Imaging of Tauopathies with PET Ligands: State of the Art and Future Outlook
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Quality of the Selected Studies
3. Results
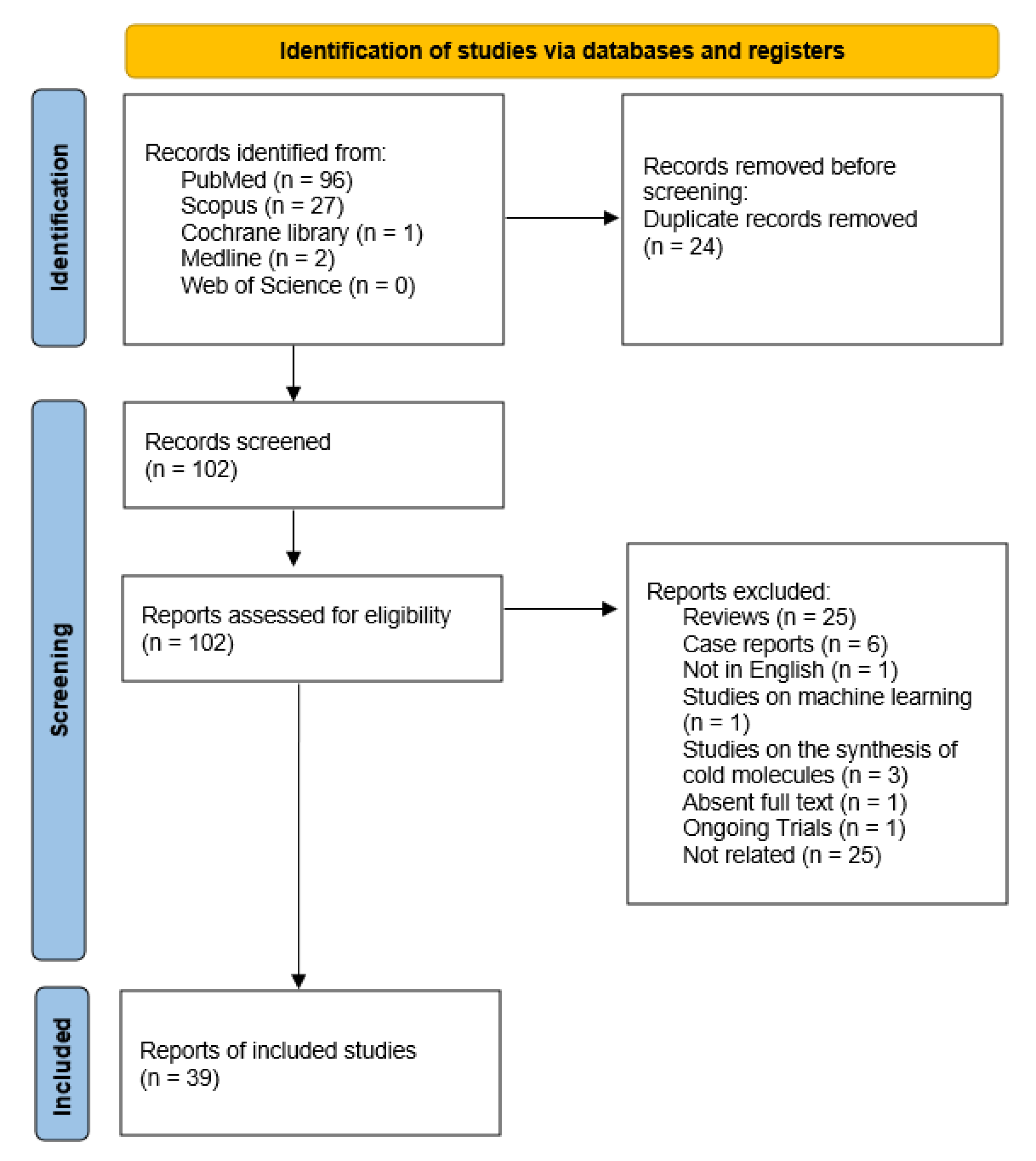
3.1. Search Results
3.2. Study Characteristics
3.3. Methodological Quality Assessment
4. Discussion
4.1. Studies on Microglia Activation
4.2. PET Tracers for Specific Tau Filaments
4.3. Protofibril Tracers
4.4. Comparative Studies between Amyloid Tracers
4.5. Distribution Studies in Patients with Tauopathies
4.6. Novel Radiotracers to Image Cognitive Decline
4.7. Clearance and Biodistribution Studies of Two Amyloid and Tau Radiopharmaceuticals
4.8. Studies concerning Important Genes and Enzymes Involved in Neurodegeneration
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sexton, C.; Snyder, H.; Beher, D.; Boxer, A.L.; Brannelly, P.; Brion, J.P.; Buée, L.; Cacace, A.M.; Chételat, G.; Citron, M.; et al. Current directions in tau research: Highlights from Tau 2020. Alzheimer’s Dement. 2022, 18, 988–1007. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G. Tauopathies. Handb. Clin. Neurol. 2017, 145, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Frantellizzi, V.; Conte, M.; De Vincentis, G. Hybrid Imaging of Vascular Cognitive Impairment. Semin. Nucl. Med. 2021, 51, 286–295. [Google Scholar] [CrossRef]
- Khan, A.; Kalaria, R.N.; Corbett, A.; Ballard, C. Update on Vascular Dementia. J. Geriatr. Psychiatry Neurol. 2016, 29, 281–301. [Google Scholar] [CrossRef] [PubMed]
- Ricci, M.; Cimini, A.; Chiaravalloti, A.; Filippi, L.; Schillaci, O. Positron Emission Tomography (PET) and Neuroimaging in the Personalized Approach to Neurodegenerative Causes of Dementia. Int. J. Mol. Sci. 2020, 21, 7481. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, K.M.; Yang, L.; Dong, Q.; Yu, J.T. Tauopathies: New perspectives and challenges. Mol. Neurodegener. 2022, 17, 28. [Google Scholar] [CrossRef]
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; van Swieten, J.C.; Seelaar, H.; Dopper, E.G.; Onyike, C.U.; et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011, 134, 2456–2477. [Google Scholar] [CrossRef]
- Leuzy, A.; Chiotis, K.; Lemoine, L.; Gillberg, P.G.; Almkvist, O.; Rodriguez-Vieitez, E.; Nordberg, A. Tau PET imaging in neurodegenerative tauopathies-still a challenge. Mol. Psychiatry 2019, 24, 1112–1134. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Jagust, W. Imaging the evolution and pathophysiology of Alzheimer disease. Nat. Rev. Neurosci. 2018, 19, 687–700. [Google Scholar] [CrossRef]
