New Concepts for the Diagnosis of Polypoidal Choroidal Vasculopathy
Abstract
1. Introduction
2. Optical Coherence Tomography in PCV
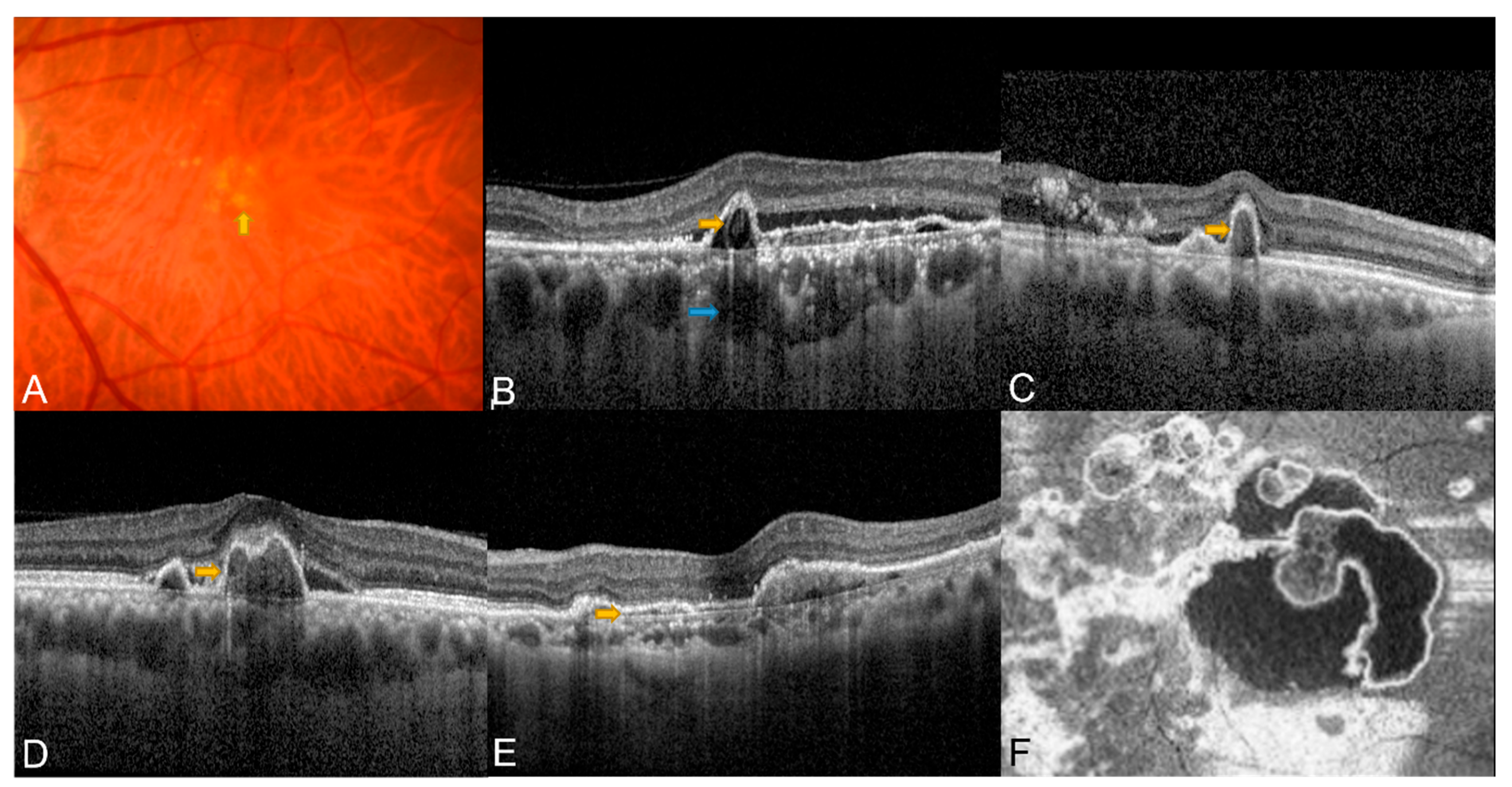
2.1. Classic OCT Features of PCV
2.2. En Face OCT in PCV
2.3. OCT Guiding Treatment for PCV
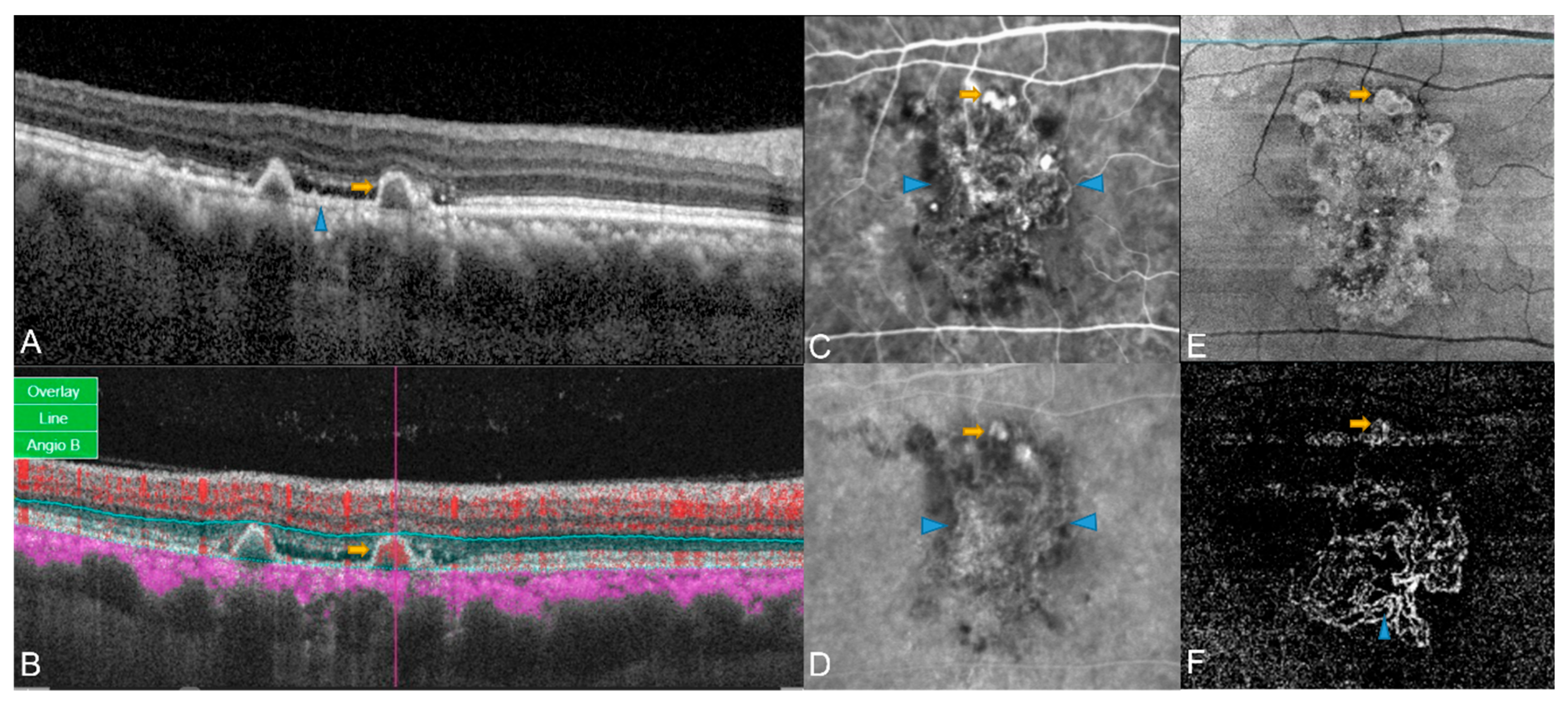
3. OCT Angiography in PCV
3.1. Comparison of OCTA and ICGA in PCV
3.2. Three-Dimensional Anatomical Characterization of PCV Complex by OCTA
3.3. Patterns of BVN in OCTA
3.4. Accuracy of OCTA in Differentiating PCV from Typical nAMD
3.5. OCTA in PCV after Treatment with anti-VEGF Monotherapy or Combination Therapy
4. Color Fundus Photography in PCV
4.1. Classic PCV Features on CFP
4.2. Role of CFP in Classic PCV Diagnostic Criteria
4.3. CFP in Non-ICGA Diagnostic Criteria
5. Fundus Autofluorescence in PCV
6. Fundus Fluorescein Angiography in PCV
7. Multimodal Imaging in PCV
7.1. Multimodal Imaging in the Diagnosis of PCV
7.2. Future Angles—Machine Learning Approaches
8. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yannuzi, L. Ideopathic polypoidal choroidal vasculopathy (IPCV). Retina 1990, 10, 1–8. [Google Scholar] [CrossRef]
- Wong, R.L.; Lai, T.Y. Polypoidal Choroidal Vasculopathy: An Update on Therapeutic Approaches. J. Ophthalmic. Vis. Res. 2013, 8, 359–371. [Google Scholar] [PubMed]
- Chaikitmongkol, V.; Cheung, C.M.G.; Koizumi, H.; Govindahar, V.; Chhablani, J.; Lai, T.Y.Y. Latest Developments in Polypoidal Choroidal Vasculopathy: Epidemiology, Etiology, Diagnosis, and Treatment. Asia Pac. J. Ophthalmol. 2020, 9, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Coscas, G.; Lupidi, M.; Coscas, F.; Benjelloun, F.; Zerbib, J.; Dirani, A.; Semoun, O.; Souied, E.H. Toward a specific classification of polypoidal choroidal vasculopathy: Idiopathic disease or subtype of age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3187–3195. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Chong, V.; Loewenstein, A.; Larsen, M.; Souied, E.; Schlingemann, R.; Eldem, B.; Monés, J.; Richard, G.; Bandello, F. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br. J. Ophthalmol. 2014, 98, 1144–1167. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumawat, D.; Sundar, M.D.; Gagrani, M.; Gupta, B.; Roop, P.; Hasan, N.; Sharma, A.; Chawla, R. Polypoidal choroidal vasculopathy: A comprehensive clinical update. Ther. Adv. Ophthalmol. 2019, 11, 2515841419831152. [Google Scholar] [CrossRef]
- Zhalka, F.E.; Moisseiev, E.; Rubowitz, A. Polypoidal choroidal vasculopathy-characteristics and response to treatment with bevacizumab in caucasian patients. Int. J. Retin. Vitr. 2022, 8, 82. [Google Scholar] [CrossRef]
- Tan, C.S.; Ngo, W.K.; Lim, L.W.; Tan, N.W.; Lim, T.H. EVEREST study report 3: Diagnostic challenges of polypoidal choroidal vasculopathy. Lessons learnt from screening failures in the EVEREST study. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 1923–1930. [Google Scholar] [CrossRef]
- Ho, C.P.S.; Lai, T.Y.Y. Current management strategy of polypoidal choroidal vasculopathy. Indian J. Ophthalmol. 2018, 66, 1727–1735. [Google Scholar] [CrossRef]
