ERα, generally clustering between amino acids 534 and 538. D538G, Y537S, and E380Q are the major hotspot mutations in ERα LBDs, and others detected include L536Q, Y537N, and Y537C. Preclinical studies have shown that these mutations confer resistance to hormone therapy [5,12,13,14,15]. Polyclonal detection of these genomic hotspot mutations after endocrine therapy has been reported [16]. Evaluating ESR1 mutations in ctDNA using NGS technology can detect rare mutation sites and polyclonal mutations; however, it requires a sufficient amount of nucleic acid and is time-consuming. In contrast, a digital polymerase chain reaction (PCR) can evaluate hotspot mutations with high sensitivity, as shown in several studies [7,8,10,11,16,17,18]. However, a PCR can only detect mutations at specifically defined sites.
A peptide nucleic acid (PNA)-based PCR is a highly sensitive and specific approach for the detection of genetic mutations. This technique enhances the specificity of the PCR reaction by targeting the initial step involved in nonspecific amplification, namely, primer binding to a mismatched target sequence. PNA is a DNA analogue in which the four nucleotides, adenine (A), thymine (T), guanine (G), and cytosine (C), are attached to an N-(2-aminoethyl) glycine backbone rather than to the negatively charged deoxyribose phosphate backbone that is present in DNA [19]. Genomic analysis using the PNA-locked nucleic acid (LNA)-mediated PCR clamping (PNA-LNA PCR clamp) method has been shown to be highly sensitive in detecting gene mutations in EGFR and RAS [20,21,22,23]. Although the use of ctDNA is a less invasive and less expensive method for assessing ESR1 mutations, few studies have evaluated its feasibility compared with that of NGS. The amplified product of clamp PCR can also be directly sequenced to evaluate mutations in the defined region; therefore, this feature was considered useful for assessing polyclonal ESR1 mutations. To detect polyclonal ESR1 mutations with simplicity, low cost, and low invasiveness, we developed a novel assay for the detection of polyclonal ESR1 mutations in ctDNA using the PNA-LNA PCR clamp method. In addition, patient blood samples were prospectively collected to evaluate the feasibility of this system compared with that of NGS.
2. Materials and Methods
2.1. Design for the Detection of ESR1 Mutations
To develop a detection system for ESR1 mutations using PNA-LNA PCR clamping, we focused on three common hotspot mutations in ESR1 (E380Q, Y537S, and D538G) that have been reported to be important for acquired resistance to endocrine therapy [7,24,25]. In addition to these three hotspots, other ESR1 mutations have also been detected at lower frequencies, mainly in the region of codons 536–538 [25]. Therefore, we performed direct sequencing of the 536–538 codon region using amplified products obtained by clamp PCR targeting D538G and Y537S to identify additional mutations in this region (Figure 1).
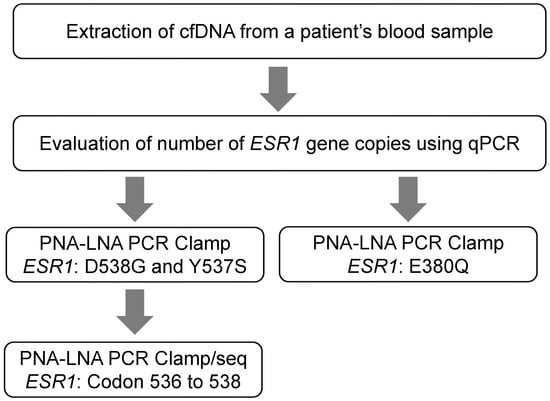
Figure 1.
Detection of ESR1 mutations in ctDNA using a PNA-LNA PCR clamp assay. cfDNA, cell free DNA; LNA, locked nucleic acid; PNA, peptide nucleic acid.
2.2. Plasmids
Wild-type fragments containing the ESR1 gene were amplified using PCR from normal human genomic DNA (gDNA). The primers used are shown in Table 1. The amplified fragments were cloned into the pGEM® T Easy vector (Promega, Madison, WI, USA), and the plasmid DNA was extracted. Plasmids with ESR1 mutations were produced by transfection of wild-type plasmid DNA into the template using the PrimeSTAR Mutagenesis Basal Kit (Takara Bio, Kusatsu, Japan).

Table 1.
Primer sequences.