- Heneka, M.T.; Sastre, M.; Dumitrescu-Ozimek, L.; Dewachter, I.; Walter, J.; Klockgether, T.; Van Leuven, F. Focal glial activation coincides with increased BACE1 activation and precedes amyloid plaque deposition in APP[V717I] transgenic mice. J. Neuroinflamm. 2005, 2, 22. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef] [PubMed]
- Solito, E.; Sastre, M. Microglia function in Alzheimer’s disease. Front. Pharmacol. 2012, 3, 14. [Google Scholar] [CrossRef]
- Malpetti, M.; Kievit, R.A.; Passamonti, L.; Jones, P.S.; Tsvetanov, K.A.; Rittman, T.; Mak, E.; Nicastro, N.; Bevan-Jones, W.R.; Su, L.; et al. Microglial activation and tau burden predict cognitive decline in Alzheimer’s disease. Brain 2020, 143, 1588–1602. [Google Scholar] [CrossRef] [PubMed]
- Scarf, A.M.; Kassiou, M. The translocator protein. J. Nucl. Med. 2011, 52, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Rauchmann, B.S.; Brendel, M.; Franzmeier, N.; Trappmann, L.; Zaganjori, M.; Ersoezlue, E.; Morenas-Rodriguez, E.; Guersel, S.; Burow, L.; Kurz, C. Microglial Activation and Connectivity in Alzheimer Disease and Aging. Ann. Neurol. 2022, 92, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Fairley, L.H.; Sahara, N.; Aoki, I.; Ji, B.; Suhara, T.; Higuchi, M.; Barron, A.M. Neuroprotective effect of mitochondrial translocator protein ligand in a mouse model of tauopathy. J. Neuroinflamm. 2021, 18, 76. [Google Scholar] [CrossRef]
- Mackenzie, I.R.; Neumann, M.; Bigio, E.H.; Cairns, N.J.; Alafuzoff, I.; Kril, J.; Kovacs, G.G.; Ghetti, B.; Halliday, G.; Holm, I.E.; et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: An update. Acta Neuropathol. 2010, 119, 1–4. [Google Scholar] [CrossRef]
- Spinelli, E.G.; Mandelli, M.L.; Miller, Z.A.; Santos-Santos, M.A.; Wilson, S.M.; Agosta, F.; Grinberg, L.T.; Huang, E.J.; Trojanowski, J.Q.; Meyer, M.; et al. Typical and atypical pathology in primary progressive aphasia variants. Ann. Neurol. 2017, 81, 430–443. [Google Scholar] [CrossRef]
- Gorno-Tempini, M.L.; Hillis, A.E.; Weintraub, S.; Kertesz, A.; Mendez, M.; Cappa, S.F.; Ogar, J.M.; Rohrer, J.D.; Black, S.; Boeve, B.F.; et al. Classification of primary progressive aphasia and its variants. Neurology 2011, 76, 1006–1014. [Google Scholar] [CrossRef]
- Bevan-Jones, W.R.; Cope, T.E.; Jones, P.S.; Kaalund, S.S.; Passamonti, L.; Allinson, K.; Green, O.; Hong, Y.T.; Fryer, T.D.; Arnold, R.; et al. Neuroinflammation and protein aggregation co-localize across the frontotemporal dementia spectrum. Brain 2020, 143, 1010–1026. [Google Scholar] [CrossRef] [PubMed]
