Effects of Cortactin Expression on Prognosis in Patients with Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Clinical Data Collection
2.2. Tissue Microarray (TMA) Construction
2.3. Immunohistochemical (IHC) Staining
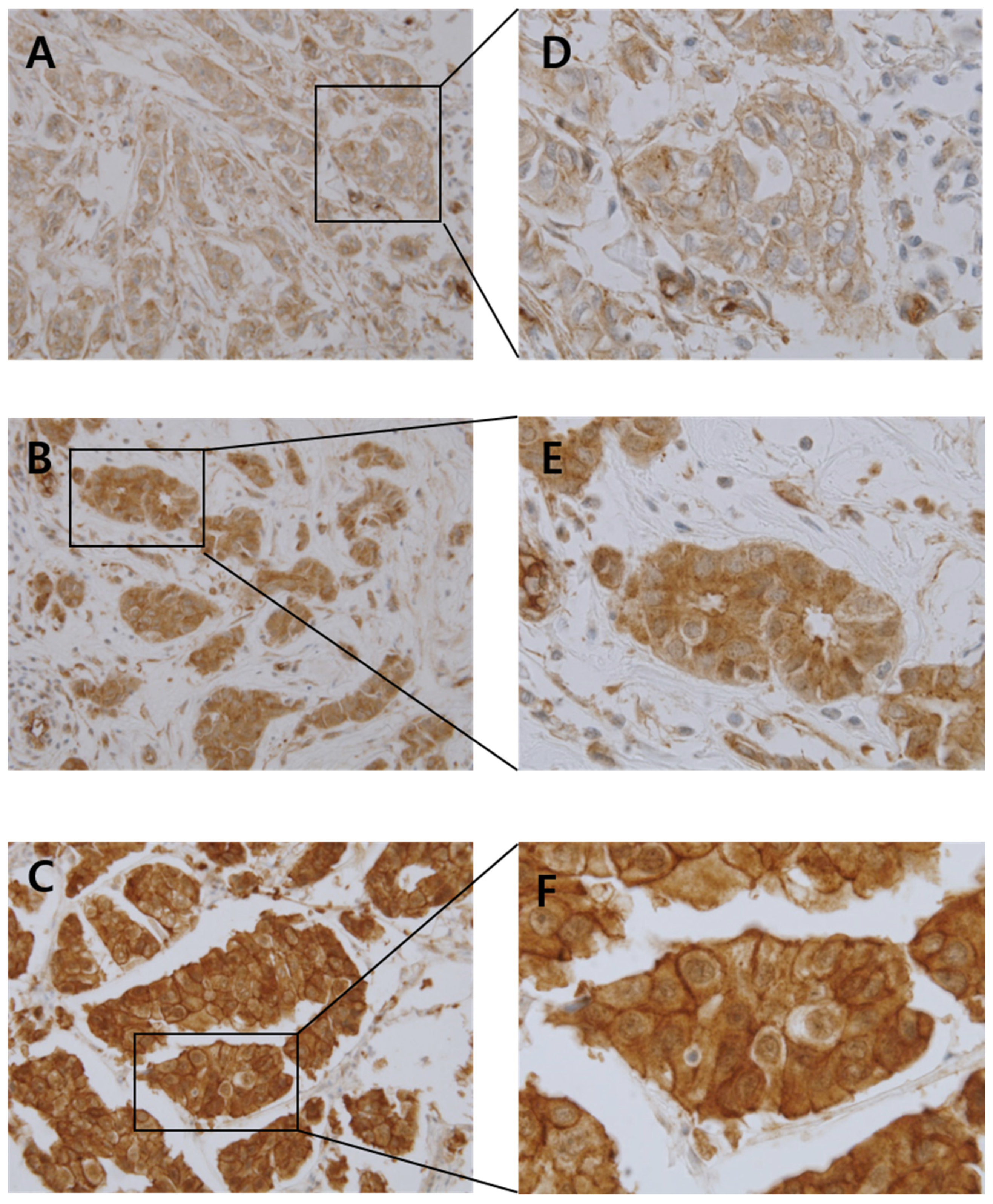
2.4. Grading for Cortactin Immunoreactivity
2.5. Statistical Analysis
3. Results
3.1. Clinicopathological Characteristics
3.2. Correlation between Cortactin Expression and Clinicopathological Parameters
3.3. Correlation between Cortactin Expression and Patient Outcomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Kim, H.; Jang, S.M.; Ahn, H.; Sim, J.; Yi, K.; Chung, Y.; Han, H.; Rehman, A.; Chung, M.S.; Jang, K.; et al. Clinicopathological significance of dual-specificity protein phosphatase 4 expression in invasive ductal carcinoma of the breast. J. Breast Cancer 2015, 18, 1–7. [Google Scholar] [CrossRef]
- Rouzier, R.; Perou, C.M.; Symmans, W.F.; Ibrahim, N.; Cristofanilli, M.; Anderson, K.; Hess, K.R.; Stec, J.; Ayers, M.; Wagner, P.; et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin. Cancer Res. 2005, 11, 5678–5685. [Google Scholar] [CrossRef] [PubMed]
- Bertolo, C.; Guerrero, D.; Vicente, F.; Cordoba, A.; Esteller, M.; Ropero, S.; Guillen-Grima, F.; Martinez-Penuela, J.M.; Lera, J.M. Differences and molecular immunohistochemical parameters in the subtypes of infiltrating ductal breast cancer. Am. J. Clin. Pathol. 2008, 130, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Schuuring, E.; Verhoeven, E.; Litvinov, S.; Michalides, R.J. The product of the EMS1 gene, amplified and overexpressed in human carcinomas, is homologous to a v-src substrate and is located in cell-substratum contact sites. Mol. Cell. Biol. 1993, 13, 2891–2898. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Reynolds, A.; Kanner, S.; Vines, R.; Parsons, J. Identification and characterization of a novel cytoskeleton-associated pp60src substrate. Mol. Cell. Biol. 1991, 11, 5113–5124. [Google Scholar]
- Weaver, A.M. Cortactin in tumor invasiveness. Cancer Lett. 2008, 265, 157–166. [Google Scholar] [CrossRef]
- Kirkbride, K.C.; Sung, B.H.; Sinha, S.; Weaver, A.M. Cortactin: A multifunctional regulator of cellular invasiveness. Cell Adhes. Migr. 2011, 5, 187–198. [Google Scholar] [CrossRef]
- Weaver, A.M.; Karginov, A.V.; Kinley, A.W.; Weed, S.A.; Li, Y.; Parsons, J.T.; Cooper, J.A. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr. Biol. 2001, 11, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Uruno, T.; Liu, J.; Zhang, P.; Fan, Y.-X.; Egile, C.; Li, R.; Mueller, S.C.; Zhan, X. Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat. Cell Biol. 2001, 3, 259–266. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Condeelis, J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim. Biophys. Acta 2007, 1773, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, J.P.; García, L.A.; Ramos, S.; Lazo, P.S.; Suárez, C. EMS1 gene amplification correlates with poor prognosis in squamous cell carcinomas of the head and neck. Clin. Cancer Res. 2000, 6, 3177–3182. [Google Scholar] [PubMed]
- Chuma, M.; Sakamoto, M.; Yasuda, J.; Fujii, G.; Nakanishi, K.; Tsuchiya, A.; Ohta, T.; Asaka, M.; Hirohashi, S. Overexpression of cortactin is involved in motility and metastasis of hepatocellular carcinoma. J. Hepatol. 2004, 41, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Ni, Q.F.; Yu, J.W.; Qian, F.; Sun, N.Z.; Xiao, J.J.; Zhu, J.W. Cortactin promotes colon cancer progression by regulating ERK pathway. Int. J. Oncol. 2015, 47, 1034–1042. [Google Scholar] [CrossRef]
- Zuo, Q.; Wu, W.; Li, X.; Zhao, L.; Chen, W. HDAC6 and SIRT2 promote bladder cancer cell migration and invasion by targeting cortactin. Oncol. Rep. 2012, 27, 819–824. [Google Scholar] [CrossRef]
- Hui, R.; Ball, J.R.; Macmillan, R.D.; Kenny, F.S.; Prall, O.W.; Campbell, D.H.; Cornish, A.L.; McClelland, R.A.; Daly, R.J.; Forbes, J.F.; et al. EMS1 gene expression in primary breast cancer: Relationship to cyclin D1 and oestrogen receptor expression and patient survival. Oncogene 1998, 17, 1053–1059. [Google Scholar] [CrossRef][Green Version]
- Chin, S.F.; Wang, Y.; Thorne, N.P.; Teschendorff, A.E.; Pinder, S.E.; Vias, M.; Naderi, A.; Roberts, I.; Barbosa-Morais, N.L.; Garcia, M.J.; et al. Using array-comparative genomic hybridization to define molecular portraits of primary breast cancers. Oncogene 2007, 26, 1959–1970. [Google Scholar] [CrossRef]
- Schuuring, E.; Verhoeven, E.; van Tinteren, H.; Peterse, J.L.; Nunnink, B.; Thunnissen, F.B.; Devilee, P.; Cornelisse, C.J.; van de Vijver, M.J.; Mooi, W.J. Amplification of genes within the chromosome 11q13 region is indicative of poor prognosis in patients with operable breast cancer. Cancer Res. 1992, 52, 5229–5234. [Google Scholar]
- Yin, M.; Ma, W.; An, L. Cortactin in cancer cell migration and invasion. Oncotarget 2017, 8, 88232–88243. [Google Scholar] [CrossRef]
- Cai, J.H.; Zhao, R.; Zhu, J.W.; Jin, X.L.; Wan, F.J.; Liu, K.; Ji, X.P.; Zhu, Y.B.; Zhu, Z.G. Expression of cortactin correlates with a poor prognosis in patients with stages II-III colorectal adenocarcinoma. J. Gastrointest. Surg. 2010, 14, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Cheng, X.; Ji, X.; He, Y.; Jing, X.; Wu, H.; Zhao, R. Cortactin contributes to the tumorigenicity of colorectal cancer by promoting cell proliferation. Oncol. Rep. 2016, 36, 3497–3503. [Google Scholar] [CrossRef] [PubMed]
- van Rossum, A.G.; van Bragt, M.P.; Schuuring-Scholtes, E.; van der Ploeg, J.C.; van Krieken, J.H.; Kluin, P.M.; Schuuring, E. Transgenic mice with mammary gland targeted expression of human cortactin do not develop (pre-malignant) breast tumors: Studies in MMTV-cortactin and MMTV-cortactin/-cyclin D1 bitransgenic mice. BMC Cancer 2006, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tondravi, M.; Liu, J.; Smith, E.; Haudenschild, C.C.; Kaczmarek, M.; Zhan, X. Cortactin Potentiates Bone Metastasis of Breast Cancer Cells1. Cancer Res. 2001, 61, 6906–6911. [Google Scholar] [PubMed]
- Bowden, E.T.; Onikoyi, E.; Slack, R.; Myoui, A.; Yoneda, T.; Yamada, K.M.; Mueller, S.C. Co-localization of cortactin and phosphotyrosine identifies active invadopodia in human breast cancer cells. Exp. Cell Res. 2006, 312, 1240–1253. [Google Scholar] [CrossRef] [PubMed]
- Dedes, K.J.; Lopez-Garcia, M.A.; Geyer, F.C.; Lambros, M.B.; Savage, K.; Vatcheva, R.; Wilkerson, P.; Wetterskog, D.; Lacroix-Triki, M.; Natrajan, R.; et al. Cortactin gene amplification and expression in breast cancer: A chromogenic in situ hybridisation and immunohistochemical study. Breast Cancer Res. Treat. 2010, 124, 653–666. [Google Scholar] [CrossRef]
- Sheen-Chen, S.-M.; Huang, C.-Y.; Liu, Y.-Y.; Huang, C.-C.; Tang, R.-P. Cortactin in Breast Cancer: Analysis with Tissue Microarray. Anticancer Res. 2011, 31, 293–297. [Google Scholar]
- Moon, S.-J.; Choi, H.-J.; Kye, Y.-H.; Jeong, G.-Y.; Kim, H.-Y.; Myung, J.-K.; Kong, G. CTTN Overexpression Confers Cancer Stem Cell-like Properties and Trastuzumab Resistance via DKK-1/WNT Signaling in HER2 Positive Breast Cancer. Cancer 2023, 15, 1168. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch. Pathol. Lab. Med. 2018, 142, 1364–1382. [Google Scholar] [CrossRef]
- Reis-Filho, J.S.; Pusztai, L. Gene expression profiling in breast cancer: Classification, prognostication, and prediction. Lancet 2011, 378, 1812–1823. [Google Scholar] [CrossRef]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef]
- Criscitiello, C.; Azim, H.A., Jr.; Schouten, P.C.; Linn, S.C.; Sotiriou, C. Understanding the biology of triple-negative breast cancer. Ann. Oncol. 2012, 23 (Suppl. S6), vi13–vi18. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Claus, E.; Sohl, J.; Razzak, A.R.; Arnaout, A.; Winer, E.P. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: High incidence of central nervous system metastases. Cancer 2008, 113, 2638–2645. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, L.N.; Wilkinson, K.H.; Kong, A. Triple-Negative Breast Cancer: Who Should Receive Neoadjuvant Chemotherapy? Surg. Oncol. Clin. N. Am. 2018, 27, 141–153. [Google Scholar] [CrossRef] [PubMed]

| Clinicopathological Characteristics | Value (%) | |
|---|---|---|
| Age (years, mean ± SD), (range) | 52.6 ± 11.1, (27–83) | |
| Size (cm, mean ± SD), (range) | 2.6 ± 1.8, (0.12–16) | |
| Sex | ||
| Male | 0 (0%) | |
| Female | 506 (100%) | |
| Histologic grade | ||
| Grade 1 | 112 (22.1%) | |
| Grade 2 | 226 (44.7%) | |
| Grade 3 | 168 (33.2%) | |
| Histologic type | ||
| Invasive breast carcinoma, No special type | 474 (93.7%) | |
| Lobular carcinoma | 22 (4.3%) | |
| Other | 10 (2.0%) | |
| Molecular type | ||
| Luminal A | 202 (40.0%) | |
| Luminal B | 109 (21.5%) | |
| HER2 | 87 (17.2%) | |
| Triple negative | 108 (21.3%) | |
| Unknown | 0 (0.0%) | |
| pT stage | ||
| T1 | 229 (45.3%) | |
| T2 | 233 (46.0%) | |
| T3 | 30 (5.9%) | |
| T4 | 14 (2.8%) | |
| pN stage | ||
| N0 | 319 (63.0%) | |
| N1 | 108 (21.4%) | |
| N2 | 44 (8.7%) | |
| N3 | 35 (6.9%) | |
| AJCC stage (8th) | ||
| I | 178 (35.2%) | |
| II | 232 (45.8%) | |
| III | 96 (19.0%) | |
| Recurrence | ||
| Recurrence | 93 (18.4%) | |
| No recurrence | 413 (81.6%) | |
| Death | ||
| Death | 57 (11.2%) | |
| Alive | 449 (88.7%) | |
| Clinocpathological Characteristics | Value (%) | |
|---|---|---|
| Age (years, mean ± SD), (range) | 50.9 ± 10.6 (27–75) | |
| Size (cm, mean ± SD), (range) | 3.1 ± 2.3 (0.7–16) | |
| Sex | ||
| Male | 0 (0%) | |
| Female | 108 (100%) | |
| Histologic grade | ||
| Grade 1 | 9 (8.3%) | |
| Grade 2 | 23 (21.3%) | |
| Grade 3 | 76 (70.4%) | |
| Histologic type | ||
| Invasive breast carcinoma, No special type | 101 (93.5%) | |
| Lobular carcinoma | 2 (1.9%) | |
| Other | 5 (4.6%) | |
| pT stage | ||
| T1 | 30 (27.8%) | |
| T2 | 65 (60.2%) | |
| T3 | 8 (7.4%) | |
| T4 | 5 (4.6%) | |
| pN stage | ||
| N0 | 69 (63.9%) | |
| N1 | 22 (20.4%) | |
| N2 | 7 (6.5%) | |
| N3 | 10 (9.2%) | |
| AJCC stage (8th) | ||
| I | 24 (22.2%) | |
| II | 62 (57.4%) | |
| III | 22 (20.4%) | |
| Recurrence | ||
| Recurrence | 19 (17.6%) | |
| No recurrence | 89 (82.4%) | |
| Death | ||
| Death | 17 (15.7%) | |
| Alive | 91 (84.3%) | |
| Parameter | Cortactin Expression | p Value | ||
|---|---|---|---|---|
| High Group (n = 333) No. (%) | Low Group (n = 173) No. (%) | |||
| Age | 0.597 † | |||
| (years, mean ± SD), (range) | 53.1 ± 11.3 (28–83) | 52.4 ± 10.9 (27–78) | ||
| Tumor size | 0.775 † | |||
| (cm, mean ± SD), (range) | 2.6 ± 1.9 (0.12–16) | 2.6 ± 1.6 (0.5–11) | ||
| Histologic grade | 0.238 | |||
| Grade 1 | 70 (21.0%) | 42 (24.3%) | ||
| Grade 2 | 144 (43.3%) | 82 (47.4%) | ||
| Grade 3 | 119 (35.7%) | 49 (28.3%) | ||
| Molecular type | 0.312 | |||
| Luminal A | 125 (37.6%) | 77 (44.5%) | ||
| Luminal B | 79 (23.7%) | 30 (17.3%) | ||
| HER2 | 58 (17.4%) | 29 (16.8%) | ||
| Triple negative | 71 (21.3%) | 37 (21.4%) | ||
| pT stage | 0.615 | |||
| T1 | 151 (45.4%) | 78 (45.1%) | ||
| T2 | 150 (45.0%) | 83 (48.0%) | ||
| T3 | 23 (6.9%) | 7 (4.0%) | ||
| T4 | 9 (2.7%) | 5 (2.9%) | ||
| pN stage | 0.761 | |||
| N0 | 209 (62.8%) | 110 (63.6%) | ||
| N1 | 75 (22.5%) | 33 (19.1%) | ||
| N2 | 27 (8.1%) | 17 (9.8%) | ||
| N3 | 22 (6.6%) | 13 (7.5%) | ||
| AJCC stage (8th) | 0.623 | |||
| I | 122 (36.6%) | 56 (32.4%) | ||
| II | 150 (45.1%) | 82 (47.4%) | ||
| III | 61 (18.3%) | 35 (20.2%) | ||
| Variables | Disease-Free Survival | Overall Survival | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Univariate analysis | ||||||
| Age (per 1 year) | 0.990 | (0.968–1.011) | 0.307 | 1 | (0.976–1.025) | 0.998 |
| Size (per 1 cm) | 1.207 | (1.111–1.311) | <0.001 | 1.255 | (1.144–1.376) | <0.001 |
| Histologic grade 2 | 2.670 | (1.190–5.989) | 0.017 | 2.766 | (0.957–8.000) | 0.060 |
| (vs. 1) | ||||||
| Histologic grade 3 | 3.923 | (1.746–8.816) | 0.001 | 5.624 | (1.981–15.966) | 0.001 |
| (vs. 1) | ||||||
| TNBC subtype | 1.095 | (0.641–1.871) | 0.741 | 1.764 | (0.998–3.112) | 0.051 |
| (vs. Luminal A, Luminal B, HER2) | ||||||
| pT stage 4 | 5.058 | (2.042–12.53) | <0.001 | 6.779 | (2.695–17.05) | <0.001 |
| (vs. 1, 2, 3) | ||||||
| pN stage 1, 2, 3 | 3.114 | (1.979–4.897) | <0.001 | 2.802 | (1.643–4.777) | <0.001 |
| (vs. 0) | ||||||
| AJCC stage II (vs. I) | 2.581 | (1.351–4.929) | 0.004 | 2.401 | (1.083–5.323) | 0.031 |
| AJCC stage III (vs. I) | 5.210 | (2.658–10.214) | <0.001 | 5.967 | (2.679–13.287) | <0.001 |
| Cortactin expression low (vs. high) | 1.218 | (0.774–1.915) | 0.394 | 1.069 | (0.620–1.842) | 0.811 |
| Multivariate | ||||||
| analysis | ||||||
| Age (per 1 year) | 0.998 | 0.978–1.019 | 0.874 | 1.010 | 0.986–1.034 | 0.426 |
| Size (per 1 cm) | 1.076 | 0.969–1.195 | 0.161 | 1.119 | 0.997–1.257 | 0.057 |
| Histologic grade 2 (vs. 1) | 2.320 | 1.028–5.235 | 0.045 | 2.455 | 0.843–7.153 | 0.103 |
| Histologic grade 3 (vs. 1) | 2.953 | 1.286–6.778 | 0.011 | 4.152 | 1.434–12.026 | 0.009 |
| pT stage 4 (vs. 1, 2, 3) | 2.325 | 0.835–6.471 | 0.108 | 2.905 | 1.008–8.370 | 0.048 |
| pN stage 1, 2, 3 (vs. 0) | 2.595 | 1.624–4.146 | <0.001 | 2.139 | 1.230–3.718 | 0.007 |
| Cortactin expression low (vs. high) | 1.360 | (0.861–2.149) | 0.187 | 1.221 | 0.705–2.117 | 0.476 |
| Variables | Disease-Free Survival | Overall Survival | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Univariate analysis | ||||||
| Age (per 1 year) | 1.005 | (0.959–1.052) | 0.843 | 0.997 | (0.951–1.045) | 0.903 |
| Size (per 1 cm) | 1.005 | (0.810–1.247) | 0.965 | 0.997 | (0.791–1.257) | 0.981 |
| Histologic grade 2 (vs. 1) | 2.232 | (0.261–19.11) | 0.464 | 1.752 | (0.195–15.73) | 0.617 |
| Histologic grade 3 (vs. 1) | 1.477 | (0.191–11.44) | 0.709 | 1.666 | (0.216–12.84) | 0.624 |
| pT stage 4 (vs. 1,2,3) | 3.921 | (0.889–17.29) | 0.071 | 4.251 | (0.955–18.92) | 0.058 |
| pN stage 1, 2, 3 (vs. 0) | 2.323 | (0.8946–6.034) | 0.083 | 1.502 | (0.571–3.952) | 0.41 |
| AJCC stage II (vs. I) | 1.720 | (0.377–7.853) | 0.484 | 0.6478 | (0.195–2.154) | 0.479 |
| AJCC stage III (vs. I) | 2.575 | (0.499–13.293) | 0.259 | 1.2367 | (0.332–4.611) | 0.752 |
| Cortactin expression | 2.199 | (0.847–5.71) | 0.105 | 3.511 | (1.331–9.258) | 0.011 |
| low (vs. high) | ||||||
| Multivariate analysis | ||||||
| Age (per 1 year) | 1.003 | 0.958–1.050 | 0.891 | 0.997 | 0.951–1.045 | 0.910 |
| Size (per 1 cm) | 0.821 | 0.619–1.087 | 0.169 | 0.827 | 0.606–1.127 | 0.228 |
| Histologic grade 2 (vs. 1) | 2.001 | 0.219–18.263 | 0.539 | 2.078 | 0.214–20.175 | 0.528 |
| Histologic grade 3 (vs. 1) | 1.100 | 0.129–9.354 | 0.933 | 1.816 | 0.214–15.403 | 0.584 |
| pT stage 4 (vs. 1, 2, 3) | 16.385 | 2.322–115.604 | 0.005 | 13.419 | 1.917–93.936 | 0.009 |
| pN stage 1, 2, 3 (vs. 0) | 2.926 | (1.036–8.262) | 0.043 | 1.556 | 0.560–4.320 | 0.396 |
| Cortactin expression | 2.529 | (0.946–6.763) | 0.065 | 4.058 | 1.489–11.056 | 0.006 |
| low (vs. high) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, H.; Jee, S.; Cha, H.; Song, K.; Bang, S.; Kim, H.; Paik, S.; Park, H.; Myung, J. Effects of Cortactin Expression on Prognosis in Patients with Breast Cancer. Diagnostics 2023, 13, 2876. https://doi.org/10.3390/diagnostics13182876
Son H, Jee S, Cha H, Song K, Bang S, Kim H, Paik S, Park H, Myung J. Effects of Cortactin Expression on Prognosis in Patients with Breast Cancer. Diagnostics. 2023; 13(18):2876. https://doi.org/10.3390/diagnostics13182876
Chicago/Turabian StyleSon, Hwangkyu, Seungyun Jee, Hyebin Cha, Kihyuk Song, Seongsik Bang, Hyunsung Kim, Seungsam Paik, Hosub Park, and Jaekyung Myung. 2023. "Effects of Cortactin Expression on Prognosis in Patients with Breast Cancer" Diagnostics 13, no. 18: 2876. https://doi.org/10.3390/diagnostics13182876
APA StyleSon, H., Jee, S., Cha, H., Song, K., Bang, S., Kim, H., Paik, S., Park, H., & Myung, J. (2023). Effects of Cortactin Expression on Prognosis in Patients with Breast Cancer. Diagnostics, 13(18), 2876. https://doi.org/10.3390/diagnostics13182876