2.3. PNA-LNA PCR Clamp Assay
The number of ESR1 copies per 2 μL of ctDNA of each sample was calculated based on a standard by real-time PCR. A standard was prepared with 1.0 × 102 to 1.0 × 105 copies from wild-type gDNA. The primers used are shown in Table S1. Real-time PCR was performed using the LightCycler® 480 System II (Roche Life Science, Penzberg, Germany). The thermal cycling profile was 95 °C for 5 min, followed by 50 cycles of 95 °C for 5 s and 62 °C for 30 s. Genomic DNA was purified using a QIAamp DNA Blood Kit (Qiagen, Hilden, Germany).
ESR1 mutations E380Q, Y537S, and D538G were analysed. PNA-LNA clamp PCR was performed using 4 μL of ctDNA from each patient sample as template DNA. Mutational analyses of Y537S and D538G were performed simultaneously. PNA-LNA clamp PCR was performed using the LightCycler® 480 System II (Roche Life Science, Germany). PCR cycling commenced at 95 °C for 5 min, followed by 50 cycles of 95 °C for 5 s and 62 °C for 30 s.
PNA-LNA clamp PCR products obtained during the mutational analysis of Y537S and D538G were used as template DNAs for amplifying the regions containing codons 536, 537, and 538 using nested PCR. These PCR products were used as template DNA and then sequenced by direct sequencing. The sequencing reagent used was the BigDye Terminator V3.1 Cycle Sequencing kit (Applied Biosystems/Thermo Fisher Scientific, Waltham, MA, USA). Sequencing reaction samples were purified using the BigDye X Terminator Purification Kit (Applied Biosystems/Thermo Fisher Scientific, MA, USA) and then analysed using the PRISM 3730XL (Applied Biosystems/Thermo Fisher Scientific, MA, USA) to detect mutations in codons 536, 537, and 538 of the ESR1 gene.
The efficiency of PCR clamping was determined by measuring the crossing point (Cp) value. The delta Cp (ΔCp) value was calculated as follows: Cp of sample with PNA—Cp of sample without PNA. ‘Cp of sample without PNA’ was calculated as a conversion from the ESR1 copy number.
2.4. Clinical Samples
To evaluate methods for the detection of ESR1 gene mutations in blood samples, a prospective study was conducted in patients with recurrent metastatic hormone receptor-positive breast cancer at the National Cancer Center Hospital, Japan, from June 2019 to January 2021. The study included two cohorts: (1) the developmental cohort and (2) the prospective validation cohort (Figure 2). Patient characteristics are shown in Table S1. Patients who consented to the study had 12 mL of blood collected when they were diagnosed with progressive disease (PD) following endocrine therapy or chemotherapy. Ethylene-diaminetetraacetic acid (EDTA) 2K tubes were used as blood collection tubes. Institutional review board and ethics committee approval were obtained from our institutions. This study was conducted in accordance with the Declaration of Helsinki [26], and the study protocol was approved by the Institutional Review Board of the National Cancer Center Hospital, Tokyo, Japan (approval number: 2016-024). All participants provided written informed consent prior to the study-related procedures. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines (http://www.strobe-statement.org/ (accessed on 29 May 2023)). No statistical methods were used to determine the sample size. Mutations obtained from clinical samples were blinded from the investigators to avoid bias.
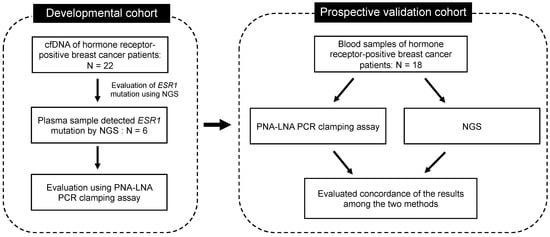
Figure 2.
Schematic representation of the developmental and prospective validation cohorts of patients with breast cancer.
2.5. Clinical Sample Collection and Cell-Free DNA/TNA Extraction
Plasma samples were collected in EDTA tubes and stored at −80 °C. For the developmental cohort, cell-free DNA (cfDNA) was obtained from collected peripheral blood using the MagMAXTM Cell-Free DNA Isolation kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. For the prospective validation cohort, cell-free total nucleic acid (cfTNA) was extracted from blood using the MagMAX™ Cell-Free Total Nucleic Acid Isolation Kit (Thermo Fisher Scientific, MA, USA). The amounts of cfDNA and cfTNA were quantified using the Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific, MA, USA).