- Bevan-Jones, R.W.; Cope, T.E.; Jones, S.P.; Passamonti, L.; Hong, Y.T.; Fryer, T.; Arnold, R.; Coles, J.P.; Aigbirhio, F.A.; Patterson, K.; et al. [18F]AV-1451 binding is increased in frontotemporal dementia due to C9orf72 expansion. Ann. Clin. Transl. Neurol. 2018, 5, 1292–1296. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Ono, M.; Yamasaki, T.; Fujinaga, M.; Zhang, M.R.; Seki, C.; Aoki, I.; Kito, S.; Sawada, M.; Suhara, T.; et al. Detection of Alzheimer’s disease-related neuroinflammation by a PET ligand selective for glial versus vascular translocator protein. J. Cereb. Blood Flow Metab. 2021, 41, 2076–2089. [Google Scholar] [CrossRef]
- Qiao, L.; Fisher, E.; McMurray, L.; Milicevic Sephton, S.; Hird, M.; Kuzhuppilly-Ramakrishnan, N.; Williamson, D.J.; Zhou, X.; Werry, E.; Kassiou, M.; et al. Radiosynthesis of (R,S)-[18F]GE387: A Potential PET Radiotracer for Imaging Translocator Protein 18 kDa (TSPO) with Low Binding Sensitivity to the Human Gene Polymorphism rs6971. ChemMedChem 2019, 14, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Horti, A.G.; Naik, R.; Foss, C.A.; Minn, I.; Misheneva, V.; Du, Y.; Wang, Y.; Mathews, W.B.; Wu, Y.; Hall, A.; et al. PET imaging of microglia by targeting macrophage colony-stimulating factor 1 receptor (CSF1R). Proc. Natl. Acad. Sci. USA 2019, 116, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Nishimura, T.; Kondo, H.; Ikeda, K.; Hayashi, Y.; McGeer, P.L. Expression of the receptor for macrophage colony stimulating factor by brain microglia and its upregulation in brains of patients with Alzheimer’s disease and amyotrophic lateral sclerosis. Brain Res. 1994, 639, 171–174. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef]
- Peyraud, F.; Cousin, S.; Italiano, A. CSF-1R Inhibitor Development: Current Clinical Status. Curr. Oncol. Rep. 2017, 19, 70. [Google Scholar] [CrossRef]
- Chitu, V.; Gokhan, Ş.; Nandi, S.; Mehler, M.F.; Stanley, E.R. Emerging Roles for CSF-1 Receptor and its Ligands in the Nervous System. Trends Neurosci. 2016, 39, 378–393. [Google Scholar] [CrossRef]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef]
- Elmore, M.R.; Najafi, A.R.; Koike, M.A.; Dagher, N.N.; Spangenberg, E.E.; Rice, R.A.; Kitazawa, M.; Matusow, B.; Nguyen, H.; West, B.L.; et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 2014, 82, 380–397. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.G.; Tang, T.M.; Lue, L.F. Studies on Colony Stimulating Factor Receptor-1 and Ligands Colony Stimulating Factor-1 and Interleukin-34 in Alzheimer’s Disease Brains and Human Microglia. Front. Aging Neurosci. 2017, 9, 244. [Google Scholar] [CrossRef]
- Smith, A.M.; Gibbons, H.M.; Oldfield, R.L.; Bergin, P.M.; Mee, E.W.; Curtis, M.A.; Faull, R.L.; Dragunow, M. M-CSF increases proliferation and phagocytosis while modulating receptor and transcription factor expression in adult human microglia. J. Neuroinflamm. 2013, 10, 85. [Google Scholar] [CrossRef]