- Medina-Baena, M.; Huertos-Carrillo, M.J.; Rodríguez, L.; García-Pulido, J.I.; Cornejo-Castillo, C.; Calandria-Amiguetti, J.M. One-Year Outcome of Aflibercept and Photodynamic Therapy in a Caucasian Patient with Polypoidal Choroidal Vasculopathy Refractory to Ranibizumab and Photodynamic Therapy. Case. Rep. Ophthalmol. 2018, 9, 172–178. [Google Scholar] [CrossRef]
- Kokame, G.T.; Shantha, J.G.; Hirai, K.; Ayabe, J. En Face Spectral-Domain Optical Coherence Tomography for the Diagnosis and Evaluation of Polypoidal Choroidal Vasculopathy. Ophthalmic. Surg. Lasers. Imaging Retin. 2016, 47, 737–744. [Google Scholar] [CrossRef]
- de Carlo, T.E.; Kokame, G.T.; Shantha, J.G.; Lai, J.C.; Wee, R. Spectral-Domain Optical Coherence Tomography Angiography for the Diagnosis and Evaluation of Polypoidal Choroidal Vasculopathy. Ophthalmologica 2018, 239, 103–109. [Google Scholar] [CrossRef]
- Cheung, C.M.G.; Lai, T.Y.Y.; Ruamviboonsuk, P.; Chen, S.J.; Chen, Y.; Freund, K.B.; Gomi, F.; Koh, A.H.; Lee, W.K.; Wong, T.Y. Polypoidal Choroidal Vasculopathy: Definition, Pathogenesis, Diagnosis, and Management. Ophthalmology 2018, 125, 708–724. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.M.; Li, X.; Mathur, R.; Lee, S.Y.; Chan, C.M.; Yeo, I.; Loh, B.K.; Williams, R.; Wong, E.Y.; Wong, D.; et al. A prospective study of treatment patterns and 1-year outcome of Asian age-related macular degeneration and polypoidal choroidal vasculopathy. PLoS ONE 2014, 9, e101057. [Google Scholar] [CrossRef] [PubMed]
- Meira, J.; Marques, M.L.; Falcão-Reis, F.; Rebelo Gomes, E.; Carneiro, Â. Immediate Reactions to Fluorescein and Indocyanine Green in Retinal Angiography: Review of Literature and Proposal for Patient's Evaluation. Clin. Ophthalmol. 2020, 14, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, J.; Li, Z.; Yu, S.; Yang, Y.; Yan, H.; Zeng, J.; Tang, S.; Ding, X. Distinguishing polypoidal choroidal vasculopathy from typical neovascular age-related macular degeneration based on spectral domain optical coherence tomographY. Retina 2016, 36, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.M.G.; Lai, T.Y.Y.; Teo, K.; Ruamviboonsuk, P.; Chen, S.J.; Kim, J.E.; Gomi, F.; Koh, A.H.; Kokame, G.; Jordan-Yu, J.M.; et al. Polypoidal Choroidal Vasculopathy: Consensus Nomenclature and Non-Indocyanine Green Angiograph Diagnostic Criteria from the Asia-Pacific Ocular Imaging Society PCV Workgroup. Ophthalmology 2021, 128, 443–452. [Google Scholar] [CrossRef]
- Fujimoto, J.G.; Pitris, C.; Boppart, S.A.; Brezinski, M.E. Optical coherence tomography: An emerging technology for biomedical imaging and optical biopsy. Neoplasia 2000, 2, 9–25. [Google Scholar] [CrossRef]
- Banister, K.; Cook, J.A.; Scotland, G.; Azuara-Blanco, A.; Goulão, B.; Heimann, H.; Hernández, R.; Hogg, R.; Kennedy, C.; Sivaprasad, S.; et al. Non-invasive testing for early detection of neovascular macular degeneration in unaffected second eyes of older adults: EDNA diagnostic accuracy study. Health Technol. Assess. 2022, 26, 1–142. [Google Scholar] [CrossRef]
- Al-Mujaini, A.; Wali, U.K.; Azeem, S. Optical coherence tomography: Clinical applications in medical practice. Oman. Med. J. 2013, 28, 86–91. [Google Scholar] [CrossRef]
- Hood, D.C.; Raza, A.S.; Kay, K.Y.; Sandler, S.F.; Xin, D.; Ritch, R.; Liebmann, J.M. A comparison of retinal nerve fiber layer (RNFL) thickness obtained with frequency and time domain optical coherence tomography (OCT). Opt. Express 2009, 17, 3997–4003. [Google Scholar] [CrossRef]
- Adsuara, C.M.; Sargues, L.R.; Hernández, J.M.; Garfella, M.H.; Bel, L.H.; Navarro, V.C.; Taulet, E.C. Multimodal imaging in multiple evanescent white dot syndrome and new insights in pathogenesis. J. Fr. Ophtalmol. 2021, 44, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Farci, R.; Sellam, A.; Coscas, F.; Coscas, G.J.; Diaz, G.; Napoli, P.E.; Souied, E.; Galantuomo, M.S.; Fossarello, M. Multimodal OCT Reflectivity Analysis of the Cystoid Spaces in Cystoid Macular Edema. BioMed Res. Int. 2019, 2019, 7835372. [Google Scholar] [CrossRef] [PubMed]
- Permadi, A.C.; Djatikusumo, A.; Adriono, G.A. Optical coherence tomography in diagnosing polypoidal choroidal vasculopathy. Looking into the future: A systematic review and meta-analysis. Int. J. Retin. Vitr. 2022, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- De Salvo, G.; Vaz-Pereira, S.; Keane, P.A.; Tufail, A.; Liew, G. Sensitivity and specificity of spectral-domain optical coherence tomography in detecting idiopathic polypoidal choroidal vasculopathy. Am. J. Ophthalmol. 2014, 158, 1228–1238.e1221. [Google Scholar] [CrossRef]
- Chang, Y.S.; Kim, J.H.; Kim, J.W.; Lee, T.G.; Kim, C.G. Optical Coherence Tomography-based Diagnosis of Polypoidal Choroidal Vasculopathy in Korean Patients. Korean J. Ophthalmol. 2016, 30, 198–205. [Google Scholar] [CrossRef]
- Cheung, C.M.G.; Yanagi, Y.; Akiba, M.; Tan, A.; Mathur, R.; Chan, C.M.; Yeo, I.; Wong, T.Y. Improved detection and diagnosis of polypoidal choroidal vasculopathy using a combination of optical coherence tomography and optical coherence tomography angiography. Retina 2019, 39, 1655–1663. [Google Scholar] [CrossRef]
- de Carlo, T.E.; Kokame, G.T.; Kaneko, K.N.; Lian, R.; Lai, J.C.; Wee, R. Sensitivity and specificity of detecting polypoidal choroidal vasculopathy with en face optical coherence tomography and optical coherence tomography angiography. Retina 2019, 39, 1343–1352. [Google Scholar] [CrossRef]
- Iijima, H.; Iida, T.; Imai, M.; Gohdo, T.; Tsukahara, S. Optical coherence tomography of orange-red subretinal lesions in eyes with idiopathic polypoidal choroidal vasculopathy. Am. J. Ophthalmol. 2000, 129, 21–26. [Google Scholar] [CrossRef]
- Otsuji, T.; Takahashi, K.; Fukushima, I.; Uyama, M. Optical coherence tomographic findings of idiopathic polypoidal choroidal vasculopathy. Ophthalmic. Surg. Lasers 2000, 31, 210–214. [Google Scholar] [CrossRef]
- Cheung, C.M.G.; Yanagi, Y.; Mohla, A.; Lee, S.Y.; Mathur, R.; Chan, C.M.; Yeo, I.; Wong, T.Y. Characterization and differentiation of polypoidal choroidal vasculopathy using swept source optical coherence tomography angiography. Retina 2017, 37, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Tsujikawa, A.; Sasahara, M.; Otani, A.; Gotoh, N.; Kameda, T.; Iwama, D.; Yodoi, Y.; Tamura, H.; Mandai, M.; Yoshimura, N. Pigment epithelial detachment in polypoidal choroidal vasculopathy. Am. J. Ophthalmol. 2007, 143, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Kishi, S.; Watanabe, G.; Matsumoto, H.; Mukai, R. Tomographic features of branching vascular networks in polypoidal choroidal vasculopathy. Retina 2007, 27, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Ojima, Y.; Hangai, M.; Sakamoto, A.; Tsujikawa, A.; Otani, A.; Tamura, H.; Yoshimura, N. Improved visualization of polypoidal choroidal vasculopathy lesions using spectral-domain optical coherence tomography. Retina 2009, 29, 52–59. [Google Scholar] [CrossRef]
- Khan, S.; Engelbert, M.; Imamura, Y.; Freund, K.B. Polypoidal choroidal vasculopathy: Simultaneous indocyanine green angiography and eye-tracked spectral domain optical coherence tomography findings. Retina 2012, 32, 1057–1068. [Google Scholar] [CrossRef]
- Kawamura, A.; Yuzawa, M.; Mori, R.; Haruyama, M.; Tanaka, K. Indocyanine green angiographic and optical coherence tomographic findings support classification of polypoidal choroidal vasculopathy into two types. Acta Ophthalmol. 2013, 91, e474–e481. [Google Scholar] [CrossRef]
- Song, Y.; Tham, Y.C.; Chong, C.; Ong, R.; Fenner, B.J.; Cheong, K.X.; Takahashi, K.; Jordan-Yu, J.M.; Teo, K.Y.C.; Tan, A.C.S.; et al. Patterns and Determinants of Choroidal Thickness in a Multiethnic Asian Population: The Singapore Epidemiology of Eye Diseases Study. Ophthalmol. Retin. 2021, 5, 458–467. [Google Scholar] [CrossRef]
- Cheong, K.X.; Lim, L.W.; Li, K.Z.; Tan, C.S. A novel and faster method of manual grading to measure choroidal thickness using optical coherence tomography. Eye 2018, 32, 433–438. [Google Scholar] [CrossRef]
- Cheung, C.M.G.; Lee, W.K.; Koizumi, H.; Dansingani, K.; Lai, T.Y.Y.; Freund, K.B. Pachychoroid disease. Eye 2019, 33, 14–33. [Google Scholar] [CrossRef]
- Seong, S.; Choo, H.G.; Kim, Y.J.; Kim, J.Y.; Lee, J.H.; Oh, H.S.; You, Y.S.; Kim, S.H.; Kwon, O.W. Novel Findings of Polypoidal Choroidal Vasculopathy via Optical Coherence Tomography Angiography. Korean J. Ophthalmol. 2019, 33, 54–62. [Google Scholar] [CrossRef]
- Kameda, T.; Tsujikawa, A.; Otani, A.; Sasahara, M.; Gotoh, N.; Tamura, H.; Yoshimura, N. Polypoidal choroidal vasculopathy examined with en face optical coherence tomography. Clin. Exp. Ophthalmol. 2007, 35, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Manayath, G.; Shroff, D.; Salloju, V.; Dhar, P. Polypoidal Choroidal Vasculopathy: An Update on Diagnosis and Treatment. Clin. Ophthalmol. 2023, 17, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Ritter, M.; Simader, C.; Bolz, M.; Deák, G.G.; Mayr-Sponer, U.; Sayegh, R.; Kundi, M.; Schmidt-Erfurth, U.M. Intraretinal cysts are the most relevant prognostic biomarker in neovascular age-related macular degeneration independent of the therapeutic strategy. Br. J. Ophthalmol. 2014, 98, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- Vyas, C.H.; Cheung, C.M.G.; Jordan-Yu, J.M.N.; Shimizu, H.; Tan, A.C.S.; Sim, S.S.; Fenner, B.J.; Akiba, M.; Chakravarthy, U.; Teo, K.Y.C. Novel volumetric imaging biomarkers for assessing disease activity in eyes with PCV. Sci. Rep. 2022, 12, 2993. [Google Scholar] [CrossRef]
- Teo, K.Y.C.; Yanagi, Y.; Lee, S.Y.; Yeo, I.Y.S.; Tan, G.S.W.; Mathur, R.; Chan, C.M.; Wong, T.Y.; Cheung, C.M.G. Comparison of optical coherence tomography angiographic changes after anti-vascular endothelial growth factor therapy alone or in combination with photodynamic therapy in polypoidal choroidal vasculopathy. Retina 2018, 38, 1675–1687. [Google Scholar] [CrossRef]
- Chong Teo, K.Y.; Sadda, S.R.; Cheung, C.M.G.; Chakravarthy, U.; Staurenghi, G.; Invernizzi, A.; Ogura, Y.; Ruamviboonsuk, P.; Chen, S.J.; Gupta, V.; et al. Non-ICGA treatment criteria for Suboptimal Anti-VEGF Response for Polypoidal Choroidal Vasculopathy: APOIS PCV Workgroup Report 2. Ophthalmol. Retina 2021, 5, 945–953. [Google Scholar] [CrossRef]
- Ferrara, D. Image artifacts in optical coherence tomography angiography. Clin. Exp. Ophthalmol. 2016, 44, 367–368. [Google Scholar] [CrossRef]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K. Image artifacts in optical coherence tomography angiography. Retina 2015, 35, 2163–2180. [Google Scholar] [CrossRef]
- Arrigo, A.; Aragona, E.; Bordato, A.; Amato, A.; Borghesan, F.; Bandello, F.; Battaglia Parodi, M. Morphological and Functional Relationship Between OCTA and FA/ICGA Quantitative Features in AMD-Related Macular Neovascularization. Front. Med. 2021, 8, 758668. [Google Scholar] [CrossRef]
- Takayama, K.; Ito, Y.; Kaneko, H.; Kataoka, K.; Sugita, T.; Maruko, R.; Hattori, K.; Ra, E.; Haga, F.; Terasaki, H. Comparison of indocyanine green angiography and optical coherence tomographic angiography in polypoidal choroidal vasculopathy. Eye 2017, 31, 45–52. [Google Scholar] [CrossRef]
- Inoue, M.; Balaratnasingam, C.; Freund, K.B. Optical coherence tomography angiography of polypoidal choroidal vasculopathy and polypoidal choroidal neovascularization. Retina 2015, 35, 2265–2274. [Google Scholar] [CrossRef] [PubMed]
- Srour, M.; Querques, G.; Semoun, O.; El Ameen, A.; Miere, A.; Sikorav, A.; Zambrowski, O.; Souied, E.H. Optical coherence tomography angiography characteristics of polypoidal choroidal vasculopathy. Br. J. Ophthalmol. 2016, 100, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Mori, R.; Kawamura, A.; Nakashizuka, H.; Wakatsuki, Y.; Yuzawa, M. Comparison of OCT angiography and indocyanine green angiographic findings with subtypes of polypoidal choroidal vasculopathy. Br. J. Ophthalmol. 2017, 101, 51–55. [Google Scholar] [CrossRef]
- Chi, Y.T.; Yang, C.H.; Cheng, C.K. Optical Coherence Tomography Angiography for Assessment of the 3-Dimensional Structures of Polypoidal Choroidal Vasculopathy. JAMA Ophthalmol. 2017, 135, 1310–1316. [Google Scholar] [CrossRef]
- Huang, C.H.; Yeh, P.T.; Hsieh, Y.T.; Ho, T.C.; Yang, C.M.; Yang, C.H. Characterizing Branching Vascular Network Morphology in Polypoidal Choroidal Vasculopathy by Optical Coherence Tomography Angiography. Sci. Rep. 2019, 9, 595. [Google Scholar] [CrossRef]
- Pang, C.E.; Freund, K.B. Pachychoroid neovasculopathy. Retina 2015, 35, 1–9. [Google Scholar] [CrossRef]
- Koizumi, H.; Yamagishi, T.; Yamazaki, T.; Kinoshita, S. Relationship between clinical characteristics of polypoidal choroidal vasculopathy and choroidal vascular hyperpermeability. Am. J. Ophthalmol. 2013, 155, 305–313.e301. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhou, Y.; Gao, S.S.; Liu, W.; Huang, Y.; Huang, D.; Jia, Y. Evaluating Polypoidal Choroidal Vasculopathy With Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, 526–532. [Google Scholar] [CrossRef]
- Koh, A.H.; Chen, L.J.; Chen, S.J.; Chen, Y.; Giridhar, A.; Iida, T.; Kim, H.; Lai, T.Y.Y.; Lee, W.K.; Li, X.; et al. Polypoidal choroidal vasculopathy: Evidence-based guidelines for clinical diagnosis and treatment. Retina 2013, 33, 686–716. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W.; Wong, T.Y.; Cheung, C.M. Polypoidal Choroidal Vasculopathy in Asians. J. Clin. Med. 2015, 4, 782–821. [Google Scholar] [CrossRef]
- Japanese Study Group of Polypoidal Choroidal Vasculopathy. Criteria for diagnosis of polypoidal choroidal vasculopathy. Nippon Ganka Gakkai Zasshi 2005, 109, 417–427. [Google Scholar]
- Lim, T.H.; Lai, T.Y.Y.; Takahashi, K.; Wong, T.Y.; Chen, L.J.; Ruamviboonsuk, P.; Tan, C.S.; Lee, W.K.; Cheung, C.M.G.; Ngah, N.F.; et al. Comparison of Ranibizumab With or Without Verteporfin Photodynamic Therapy for Polypoidal Choroidal Vasculopathy: The EVEREST II Randomized Clinical Trial. JAMA Ophthalmol. 2020, 138, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Chaikitmongkol, V.; Khunsongkiet, P.; Patikulsila, D.; Ratanasukon, M.; Watanachai, N.; Jumroendararasame, C.; Mayerle, C.B.; Han, I.C.; Chen, C.J.; Winaikosol, P.; et al. Color Fundus Photography, Optical Coherence Tomography, and Fluorescein Angiography in Diagnosing Polypoidal Choroidal Vasculopathy. Am. J. Ophthalmol. 2018, 192, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Chaikitmongkol, V.; Kong, J.; Khunsongkiet, P.; Patikulsila, D.; Sachdeva, M.; Chavengsaksongkram, P.; Dejkriengkraikul, C.; Winaikosol, P.; Choovuthayakorn, J.; Watanachai, N.; et al. Sensitivity and Specificity of Potential Diagnostic Features Detected Using Fundus Photography, Optical Coherence Tomography, and Fluorescein Angiography for Polypoidal Choroidal Vasculopathy. JAMA Ophthalmol. 2019, 137, 661–667. [Google Scholar] [CrossRef]
- Yang, J.; Yuan, M.; Wang, E.; Xia, S.; Chen, Y. Noninvasive multimodal imaging in diagnosing polypoidal choroidal vasculopathy. BMC Ophthalmol. 2019, 19, 229. [Google Scholar] [CrossRef]
- Sho, K.; Takahashi, K.; Yamada, H.; Wada, M.; Nagai, Y.; Otsuji, T.; Nishikawa, M.; Mitsuma, Y.; Yamazaki, Y.; Matsumura, M.; et al. Polypoidal choroidal vasculopathy: Incidence, demographic features, and clinical characteristics. Arch. Ophthalmol. 2003, 121, 1392–1396. [Google Scholar] [CrossRef]
- Pole, C.; Ameri, H. Fundus Autofluorescence and Clinical Applications. J. Ophthalmic. Vis. Res. 2021, 16, 432–461. [Google Scholar] [CrossRef]
- Delori, F.C.; Dorey, C.K.; Staurenghi, G.; Arend, O.; Goger, D.G.; Weiter, J.J. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Investig. Ophthalmol. Vis. Sci. 1995, 36, 718–729. [Google Scholar]
- Zhao, X.; Xia, S.; Chen, Y. Characteristic appearances of fundus autofluorescence in treatment-naive and active polypoidal choroidal vasculopathy: A retrospective study of 170 patients. Graefes. Arch. Clin. Exp. Ophthalmol. 2018, 256, 1101–1110. [Google Scholar] [CrossRef]
- Yamagishi, T.; Koizumi, H.; Yamazaki, T.; Kinoshita, S. Fundus autofluorescence in polypoidal choroidal vasculopathy. Ophthalmology 2012, 119, 1650–1657. [Google Scholar] [CrossRef]
- Suzuki, M.; Gomi, F.; Sawa, M.; Ueno, C.; Nishida, K. Changes in fundus autofluorescence in polypoidal choroidal vasculopathy during 3 years of follow-up. Graefes. Arch. Clin. Exp. Ophthalmol. 2013, 251, 2331–2337. [Google Scholar] [CrossRef] [PubMed]
- Öztaş, Z.; Menteş, J.; Nalçacı, S.; Barış, M. Characteristics of Fundus Autofluorescence in Active Polypoidal Choroidal Vasculopathy. Turk. J. Ophthalmol. 2016, 46, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.Y.Y.; Tang, Z.; Lai, A.C.W.; Szeto, S.K.H.; Lai, R.Y.K.; Cheung, C.Y. Association of Fundus Autofluorescence Abnormalities and Pachydrusen in Central Serous Chorioretinopathy and Polypoidal Choroidal Vasculopathy. J. Clin. Med. 2022, 11, 5370. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, T.; Koizumi, H.; Yamazaki, T.; Kinoshita, S. Changes in fundus autofluorescence after treatments for polypoidal choroidal vasculopathy. Br. J. Ophthalmol. 2014, 98, 780–784. [Google Scholar] [CrossRef]
- Ueno, C.; Gomi, F.; Sawa, M.; Nishida, K. Correlation of indocyanine green angiography and optical coherence tomography findings after intravitreal ranibizumab for polypoidal choroidal vasculopathy. Retina 2012, 32, 2006–2013. [Google Scholar] [CrossRef]
- Nakashizuka, H.; Mitsumata, M.; Okisaka, S.; Shimada, H.; Kawamura, A.; Mori, R.; Yuzawa, M. Clinicopathologic findings in polypoidal choroidal vasculopathy. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4729–4737. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, W.; Yang, J.; Zhao, J.; Ding, D.; He, F.; Chen, D.; Yang, Z.; Li, X.; Yu, W.; et al. Automated diagnoses of age-related macular degeneration and polypoidal choroidal vasculopathy using bi-modal deep convolutional neural networks. Br. J. Ophthalmol. 2021, 105, 561–566. [Google Scholar] [CrossRef]
- Tsiknakis, N.; Theodoropoulos, D.; Manikis, G.; Ktistakis, E.; Boutsora, O.; Berto, A.; Scarpa, F.; Scarpa, A.; Fotiadis, D.I.; Marias, K. Deep learning for diabetic retinopathy detection and classification based on fundus images: A review. Comput. Biol. Med. 2021, 135, 104599. [Google Scholar] [CrossRef]
- Alghamdi, H.S. Towards Explainable Deep Neural Networks for the Automatic Detection of Diabetic Retinopathy. Appl. Sci. 2022, 12, 9435. [Google Scholar] [CrossRef]
- Chou, Y.B.; Hsu, C.H.; Chen, W.S.; Chen, S.J.; Hwang, D.K.; Huang, Y.M.; Li, A.F.; Lu, H.H. Deep learning and ensemble stacking technique for differentiating polypoidal choroidal vasculopathy from neovascular age-related macular degeneration. Sci. Rep. 2021, 11, 7130. [Google Scholar] [CrossRef]
| Year | Author | Terminology | Non-ICGA Modality | Corresponding Lesion Component in ICGA | |
|---|---|---|---|---|---|
| 1 | 2016 | Liu et al. [16] | Thumb-like projection | OCT | Polypoidal lesion |
| 2 | 2018 | Cheung et al. [13] | Orange nodule | CFP | Polypoidal lesion |
| 3 | 2021 | APOIS [17] | Sharp-peaked PED | OCT | Polypoidal lesion |
| 4 | 2021 | APOIS [17] | Sub-RPE ring-like lesion | OCT | Polypoidal lesion |
| 5 | 2021 | APOIS [17] | Multilobular PED | OCT | Polypoidal lesion |
| 6 | 2018 | de Carlo et al. [12] | Notched PED | OCT | Polypoidal lesion |
| 7 | 2015 | Coscas G et al. [4] | Double-layer sign | OCT | BVN |
| 8 | 2021 | APOIS [17] | Complex RPE elevation | En face OCT | Polypoidal lesion+ BVN |
| 9 | 2018 | Cheung et al. [13] | Thick choroid with dilated Haller’s layer | OCT | Pachychoroid |
| Year | Author | Modality | Features | Standard | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|---|---|
| 1 | 2014 | De Salvo et al. [25] | SD-OCT | Multiple PEDs, sharp PED peak, PED notch, hyporeflective lumen within hyperreflective lesions adherent to RPE | Three or more OCT features | 94.6 | 92.9 |
| 2 | 2016 | Liu et al. [16] | SD-OCT | Local PED; DLS; TLP | At least two signs positive | 87.5 | 87.5 |
| 3 | 2016 | Chang et al. [26] | SD-OCT | Multiple RPEDs, sharp RPED peak, RPED notch, rounded hyporeflective area representing the polyp lumen within hyperreflective lesions adhered beneath RPE, presence of hyperreflective intraretinal hard exudates | Three or more OCT features | 85.7 | 86.2 |
| 4 | 2017 | Cheung et al. [31] | SS-OCTA | Shape; branching; presence of anastomoses and loops; morphology (vascular net with a peripheral arcade vs. a “dead tree” appearance) | All | 83.0 (vascular network) | 57.1 (vascular network) |
| 40.5 (polyps) | 66.7 (polyps) | ||||||
| 5 | 2019 | Cheung et al. [27] | SD-OCT | Notched/narrow-peaked PED; round sub-RPE hyporeflective lesion; RPE detachment (including PED/DLS) | At least 2 signs positive | 82.6 | 51.9 |
| OCTA | Localized sub-RPE hyperflow lesion in the cross-sectional OCTA; nodular hyperflow lesion in the en face OCTA | At least 1 sign positive | 82.6 | 92.6 | |||
| OCT + OCTA | Combined OCT/OCTA diagnosis of PCV | All | 82.6 | 100.0 | |||
| 6 | 2019 | de Carlo et al. [28] | Structural en face OCT | Presence of the PCV complex (BVN and polyps) | All | 30.0 | 85.7 |
| OCTA | 43.9 | 87.1 |
| Author | Non-ICGA Criteria | Optimal Combination | Sensitivity | Specificity | PPV | NPV | AUC (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| 1 | Chaikitmongkol et al. [63] | 4 Major criteria 1. Notched or hemorrhagic PED (CFP) 2. Sharply peaked PED (OCT) 3. Hyperreflective ring (OCT) 4. Notched PED (OCT) | ≥2 of 4 major criteria | 0.95 | 0.95 | 0.92 | 0.95 | 0.93 (0.89–0.98) |
| 2 | Yang et al. [65] | 5 Major criteria 1. Subretinal orange nodule (CFP) 2. Thumb-like PED (OCT) 3. Notched PED (OCT) 4. Bubble sign (OCT) 5. Bruch’s membrane depression under serosanguinous PED in OCT | ≥2 of 5 major criteria | 0.88 | 0.92 | 0.79 | 0.89 | 0.90 (0.84–0.97) |
| 3 | Cheung et al. [17] | 3 Major criteria 1. Sub-RPE ring-like lesion (OCT) 2. En face OCT-complex RPE elevation (en face OCT) 3. Sharp-peaked PED (OCT) 4 Minor criteria 1. Orange nodule (CFP) 2. Thick choroid with dilated Haller’s layer (OCT) 3. Complex/Multilobular PED (OCT) 4. Double-layer sign (OCT) | 3 Major + any 1 minor | 0.78 | 0.91 | 0.94 | 0.68 | 0.91 (0.86–0.95) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Chandrasekaran, P.R.; Cheong, K.X.; Wong, M.; Teo, K. New Concepts for the Diagnosis of Polypoidal Choroidal Vasculopathy. Diagnostics 2023, 13, 1680. https://doi.org/10.3390/diagnostics13101680
Zhao J, Chandrasekaran PR, Cheong KX, Wong M, Teo K. New Concepts for the Diagnosis of Polypoidal Choroidal Vasculopathy. Diagnostics. 2023; 13(10):1680. https://doi.org/10.3390/diagnostics13101680
Chicago/Turabian StyleZhao, Jinzhi, Priya R Chandrasekaran, Kai Xiong Cheong, Mark Wong, and Kelvin Teo. 2023. "New Concepts for the Diagnosis of Polypoidal Choroidal Vasculopathy" Diagnostics 13, no. 10: 1680. https://doi.org/10.3390/diagnostics13101680
APA StyleZhao, J., Chandrasekaran, P. R., Cheong, K. X., Wong, M., & Teo, K. (2023). New Concepts for the Diagnosis of Polypoidal Choroidal Vasculopathy. Diagnostics, 13(10), 1680. https://doi.org/10.3390/diagnostics13101680