2.6. Targeted NGS
The method of NGS for evaluating ESR1 mutation in patient samples is shown in the Supplementary Information.
3. Results
3.1. PNA-LNA PCR Clamp Reaction for the Detection of ESR1 Mutations
We checked the amplification of the target product using electrophoresis to evaluate the performance of the designed primers. Specific amplification of the target product was confirmed (Figure 3A). We performed PNA clamp assays to confirm that the mutant allele was not amplified, and that the wild-type allele was amplified (Figure 3B). The specificity of the LNA probes for mutation detection was evaluated. Without the addition of PNA, the wild-type gDNA showed a slight signal for D538G, which was suppressed by the addition of PNA (Figure 3C). Finally, the sensitivity of the probes for the detection of ESR1 gene mutations was evaluated using standard samples (Figure 3D). The probes for all ESR1 gene mutations showed a detection sensitivity of 0.1%. Standard samples of ESR1 gene mutations in Y537S and L536R were evaluated by direct sequencing, and mutations were detected with a sensitivity of 0.1%.
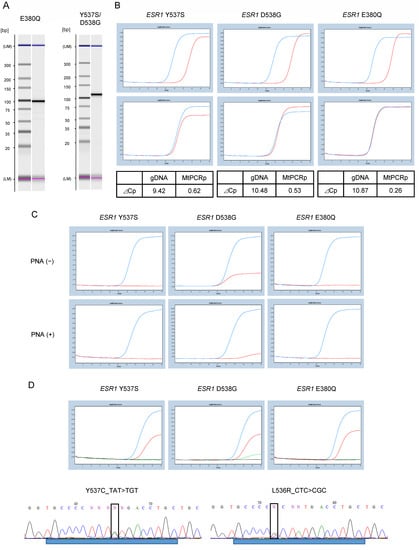
Figure 3.
PNA-LNA PCR clamp assay for the detection of ESR1 mutations. (A) Evaluation of primer performance. Amplification of the target product is confirmed by electrophoresis. Specific amplification of the target product is confirmed at an elongation temperature of 62 °C. Left: Primer for E380Q, 100 bp. Right: Primer for Y537S and D538G, 111 bp. (B) Performance evaluation of the PNA clamp assay. Top: wild-type allele (1.0 × 104). Bottom: mutant allele (1.0 × 104). Amplification was suppressed for the wild-type allele although not for the mutant allele. ⊿Cp value is calculated at threshold 0.5. (C) Evaluation of the specificity of LNA probes for mutation detection. The upper figure shows the analysis without PNA, and the lower figure shows the analysis with PNA. Blue line: mutant allele; red line: wild-type allele. (D) Standard samples of 1% and 0.1% were assayed with the probe to confirm the sensitivity of mutation detection. Analysis of 1.0% (blue line) and 0.1% (red line) of standard samples with mutations. Green line: wild-type ESR1; black line: distilled water. The bottom figure shows the sequence results from direct sequencing for 0.1% of the standard sample of Y537C and L536R mutations. ⊿Cp, change in crossing point; LNA, locked nucleic acid; PNA, peptide nucleic acid.
3.2. Developmental Cohort
We then validated the assay using clinical samples. cfDNA was extracted from the blood samples of patients with hormone receptor-positive breast cancer, and ESR1 mutations were identified by NGS using the Oncomine pan-cancer cell-free assay. ESR1 mutations were detected in 6 out of 22 patients. The sites and allele frequencies of the mutations detected in each patient sample are shown in Table S2. Of the six patients with ESR1 mutations, five had polyclonal mutations. The concordance of detection of ESR1 mutations between the PNA-LNA PCR clamp method and NGS was evaluated for these six samples with ESR1 mutations and four samples without ESR1 mutations. The median Cp value of the samples in which ESR1 gene mutations were detected was 28.02, compared with a Cp value of 29.70 for samples in which ESR1 gene mutations were not detected, showing no significant difference (Mann–Whitney U test).
The detection of E380Q, Y537S, and D538G mutations using the PNA-LNA PCR clamp assay was positive in all the samples confirmed by NGS (Table 2, Table 3 and Table 4). We further evaluated whether these mutations were detectable by direct sequencing and found that they were indeed present in all the samples (Figure S1A). The PNA-LNA PCR clamp assay detected a D538G mutation in one of the four samples that did not show any genetic mutations by NGS (Table 4). This specimen was evaluated by direct sequencing and was found to contain a D538G mutation (Figure S1B). Additionally, L536H, Y537N, and Y537C were detected by NGS as hotspot mutations in addition to E380Q, Y537S, and D538G (Table S2). Specimens with L536H mutations also showed Y537S and D538G mutations, and direct sequencing detected Y537S and D538G, although not L536H mutations (Sample #4, Figure S1A). In the two specimens in which Y537N mutations were identified by NGS, one was detected by the PNA-LNA PCR clamp reaction assay, while the other was not (Samples #1 and #3, Figure S1C). The Y537N mutation was not detected in either sample by direct sequencing. Samples with the Y537C mutation were confirmed to have the mutation by direct sequencing (Sample #6, Figure S1D).

Table 2.
Evaluation of E380Q detection using PNA-LNA PCR clamp reaction.

Table 3.
Evaluation of Y537S detection using PNA-LNA PCR clamp reaction.

Table 4.
Evaluation of D538G detection using PNA-LNA PCR clamp reaction.
Overall, we found that the sensitivity and accuracy of the detection of ESR1 mutations using the PNA-LNA PCR clamping assay were comparable with those of NGS.
3.3. Prospective Validation Cohort
Next, we evaluated whether PNA-LNA PCR clamping could identify ESR1 mutations as accurately as NGS in patient samples. Eighteen patients with recurrent metastatic hormone receptor-positive breast cancer who developed PD following previous treatment were enrolled, and their blood samples were analysed. The detection of ESR1 mutations and hotspot regions using PNA-LNA PCR clamping and NGS is shown in Table 5. PNA-LNA PCR clamping detected ESR1 mutations in 5 out of 18 samples. The recommended amount of cfTNA for the analysis using the Oncomine™ Precision Assay was 1.33 ng/µL (20 µL), and 6 out of the 18 samples met that criterion (Table S3). The two samples (#11 and #15) in which ESR1 mutations were detected by PNA-LNA PCR clamping and not by NGS had very low quantities of cfDNA (less than 0.5 ng/µL) (Table S4). These results indicate that our method is highly sensitive for detecting ESR1 mutations in samples where cfDNA levels are too low to be analysed by NGS.

Table 5.
Detection of ESR1 mutations using PNA-LNA PCR clamping compared with NGS.
4. Discussion
Here, we report a novel method for the detection of ESR1 mutations in ctDNA using PNA-LNA PCR clamping. Although the PNA-LNA PCR clamp method is highly sensitive, a large number of samples and multiple PCR reactions would be needed to detect a wide range of gene mutations. Because a simple, low-cost, and minimally invasive blood test is desirable for use in clinical practice, we designed an assay method specifically for the detection of high-frequency mutations and mutations associated with therapeutic resistance. The common mutations D538G, Y537S, and E380Q were detected using LNA probes, and additional mutations in codons 536–538 were detected by direct sequencing of the amplified clamp PCR product (Figure 1).
ER-positive breast cancer accounts for approximately 70% of all breast cancers, and endocrine therapies are effective in these patients. Various mechanisms of resistance to endocrine therapies have been reported, including ESR1 missense mutations [3,4,5]. More than 50 ESR1 missense mutations have been identified [27]. Although the clinical significance of all hotspot mutations is unknown, ESR1 mutations in the LBD region are associated with resistance to tamoxifen and fulvestrant [5,13,15,28]. The most common ESR1 mutations are E380Q, Y537S, and D538G, and these hotspots account for 70% of the ESR1 mutations [29]. Recently, many studies have reported the detection of ESR1 mutations using NGS and digital PCR [7,8,9,10,11,16,17,18]. Although NGS is expensive [30,31] and requires high concentrations of cfDNA, it is commonly used to detect polyclonal ESR1 mutations. We found that our method detected polyclonal ESR1 mutations with a sensitivity similar to that of NGS and could be performed using only 3 mL of plasma, thus providing a minimally invasive and cost-effective monitoring method. An inexpensive, low-invasive method for detecting ESR1 mutations in breast cancer patients on hormone therapy is very relevant because it could help in identifying patients who may benefit from potential therapeutic options, such as Elacestrant [32], in addition to assessing resistance to hormone therapy.
The PNA-LNA PCR clamp method uses a PNA complementary to the wild-type sequence and a fluorophore-labelled LNA complementary to the mutant sequence to evaluate mutations in the target gene. Because PNA probes form a high-strength double-stranded bond with DNA, a single-base mismatch causes a significant decrease in the melting temperature. In addition, a PNA is less susceptible to displacement by DNA polymerase. Thus, the addition of a PNA sequence complementary to the wild-type sequence inhibits the amplification of the wild-type sequence in the reaction mix. LNA, like PNA, also forms a high-strength double-stranded bond with DNA, and the Tm is greatly reduced by a single-base mismatch. Therefore, the use of a fluorophore-labelled LNA, which is complementary to the mutant sequence, enables the highly sensitive and specific detection of small amounts of mutant sequences in a background of wild-type sequences. While previously reported systems for detecting EGFR and RAS gene mutations using the PNA-LNA PCR clamp method detect targeted hot spot mutations [20,21,22,23], this analysis can detect mutations in the 536–538 codon region of ESR1 in addition to the target hot spot mutation. Unlike digital PCR, the PNA-LNA clamp method is qualitative rather than quantitative. Although quantitative evaluation of some mutations, such as PIK3CA, has been reported to be significant in predicting treatment response [33], the significance of quantitative evaluation of ESR1 mutations has not been clarified. However, the ESR1 mutation is a biomarker that predicts a weaker response to endocrine therapy, and its qualitative detection is considered to be significant.
While our results are promising, it is not entirely clear whether these results can be applied to the detection of all polyclonal ESR1 mutations in ctDNA. The number of patient samples used in this study was small, and validation of the polyclonal hotspot mutations was limited. In order to evaluate the detection sensitivity for several polyclonal hotspot mutations, it is necessary to validate the system with a larger number of patient specimens. Furthermore, our method cannot detect all potential ESR1 mutations across all regions of the gene as effectively as NGS.
5. Conclusions
In conclusion, our PNA-LNA-mediated PCR clamping assay is a highly sensitive and minimally invasive assay that could be a useful monitoring tool for the detection of ESR1 mutations in the cfDNA of patients with breast cancer.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/diagnostics13122040/s1, Figure S1: Identification of ESR1 mutations in clinical samples using PNA-LNA PCR clamp assay; Table S1: Patient characteristics in the developmental and prospective validation cohorts; Table S2: Sites and allele frequencies (%) of ESR1 mutations detected using next-generation sequencing; Table S3: Gene alterations detected using Oncomine™ Precision Assay; Table S4: Read number and coverage of next-generation sequencing.
Author Contributions
Concept and design, Y.K., E.N., K.T. and K.Y.; sample collection, Y.K., E.N., S.Y. (Shu Yazaki), H.S.O., T.N., M.T., K.S., T.S. and Y.F.; acquisition, analysis, or interpretation of data, all authors; drafting of the manuscript, Y.K. and K.Y.; administrative, technical, or material support, T.Y., S.Y. (Shigehiro Yagishita), A.K., H.T. and S.A.; supervision, A.H., and K.Y., Y.K. and K.Y. had full access to all the data and take responsibility for their integrity the accuracy of their analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly funded by LSI Medience Corporation and Thermo Fisher Scientific.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and was approved by the Institutional Review Board of the National Cancer Center Hospital, Tokyo, Japan (approval number: 2016-024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Sequence files are available in the NCBI database (PRJNA884163). Additional analysed data files are available in the Supplementary Information of this article.
Acknowledgments
We thank all the participants for their participation in this study. We thank Nao Nakamura and Kyoko Onozawa for their secretarial assistance. The National Cancer Center Biobank is supported by the National Cancer Center Research and Development Fund, Japan. Editage and Cactus Communications provided editorial support in the form of medical writing, assembling tables, and creating high-resolution images based on the authors’ detailed directions, collating author comments, copyediting, fact-checking, and referencing.
Conflicts of Interest
Yuki Kojima, Tomomi Yoshino, Shigehiro Yagishita, Shu Yazaki, Hitomi S. Okuma, Kazuki Sudo, Maki Tanioka, Tatsunori Shimoi, Kenji Tamura have nothing to disclose; Tadaaki Nishikawa received research funds from Takeda Pharmaceutical Company, Eisai, AstraZeneca, outside the submitted work; Emi Noguchi received research funds from Pfizer, Taiho, Eli Lilly, AstraZeneca, Chugai, Eisai, outside the submitted work; Ayaka Kazama and Hiroshi Terasaki are employees of LSI Medience Corporation; Sachiro Asano is employees of Life Technologies Japan Ltd., Thermo Fisher Scientific; Akinobu Hamada received research funds from Shimadzu Corporation, Daiichi Sankyo Company, Chugai Pharmaceutical, AstraZeneca, outside the submitted work; Yasuhiro Fujiwara received research funds from AstraZeneca, Chugai, Daiichi Sankyo, Bristol-Myers, SRL, Santen, outside the submitted work; Kan Yonemori received research funds from Pfizer, AstraZeneca, Eisai, Takeda Pharmaceutical Company, Chugai, Ono Pharmaceutical Company, Novartis, Daiichi Sankyo, outside the submitted work.
References
- Clark, G.M.; Osborne, C.K.; McGuire, W.L. Correlations between estrogen receptor, progesterone receptor, and patient characteristics in human breast cancer. J. Clin. Oncol. 1984, 2, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Rumble, R.B.; Macrae, E.; Barton, D.L.; Connolly, H.K.; Dickler, M.N.; Fallowfield, L.; Fowble, B.; Ingle, J.N.; Jahanzeb, M.; et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology Guideline. J. Clin. Oncol. 2016, 34, 3069–3103. [Google Scholar] [CrossRef]
- Najim, O.; Seghers, S.; Sergoynne, L.; Van Gaver, H.; Papadimitriou, K.; Wouters, K.; Trinh, X.B.; Huizing, M.T.; Tjalma, W. The association between type of endocrine therapy and development of estrogen receptor-1 mutation(s) in patients with hormone-sensitive advanced breast cancer: A systematic review and meta-analysis of randomized and non-randomized trials. Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 188315. [Google Scholar] [CrossRef] [PubMed]
- Jeselsohn, R.; Yelensky, R.; Buchwalter, G.; Frampton, G.; Meric-Bernstam, F.; Gonzalez-Angulo, A.M.; Ferrer-Lozano, J.; Perez-Fidalgo, J.A.; Cristofanilli, M.; Gómez, H.; et al. Emergence of constitutively active estrogen receptor- α mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin. Cancer Res. 2014, 20, 1757–1767. [Google Scholar] [CrossRef] [PubMed]
- Toy, W.; Shen, Y.; Won, H.; Green, B.; Sakr, R.A.; Will, M.; Li, Z.; Gala, K.; Fanning, S.; King, T.A.; et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat. Genet. 2013, 45, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Haber, D.A.; Velculescu, V.E. Blood-based analyses of cancer: Circulating tumor cells and circulating tumor DNA. Cancer Discov. 2014, 4, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Fribbens, C.; O’Leary, B.; Kilburn, L.; Hrebien, S.; Garcia-Murillas, I.; Beaney, M.; Cristofanilli, M.; Andre, F.; Loi, S.; Loibl, S.; et al. Plasma ESR1 mutations and the treatment of estrogen receptor-positive advanced breast cancer. J. Clin. Oncol. 2016, 34, 2961–2968. [Google Scholar] [CrossRef]
- Schiavon, G.; Hrebien, S.; Garcia-Murillas, I.; Cutts, R.J.; Pearson, A.; Tarazona, N.; Fenwick, K.; Kozarewa, I.; Lopez-Knowles, E.; Ribas, R.; et al. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci. Transl. Med. 2015, 7, 313ra182. [Google Scholar] [CrossRef]
- Guttery, D.S.; Page, K.; Hills, A.; Woodley, L.; Marchese, S.D.; Rghebi, B.; Hastings, R.K.; Luo, J.; Pringle, J.H.; Stebbing, J.; et al. Noninvasive detection of activating estrogen receptor 1 (ESR1) mutations in estrogen receptor-positive metastatic breast cancer. Clin. Chem. 2015, 61, 974–982. [Google Scholar] [CrossRef]
- Chu, D.; Paoletti, C.; Gersch, C.; VanDenBerg, D.A.; Zabransky, D.J.; Cochran, R.L.; Wong, H.Y.; Toro, P.V.; Cidado, J.; Croessmann, S.; et al. ESR1 mutations in circulating plasma tumor dna from metastatic breast cancer patients. Clin. Cancer Res. 2016, 22, 993–999. [Google Scholar] [CrossRef]
- Wang, P.; Bahreini, A.; Gyanchandani, R.; Lucas, P.C.; Hartmaier, R.J.; Watters, R.J.; Jonnalagadda, A.R.; Bittar, H.E.T.; Berg, A.; Hamilton, R.L.; et al. Sensitive detection of mono- and polyclonal ESR1 mutations in primary tumors, metastatic lesions, and cell-free DNA of breast cancer patients. Clin. Cancer Res. 2016, 22, 1130–1137. [Google Scholar] [CrossRef]
- Weis, K.E.; Ekena, K.; Thomas, J.A.; Lazennec, G.; Katzenellenbogen, B.S. Constitutively active human estrogen receptors containing amino acid substitutions for tyrosine 537 in the receptor protein. Mol. Endocrinol. 1996, 10, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.X.; Borg, A.; Wolf, D.M.; Oesterreich, S.; Fuqua, S.A. An estrogen receptor mutant with strong hormone-independent activity from a metastatic breast cancer. Cancer Res. 1997, 57, 1244–1249. [Google Scholar]
- Merenbakh-Lamin, K.; Ben-Baruch, N.; Yeheskel, A.; Dvir, A.; Soussan-Gutman, L.; Jeselsohn, R.; Yelensky, R.; Brown, M.; Miller, V.A.; Sarid, D.; et al. D538G mutation in estrogen receptor-α: A novel mechanism for acquired endocrine resistance in breast cancer. Cancer Res. 2013, 73, 6856–6864. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.R.; Wu, Y.M.; Vats, P.; Su, F.; Lonigro, R.J.; Cao, X.; Kalyana-Sundaram, S.; Wang, R.; Ning, Y.; Hodges, L.; et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat. Genet. 2013, 45, 1446–1451. [Google Scholar] [CrossRef]
- Takeshita, T.; Yamamoto, Y.; Yamamoto-Ibusuki, M.; Inao, T.; Sueta, A.; Fujiwara, S.; Omoto, Y.; Iwase, H. Droplet digital polymerase chain reaction assay for screening of ESR1 mutations in 325 breast cancer specimens. Transl. Res. 2015, 166, 540–553. [Google Scholar] [CrossRef]
- Chandarlapaty, S.; Chen, D.; He, W.; Sung, P.; Samoila, A.; You, D.; Bhatt, T.; Patel, P.; Voi, M.; Gnant, M.; et al. Prevalence of ESR1 mutations in cell-free DNA and outcomes in metastatic breast cancer: A secondary analysis of the BOLERO-2 clinical trial. JAMA Oncol. 2016, 2, 1310–1315. [Google Scholar] [CrossRef]
- Jeannot, E.; Darrigues, L.; Michel, M.; Stern, M.H.; Pierga, J.Y.; Rampanou, A.; Melaabi, S.; Benoist, C.; Bièche, I.; Vincent-Salomon, A.; et al. A single droplet digital PCR for ESR1 activating mutations detection in plasma. Oncogene 2020, 39, 2987–2995. [Google Scholar] [CrossRef]
- Giesen, U.; Kleider, W.; Berding, C.; Geiger, A.; Orum, H.; Nielsen, P.E. A formula for thermal stability (Tm) prediction of PNA/DNA duplexes. Nucleic Acids Res. 1998, 26, 5004–5006. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, H.; Tanaka, T.; Nagai, Y.; Matsuoka, M.; Huqun; Sutani, A.; Udagawa, K.; Zhang, J.; Hirama, T.; Murayama, Y.; et al. Peptide nucleic acid-locked nucleic acid polymerase chain reaction clamp-based detection test for gefitinib-refractory T790M epidermal growth factor receptor mutation. Cancer Sci. 2008, 99, 595–600. [Google Scholar] [CrossRef]
- Nagai, Y.; Miyazawa, H.; Huqun; Tanaka, T.; Udagawa, K.; Kato, M.; Fukuyama, S.; Yokote, A.; Kobayashi, K.; Kanazawa, M.; et al. Genetic heterogeneity of the epidermal growth factor receptor in non-small cell lung cancer cell lines revealed by a rapid and sensitive detection system, the peptide nucleic acid-locked nucleic acid PCR clamp. Cancer Res. 2005, 65, 7276–7282. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Lee, S.Y.; Hyun, D.S.; Lee, M.K.; Lee, H.K.; Choi, C.M.; Yang, S.H.; Kim, Y.C.; Lee, Y.C.; Kim, S.Y.; et al. Detection of EGFR mutations in circulating free DNA by PNA-mediated PCR clamping. J. Exp. Clin. Cancer Res. 2013, 32, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Itonaga, M.; Matsuzaki, I.; Warigaya, K.; Tamura, T.; Shimizu, Y.; Fujimoto, M.; Kojima, F.; Ichinose, M.; Murata, S. Novel methodology for rapid detection of KRAS mutation using PNA-LNA mediated loop-mediated isothermal amplification. PLoS ONE 2016, 11, e0151654. [Google Scholar] [CrossRef] [PubMed]
- Spoerke, J.M.; Gendreau, S.; Walter, K.; Qiu, J.; Wilson, T.R.; Savage, H.; Aimi, J.; Derynck, M.K.; Chen, M.; Chan, I.T.; et al. Heterogeneity and clinical significance of ESR1 mutations in ER-positive metastatic breast cancer patients receiving fulvestrant. Nat. Commun. 2016, 7, 11579. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.A.; Guttery, D.S.; Hills, A.; Fernandez-Garcia, D.; Page, K.; Rosales, B.M.; Goddard, K.S.; Hastings, R.K.; Luo, J.; Ogle, O.; et al. Mutation analysis of cell-free DNA and single circulating tumor cells in metastatic breast cancer patients with high circulating tumor cell counts. Clin. Cancer Res. 2017, 23, 88–96. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Dustin, D.; Gu, G.; Fuqua, S.A.W. ESR1 mutations in breast cancer. Cancer 2019, 125, 3714–3728. [Google Scholar] [CrossRef]
- Gelsomino, L.; Gu, G.; Rechoum, Y.; Beyer, A.R.; Pejerrey, S.M.; Tsimelzon, A.; Wang, T.; Huffman, K.; Ludlow, A.; Andò, S.; et al. ESR1 mutations affect anti-proliferative responses to tamoxifen through enhanced cross-talk with IGF signaling. Breast Cancer Res. Treat. 2016, 157, 253–265. [Google Scholar] [CrossRef]
- Toy, W.; Weir, H.; Razavi, P.; Lawson, M.; Goeppert, A.U.; Mazzola, A.M.; Smith, A.; Wilson, J.; Morrow, C.; Wong, W.L.; et al. Activating ESR1 mutations differentially affect the efficacy of ER antagonists. Cancer Discov. 2017, 7, 277–287. [Google Scholar] [CrossRef]
- Klouch, K.Z.; Stern, M.H.; Trabelsi-Grati, O.; Kiavue, N.; Cabel, L.; Silveira, A.B.; Hego, C.; Rampanou, A.; Popova, T.; Bataillon, G.; et al. Microsatellite instability detection in breast cancer using drop-off droplet digital PCR. Oncogene 2022, 41, 5289–5297. [Google Scholar] [CrossRef]
- Schluckebier, L.; Caetano, R.; Garay, O.U.; Montenegro, G.T.; Custodio, M.; Aran, V.; Gil Ferreira, C. Cost-effectiveness analysis comparing companion diagnostic tests for EGFR, ALK, and ROS1 versus next-generation sequencing (NGS) in advanced adenocarcinoma lung cancer patients. BMC Cancer 2020, 20, 875. [Google Scholar] [CrossRef] [PubMed]
- Bidard, F.C.; Kaklamani, V.G.; Neven, P.; Streich, G.; Montero, A.J.; Forget, F.; Mouret-Reynier, M.A.; Sohn, J.H.; Taylor, D.; Harnden, K.K. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: Results from the randomized phase III EMERALD trial. J. Clin. Oncol. 2022, 40, 3246–3256. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, B.; Hrebien, S.; Morden, J.P.; Beaney, M.; Fribbens, C.; Huang, X.; Liu, Y.; Bartlett, C.H.; Koehler, M.; Cristofanilli, M.; et al. Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer. Nat. Commun. 2018, 9, 896. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).