- Palle, P.; Monaghan, K.L.; Milne, S.M.; Wan, E.C.K. Cytokine Signaling in Multiple Sclerosis and Its Therapeutic Applications. Med. Sci. 2017, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Lue, L.F.; Rydel, R.; Brigham, E.F.; Yang, L.B.; Hampel, H.; Murphy, G.M., Jr.; Brachova, L.; Yan, S.D.; Walker, D.G.; Shen, Y.; et al. Inflammatory repertoire of Alzheimer’s disease and nondemented elderly microglia in vitro. Glia 2001, 35, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Ogata, A.; Ji, B.; Yamada, T.; Hattori, S.; Abe, J.; Ikenuma, H.; Ichise, M.; Koyama, H.; Suzuki, M.; Kato, T.; et al. [11C]NCGG401, a novel PET ligand for imaging of colony stimulating factor 1 receptors. Bioorg. Med. Chem. Lett. 2022, 65, 128704. [Google Scholar] [CrossRef]
- Dickson, D.W.; Rademakers, R.; Hutton, M.L. Progressive supranuclear palsy: Pathology and genetics. Brain Pathol. 2007, 17, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G.; Lukic, M.J.; Irwin, D.J.; Arzberger, T.; Respondek, G.; Lee, E.B.; Coughlin, D.; Giese, A.; Grossman, M.; Kurz, C.; et al. Distribution patterns of tau pathology in progressive supranuclear palsy. Acta Neuropathol. 2020, 140, 99–119. [Google Scholar] [CrossRef]
- Ishizawa, K.; Dickson, D.W. Microglial activation parallels system degeneration in progressive supranuclear palsy and corticobasal degeneration. J. Neuropathol. Exp. Neurol. 2001, 60, 647–657. [Google Scholar] [CrossRef]
- Fernández-Botrán, R.; Ahmed, Z.; Crespo, F.A.; Gatenbee, C.; Gonzalez, J.; Dickson, D.W.; Litvan, I. Cytokine expression and microglial activation in progressive supranuclear palsy. Park. Relat. Disord. 2011, 17, 683–688. [Google Scholar] [CrossRef]
- Frantellizzi, V.; Pani, A.; Ricci, M.; Locuratolo, N.; Fattapposta, F.; De Vincentis, G. Neuroimaging in Vascular Cognitive Impairment and Dementia: A Systematic Review. J. Alzheimer’s Dis. 2020, 73, 1279–1294. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.R.; de Silva, R.; Paviour, D.C.; Pittman, A.; Watt, H.C.; Kilford, L.; Holton, J.L.; Revesz, T.; Lees, A.J. Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson’s syndrome and PSP-parkinsonism. Brain 2005, 128, 1247–1258. [Google Scholar] [CrossRef]
- Höglinger, G.U.; Respondek, G.; Stamelou, M.; Kurz, C.; Josephs, K.A.; Lang, A.E.; Mollenhauer, B.; Müller, U.; Nilsson, C.; Whitwell, J.L.; et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov. Disord. 2017, 32, 853–864. [Google Scholar] [CrossRef]
- Malpetti, M.; Passamonti, L.; Rittman, T.; Jones, P.S.; Vázquez Rodríguez, P.; Bevan-Jones, W.R.; Hong, Y.T.; Fryer, T.D.; Aigbirhio, F.I.; O’Brien, J.T.; et al. Neuroinflammation and Tau Colocalize in vivo in Progressive Supranuclear Palsy. Ann. Neurol. 2020, 88, 1194–1204. [Google Scholar] [CrossRef]
- Zhu, G.; Bai, P.; Wang, K.; Mi, J.; Yang, J.; Hu, J.; Ban, Y.; Xu, R.; Chen, R.; Wang, C.; et al. Design, synthesis, and evaluation of novel O-alkyl ferulamide derivatives as multifunctional ligands for treating Alzheimer’s disease. J. Enzyme Inhib. Med. Chem. 2022, 37, 1375–1388. [Google Scholar] [CrossRef]
- Schedin-Weiss, S.; Inoue, M.; Hromadkova, L.; Teranishi, Y.; Yamamoto, N.G.; Wiehager, B.; Bogdanovic, N.; Winblad, B.; Sandebring-Matton, A.; Frykman, S.; et al. Monoamine oxidase B is elevated in Alzheimer disease neurons, is associated with γ-secretase and regulates neuronal amyloid β-peptide levels. Alzheimer’s Res. Ther. 2017, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Eisenberg, D.S.; Crowther, R.A. Propagation of Tau Aggregates and Neurodegeneration. Annu. Rev. Neurosci. 2017, 40, 189–210. [Google Scholar] [CrossRef]
- Shi, Y.; Murzin, A.G.; Falcon, B.; Epstein, A.; Machin, J.; Tempest, P.; Newell, K.L.; Vidal, R.; Garringer, H.J.; Sahara, N.; et al. Cryo-EM structures of tau filaments from Alzheimer’s disease with PET ligand APN-1607. Acta Neuropathol. 2021, 141, 697–708. [Google Scholar] [CrossRef]
- Schröter, N.; Blazhenets, G.; Frings, L.; Barkhausen, C.; Jost, W.H.; Weiller, C.; Rijntjes, M.; Meyer, P.T. Tau Imaging in the 4-Repeat-Tauopathies Progressive Supranuclear Palsy and Corticobasal Syndrome: A 11C-Pyridinyl-Butadienyl-Benzothiazole 3 PET Pilot Study. Clin. Nucl. Med. 2020, 45, 283–287. [Google Scholar] [CrossRef]
- Künze, G.; Kümpfel, R.; Rullmann, M.; Barthel, H.; Brendel, M.; Patt, M.; Sabri, O. Molecular Simulations Reveal Distinct Energetic and Kinetic Binding Properties of [18F]PI-2620 on Tau Filaments from 3R/4R and 4R Tauopathies. ACS Chem. Neurosci. 2022, 13, 2222–2234. [Google Scholar] [CrossRef]
- Kumar, J.S.D.; Molotkov, A.; Kim, J.; Carberry, P.; Idumonyi, S.; Castrillon, J.; Duff, K.; Shneider, N.A.; Mintz, A. Preclinical evaluation of a microtubule PET ligand [11C]MPC-6827 in tau and amyotrophic lateral sclerosis animal models. Pharmacol. Rep. 2022, 74, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Esparza, T.J.; Wildburger, N.C.; Jiang, H.; Gangolli, M.; Cairns, N.J.; Bateman, R.J.; Brody, D.L. Soluble Amyloid-beta Aggregates from Human Alzheimer’s Disease Brains. Sci. Rep. 2016, 6, 38187. [Google Scholar] [CrossRef]
- Meier, S.R.; Sehlin, D.; Roshanbin, S.; Falk, V.L.; Saito, T.; Saido, T.C.; Neumann, U.; Rokka, J.; Eriksson, J.; Syvänen, S. 11C-PiB and 124I-Antibody PET Provide Differing Estimates of Brain Amyloid-β after Therapeutic Intervention. J. Nucl. Med. 2022, 63, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Vassar, R.; Kuhn, P.H.; Haass, C.; Kennedy, M.E.; Rajendran, L.; Wong, P.C.; Lichtenthaler, S.F. Function, therapeutic potential and cell biology of BACE proteases: Current status and future prospects. J. Neurochem. 2014, 130, 4–28. [Google Scholar] [CrossRef] [PubMed]
- Neumann, U.; Rueeger, H.; Machauer, R.; Veenstra, S.J.; Lueoend, R.M.; Tintelnot-Blomley, M.; Laue, G.; Beltz, K.; Vogg, B.; Schmid, P.; et al. A novel BACE inhibitor NB-360 shows a superior pharmacological profile and robust reduction of amyloid-β and neuroinflammation in APP transgenic mice. Mol. Neurodegener. 2015, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Zeydan, B.; Schwarz, C.G.; Przybelski, S.A.; Lesnick, T.G.; Kremers, W.K.; Senjem, M.L.; Kantarci, O.H.; Min, P.H.; Kemp, B.J.; Jack, C.R., Jr.; et al. Comparison of 11C-Pittsburgh Compound B and 18F-Flutemetamol White Matter Binding in PET. J. Nucl. Med. 2022, 63, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Choe, Y.S.; Kim, Y.J.; Lee, B.; Kim, H.J.; Jang, H.; Kim, J.P.; Jung, Y.H.; Kim, S.J.; Kim, B.C.; et al. Concordance in detecting amyloid positivity between 18F-florbetaben and 18F-flutemetamol amyloid PET using quantitative and qualitative assessments. Sci. Rep. 2020, 10, 19576. [Google Scholar] [CrossRef]
- Cho, S.H.; Choe, Y.S.; Kim, Y.J.; Kim, H.J.; Jang, H.; Kim, Y.; Kim, S.E.; Kim, S.J.; Kim, J.P.; Jung, Y.H.; et al. Head-to-Head Comparison of 18F-Florbetaben and 18F-Flutemetamol in the Cortical and Striatal Regions. J. Alzheimer’s Dis. 2020, 76, 281–290. [Google Scholar] [CrossRef]
- Smith, R.; Schöll, M.; Leuzy, A.; Jögi, J.; Ohlsson, T.; Strandberg, O.; Hansson, O. Head-to-head comparison of tau positron emission tomography tracers [18F]flortaucipir and [18F]RO948. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 342–354. [Google Scholar] [CrossRef]
- Mormino, E.C.; Toueg, T.N.; Azevedo, C.; Castillo, J.B.; Guo, W.; Nadiadwala, A.; Corso, N.K.; Hall, J.N.; Fan, A.; Trelle, A.N.; et al. Tau PET imaging with 18F-PI-2620 in aging and neurodegenerative diseases. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2233–2244. [Google Scholar] [CrossRef]
- Gomar, J.J.; Tan, G.; Halpern, J.; Gordon, M.L.; Greenwald, B.; Koppel, J. Increased retention of tau PET ligand [18F]-AV1451 in Alzheimer’s Disease Psychosis. Transl. Psychiatry 2022, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Palleis, C.; Brendel, M.; Finze, A.; Weidinger, E.; Bötzel, K.; Danek, A.; Beyer, L.; Nitschmann, A.; Kern, M.; Biechele, G.; et al. Cortical [18F]PI-2620 Binding Differentiates Corticobasal Syndrome Subtypes. Mov. Disord. 2021, 36, 2104–2115. [Google Scholar] [CrossRef]
- Tsai, R.M.; Bejanin, A.; Lesman-Segev, O.; LaJoie, R.; Visani, A.; Bourakova, V.; O’Neil, J.P.; Janabi, M.; Baker, S.; Lee, S.E.; et al. 18F-flortaucipir (AV-1451) tau PET in frontotemporal dementia syndromes. Alzheimer’s Res. Ther. 2019, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Wren, M.C.; Lashley, T.; Årstad, E.; Sander, K. Large inter- and intra-case variability of first generation tau PET ligand binding in neurodegenerative dementias. Acta Neuropathol. Commun. 2018, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Tiepolt, S.; Becker, G.A.; Wilke, S.; Cecchin, D.; Rullmann, M.; Meyer, P.M.; Barthel, H.; Hesse, S.; Patt, M.; Luthardt, J.; et al. (+)-[18F]Flubatine as a novel α4β2 nicotinic acetylcholine receptor PET ligand-results of the first-in-human brain imaging application in patients with β-amyloid PET-confirmed Alzheimer’s disease and healthy controls. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 731–746. [Google Scholar] [CrossRef]
- Perry, E.K.; Martin-Ruiz, C.M.; Court, J.A. Nicotinic receptor subtypes in human brain related to aging and dementia. Alcohol 2001, 24, 63–68. [Google Scholar] [CrossRef]
- Perry, E.; Martin-Ruiz, C.; Lee, M.; Griffiths, M.; Johnson, M.; Piggott, M.; Haroutunian, V.; Buxbaum, J.D.; Nãsland, J.; Davis, K.; et al. Nicotinic receptor subtypes in human brain ageing, Alzheimer and Lewy body diseases. Eur. J. Pharmacol. 2000, 393, 215–222. [Google Scholar] [CrossRef]
- Lemoine, L.; Ledreux, A.; Mufson, E.J.; Perez, S.E.; Simic, G.; Doran, E.; Lott, I.; Carroll, S.; Bharani, K.; Thomas, S.; et al. Regional binding of tau and amyloid PET tracers in Down syndrome autopsy brain tissue. Mol. Neurodegener 2020, 15, 68. [Google Scholar] [CrossRef]
- Hartley, D.; Blumenthal, T.; Carrillo, M.; DiPaolo, G.; Esralew, L.; Gardiner, K.; Granholm, A.C.; Iqbal, K.; Krams, M.; Lemere, C.; et al. Down syndrome and Alzheimer’s disease: Common pathways, common goals. Alzheimer’s Dement. 2015, 11, 700–709. [Google Scholar] [CrossRef]
- Betthauser, T.J.; Cody, K.A.; Zammit, M.D.; Murali, D.; Converse, A.K.; Barnhart, T.E.; Stone, C.K.; Rowley, H.A.; Johnson, S.C.; Christian, B.T. In Vivo Characterization and Quantification of Neurofibrillary Tau PET Radioligand 18F-MK-6240 in Humans from Alzheimer Disease Dementia to Young Controls. J. Nucl. Med. 2019, 60, 93–99. [Google Scholar] [CrossRef]
- Lepelletier, F.X.; Vandesquille, M.; Asselin, M.C.; Prenant, C.; Robinson, A.C.; Mann, D.M.A.; Green, M.; Barnett, E.; Banister, S.D.; Mottinelli, M.; et al. Evaluation of 18F-IAM6067 as a sigma-1 receptor PET tracer for neurodegeneration in vivo in rodents and in human tissue. Theranostics 2020, 10, 7938–7955. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Yang, S.; Cui, J.; Wang, J.; Li, L.; Chen, Y.; Chen, J.; Feng, P.; Huang, S.; Li, H.; et al. Novel 18F-Labeled Isonicotinamide-Based Radioligands for Positron Emission Tomography Imaging of Glycogen Synthase Kinase-3β. Mol. Pharm. 2021, 18, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.; Feisullin, S. Postmortem stability of alpha-bungarotoxin binding sites in mouse and human brain. Brain Res. 1981, 216, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Wevers, A.; Monteggia, L.; Nowacki, S.; Bloch, W.; Schütz, U.; Lindstrom, J.; Pereira, E.F.; Eisenberg, H.; Giacobini, E.; de Vos, R.A.; et al. Expression of nicotinic acetylcholine receptor subunits in the cerebral cortex in Alzheimer’s disease: Histotopographical correlation with amyloid plaques and hyperphosphorylated-tau protein. Eur. J. Neurosci. 1999, 11, 2551–2565. [Google Scholar] [CrossRef]
- Wang, S.; Fang, Y.; Wang, H.; Gao, H.; Jiang, G.; Liu, J.; Xue, Q.; Qi, Y.; Cao, M.; Qiang, B.; et al. Design, synthesis and biological evaluation of 1,4-Diazobicylco[3.2.2]nonane derivatives as α7-Nicotinic acetylcholine receptor PET/CT imaging agents and agonists for Alzheimer’s disease. Eur. J. Med. Chem. 2018, 159, 255–266. [Google Scholar] [CrossRef]
- Stanimirovic, D.B.; Sandhu, J.K.; Costain, W.J. Emerging Technologies for Delivery of Biotherapeutics and Gene Therapy Across the Blood-Brain Barrier. BioDrugs 2018, 32, 547–559. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Burkhart, A.; Thomsen, L.B.; Andresen, T.L.; Moos, T. Targeting the transferrin receptor for brain drug delivery. Prog. Neurobiol. 2019, 181, 101665. [Google Scholar] [CrossRef]
- Zhao, Z.; Zlokovic, B.V. Therapeutic TVs for Crossing Barriers in the Brain. Cell 2020, 182, 267–269. [Google Scholar] [CrossRef]
- Bonvicini, G.; Syvänen, S.; Andersson, K.G.; Haaparanta-Solin, M.; López-Picón, F.; Sehlin, D. ImmunoPET imaging of amyloid-beta in a rat model of Alzheimer’s disease with a bispecific, brain-penetrating fusion protein. Transl. Neurodegener. 2022, 11, 55. [Google Scholar] [CrossRef]
- Schöll, M.; Wall, A.; Thordardottir, S.; Ferreira, D.; Bogdanovic, N.; Långström, B.; Almkvist, O.; Graff, C.; Nordberg, A. Low PiB PET retention in presence of pathologic CSF biomarkers in Arctic APP mutation carriers. Neurology 2012, 79, 229–236. [Google Scholar] [CrossRef]
- Wu, A.M. Antibodies and antimatter: The resurgence of immuno-PET. J. Nucl. Med. 2009, 50, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Schlein, E.; Syvänen, S.; Rokka, J.; Gustavsson, T.; Rossin, R.; Robillard, M.; Eriksson, J.; