Ultrasound-Guided Femoral Vascular Access for Percutaneous Coronary and Structural Interventions
Abstract
1. Introduction
2. Existing Evidence Supporting the Use of Ultrasound-Guided Femoral Access
2.1. Ultrasound-Guided Femoral Access for PCI
2.2. Ultrasound-Guided Femoral Access for TAVI
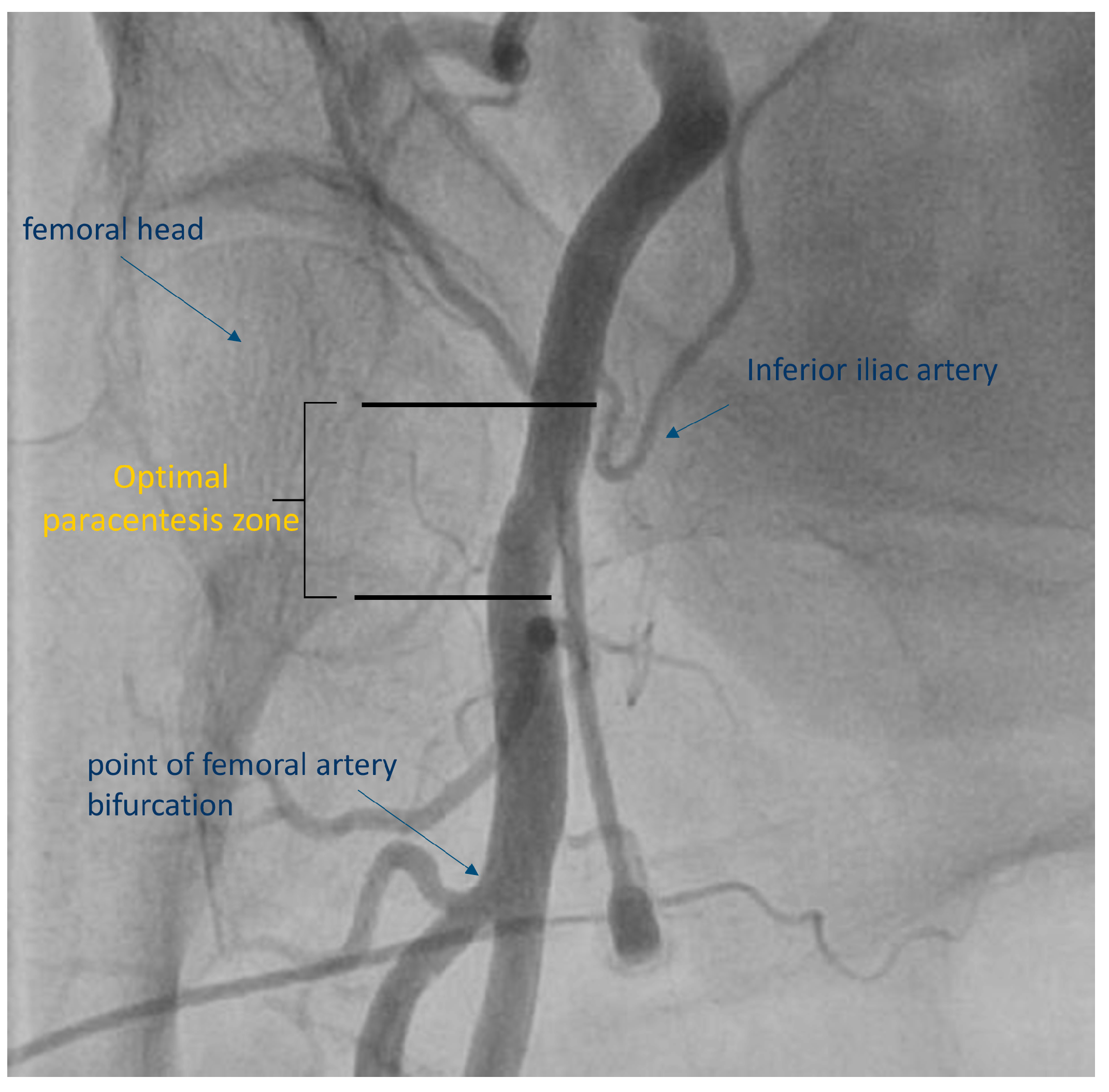
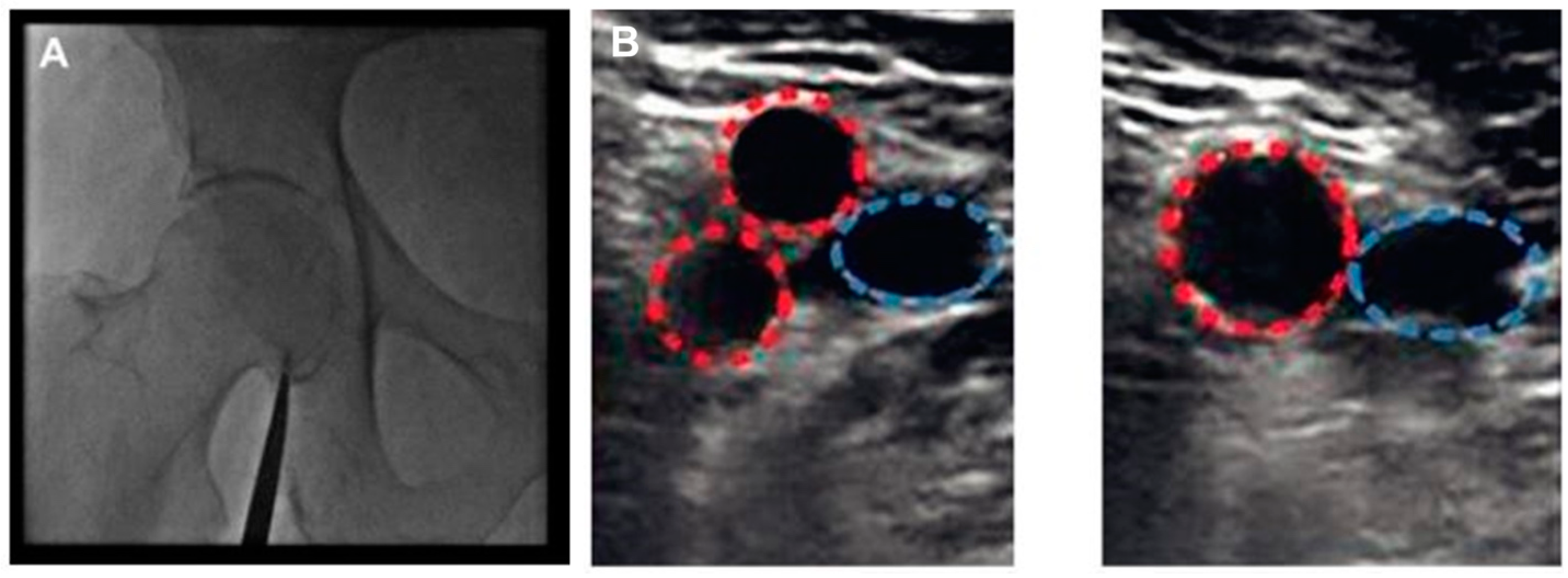
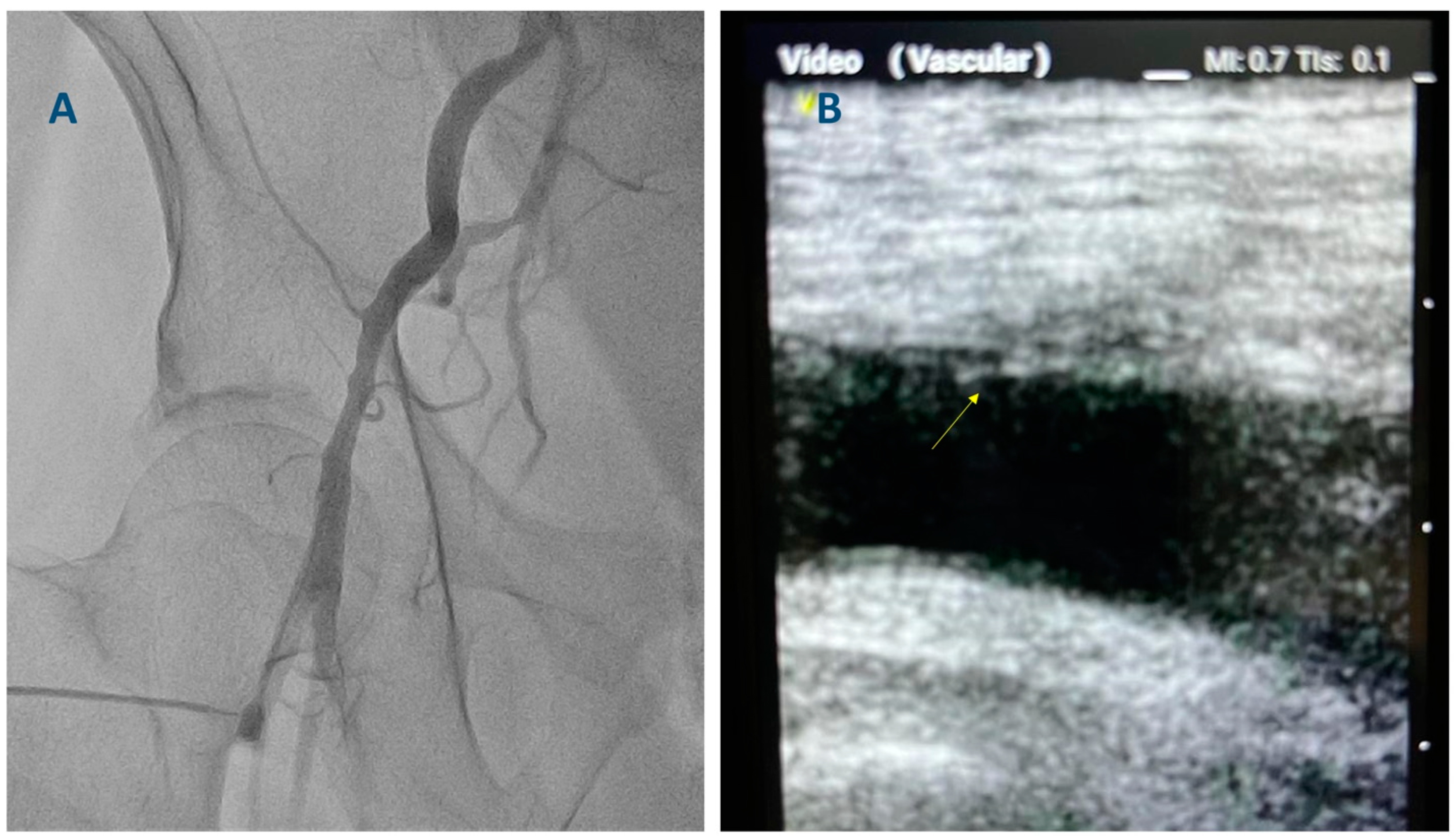
3. The Technique for Ultrasound-Guided Femoral Access
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jolly, S.S.; Yusuf, S.; Cairns, J.; Niemelä, K.; Xavier, D.; Widimsky, P.; Budaj, A.; Niemelä, M.; Valentin, V.; Lewis, B.S.; et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): A randomised, parallel group, multicentre trial. Lancet 2011, 377, 1409–1420. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Gagnor, A.; Calabró, P.; Frigoli, E.; Leonardi, S.; Zaro, T.; Rubartelli, P.; Briguori, C.; Andò, G.; Repetto, A.; et al. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: A randomised multicentre trial. Lancet 2015, 385, 2465–2476. [Google Scholar] [CrossRef] [PubMed]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e4–e17. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Biasco, L.; Ferrari, E.; Pedrazzini, G.; Faletra, F.; Moccetti, T.; Petracca, F.; Moccetti, M. Access Sites for TAVI: Patient Selection Criteria, Technical Aspects, and Outcomes. Front. Cardiovasc. Med. 2018, 5, 88. [Google Scholar] [CrossRef]
- Tajti, P.; Alaswad, K.; Karmpaliotis, D.; Jaffer, F.A.; Yeh, R.W.; Patel, M.; Mahmud, E.; Choi, J.W.; Burke, M.N.; Doing, A.H.; et al. Procedural Outcomes of Percutaneous Coronary Interventions for Chronic Total Occlusions via the Radial Approach: Insight from an International CTO Registry. JACC Cardiovasc. Interv. 2018, 12, 346–358. [Google Scholar] [CrossRef]
- Doll, J.A.; Beaver, K.; Naranjo, D.; Waldo, S.W.; Maynard, C.; Helfrich, C.D.; Rao, S.V. Trends in Arterial Access Site Selection and Bleeding Outcomes Following Coronary Procedures, 2011–2018. Circ. Cardiovasc. Qual. Outcomes 2022, 15, e008359. [Google Scholar] [CrossRef]
- Seto, A.H.; Abu-Fadel, M.S.; Sparling, J.M.; Zacharias, S.J.; Daly, T.S.; Harrison, A.T.; Suh, W.M.; Vera, J.A.; Aston, C.E.; Winters, R.J.; et al. Real-Time Ultrasound Guidance Facilitates Femoral Arterial Access and Reduces Vascular Complications: FAUST (Femoral Arterial Access with Ultrasound Trial). JACC Cardiovasc. Interv. 2010, 3, 751–758. [Google Scholar]
- Katırcıbaşı, M.T.; Güneş, H.; Aykan, A.; Aksu, E.; Özgül, S. Comparison of Ultrasound Guidance and Conventional Method for Common Femoral Artery Cannulation: A Prospective Study of 939 Patients. Acta Cardiol. Sin. 2018, 34, 394–398. [Google Scholar]
- Nguyen, P.; Makris, A.; Hennessy, A.; Jayanti, S.; Wang, A.; Park, K.; Chen, V.; Nguyen, T.; Lo, S.; Xuan, W.; et al. Standard versus ultrasound-guided radial and femoral access in coronary angiography and intervention (SURF): A randomised controlled trial. Eurointervention 2019, 15, e522–e530. [Google Scholar] [CrossRef]
- Jayanti, S.; Juergens, C.; Makris, A.; Hennessy, A.; Nguyen, P. The Learning Curves for Transradial and Ultrasound-Guided Arterial Access: An Analysis of the SURF Trial. Heart Lung Circ. 2021, 30, 1329–1336. [Google Scholar] [CrossRef]
- Jolly, S.S.; AlRashidi, S.; d’Entremont, M.A.; Alansari, O.; Brochu, B.; Heenan, L.; Skuriat, E.; Tyrwhitt, J.; Raco, M.; Tsang, M.; et al. Routine Ultrasonography Guidance for Femoral Vascular Access for Cardiac Procedures: The UNIVERSAL Randomized Clinical Trial. JAMA Cardiol. 2022, 7, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, S.; Nguyen, P.; Salerno, N.; Polimeni, A.; Sabatino, J.; Makris, A.; Hennessy, A.; Giustino, G.; Spaccarotella, C.; Mongiardo, A.; et al. Standard Versus Ultrasound-Guided Cannulation of the Femoral Artery in Patients Undergoing Invasive Procedures: A Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2020, 9, 677. [Google Scholar] [CrossRef] [PubMed]
- Iannopollo, G.; Nobile, G.; Lanzilotti, V.; Capecchi, A.; Verardi, R.; Bruno, M.; Somaschini, A.; Rubboli, A.; Di Pasquale, G.; Casella, G. Percutaneous artErial closure devices and ultrasound-guided Trans-femoRal puncture ObservatioNal InvestigatiOn: Insights from the PETRONIO registry. Catheter. Cardiovasc. Interv. 2021, 99, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Meijers, T.A.; Nap, A.; Aminian, A.; Dens, J.; Teeuwen, K.; van Kuijk, J.-P.; van Wely, M.; Schmitz, T.; Bataille, Y.; Kraaijeveld, A.O.; et al. ULTrasound-guided TRAnsfemoral puncture in COmplex Large bORe PCI: Study protocol of the UltraCOLOR trial. BMJ Open 2022, 12, e065693. [Google Scholar] [CrossRef]
- Elbaz-Greener, G.; Zivkovic, N.; Arbel, Y.; Radhakrishnan, S.; Fremes, S.E.; Wijeysundera, H.C. Use of Two-Dimensional Ultrasonographically Guided Access to Reduce Access-Related Complications for Transcatheter Aortic Valve Replacement. Can. J. Cardiol. 2017, 33, 918–924. [Google Scholar] [CrossRef]
- Khan, L.; Murdoch, D.; Savage, M.; Raffel, C.; Poon, K. Ultrasound Guided Technique for Percutaneous Transfemoral Transcatheter Aortic Valve Implantation. Heart Lung Circ. 2019, 28, S435. [Google Scholar] [CrossRef]
- Bouteau, J.; Bourguignon, T.; Caze, C.; Quilliet, L.; Ivanes, F.; Desveaux, B.; Clerc, J.M.; Etienne, C.S. P1847Comparing outcomes and complications between transfemoral TAVI performed with or without echoguided puncture. Eur. Heart J. 2019, 40, ehz748.0598. [Google Scholar] [CrossRef]
- Potluri, S.P.; Hamandi, M.; Basra, S.S.; Shinn, K.V.; Tabachnick, D.; Vasudevan, A.; Filardo, G.; DiMaio, J.M.; Brinkman, W.T.; Harrington, K.; et al. Comparison of Frequency of Vascular Complications with Ultrasound-Guided versus Fluroscopic Roadmap-Guided Femoral Arterial Access in Patients Who Underwent Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2020, 132, 93–99. [Google Scholar] [CrossRef]
- Vincent, F.; Spillemaeker, H.; Kyheng, M.; Belin-Vincent, C.; Delhaye, C.; Piérache, A.; Denimal, T.; Verdier, B.; Debry, N.; Moussa, M.; et al. Ultrasound Guidance to Reduce Vascular and Bleeding Complications of Percutaneous Transfemoral Transcatheter Aortic Valve Replacement: A Propensity Score–Matched Comparison. J. Am. Heart Assoc. 2020, 9, e014916. [Google Scholar] [CrossRef]
- Kotronias, R.; Scarsini, R.; De Maria, G.L.; Ba, S.R.; Sayeed, R.; Krasopoulos, G.; Grebenik, C.; Keiralla, A.; Newton, J.D.; Banning, A.; et al. Ultrasound guided vascular access site management and left ventricular pacing are associated with improved outcomes in contemporary transcatheter aortic valve replacement: Insights from the OxTAVI registry. Catheter. Cardiovasc. Interv. 2019, 96, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Witberg, G.; Tzalamouras, V.; Adams, H.; Patterson, T.; Roberts-Thomson, R.; Byrne, J.; Dworakowski, R.; MacCarthy, P.; Redwood, S.; Prendergast, B. Routine Ultrasound or Fluoroscopy Use and Risk of Vascular/Bleeding Complications after Transfemoral TAVR. JACC Cardiovasc. Interv. 2020, 13, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Kotronias, R.A.; Bray, J.J.; Rajasundaram, S.; Vincent, F.; Delhaye, C.; Scarsini, R.; Marin, F.; Terentes-Printzios, D.; Halcox, J.P.; Mamas, M.A.; et al. Ultrasound- versus Fluoroscopy-Guided Strategy for Transfemoral Transcatheter Aortic Valve Replacement Access: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Interv. 2021, 14, e010742. [Google Scholar] [CrossRef] [PubMed]
- Perrin, N.; Bonnet, G.; Leroux, L.; Ibrahim, R.; Modine, T.; Ben Ali, W. Transcatheter Aortic Valve Implantation: All Transfemoral? Update on Peripheral Vascular Access and Closure. Front. Cardiovasc. Med. 2021, 8, 747583. [Google Scholar] [CrossRef]
- Moriyama, N.; Dahlbacka, S.; Vähäsilta, T.; Vainikka, T.; Aho, P.; Viikilä, J.; Lammintausta, O.; Laine, M. The Efficacy of the Ultrasound-Navigated MANTA Deployment Following Transfemoral Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 2564–2566. [Google Scholar] [CrossRef]
- Miyashita, H.; Moriyama, N.; Dahlbacka, S.; Vähäsilta, T.; Vainikka, T.; Jalanko, M.; Viikilä, J.; Laine, M. Ultrasound-Guided versus Conventional MANTA Vascular Closure Device Deployment after Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2022, 180, 116–123. [Google Scholar] [CrossRef]
- Soverow, J.; Oyama, J.; Lee, M.S. Adoption of Routine Ultrasound Guidance for Femoral Arterial Access for Cardiac Catheterization. J. Invasive Cardiol. 2016, 28, 311–314. [Google Scholar]
- Damluji, A.A.; Nelson, D.W.; Valgimigli, M.; Windecker, S.; Byrne, R.A.; Cohen, F.; Patel, T.; Brilakis, E.S.; Banerjee, S.; Mayol, J.; et al. Transfemoral approach for coronary angiography and intervention: A collaboration of international cardiovascular societies. JACC Cardiovasc. Interv. 2017, 10, 2269–2279. [Google Scholar] [CrossRef]
- Brilakis, E.S. Manual of Percutaneous Coronary Interventions: A Step-by-Step Approach; Elsevier/Academic Press: London, UK, 2020. [Google Scholar]
- Schnyder, G.; Sawhney, N.; Whisenant, B.; Tsimikas, S.; Turi, Z.G. Common femoral artery anatomy is influenced by demographics and comorbidity: Implications for cardiac and peripheral invasive studies. Catheter. Cardiovasc. Interv. 2001, 53, 289–295. [Google Scholar] [CrossRef]
- Sandoval, Y.; Burke, M.N.; Lobo, A.S.; Lips, D.L.; Seto, A.H.; Chavez, I.; Sorajja, P.; Abu-Fadel, M.S.; Wang, Y.; Poulouse, A.; et al. Contemporary Arterial Access in the Cardiac Catheterization Laboratory. JACC Cardiovasc. Interv. 2017, 10, 2233–2241. [Google Scholar] [CrossRef]
- Kern, M.J. The Interventional Cardiac Catheterization Handbook, 4th ed.; Elsevier: Philadelphia, PA, USA, 2018; p. 512. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xenogiannis, I.; Varlamos, C.; Keeble, T.R.; Kalogeropoulos, A.S.; Karamasis, G.V. Ultrasound-Guided Femoral Vascular Access for Percutaneous Coronary and Structural Interventions. Diagnostics 2023, 13, 2028. https://doi.org/10.3390/diagnostics13122028
Xenogiannis I, Varlamos C, Keeble TR, Kalogeropoulos AS, Karamasis GV. Ultrasound-Guided Femoral Vascular Access for Percutaneous Coronary and Structural Interventions. Diagnostics. 2023; 13(12):2028. https://doi.org/10.3390/diagnostics13122028
Chicago/Turabian StyleXenogiannis, Iosif, Charalampos Varlamos, Thomas R. Keeble, Andreas S. Kalogeropoulos, and Grigoris V. Karamasis. 2023. "Ultrasound-Guided Femoral Vascular Access for Percutaneous Coronary and Structural Interventions" Diagnostics 13, no. 12: 2028. https://doi.org/10.3390/diagnostics13122028
APA StyleXenogiannis, I., Varlamos, C., Keeble, T. R., Kalogeropoulos, A. S., & Karamasis, G. V. (2023). Ultrasound-Guided Femoral Vascular Access for Percutaneous Coronary and Structural Interventions. Diagnostics, 13(12), 2028. https://doi.org/10.3390/diagnostics13122028





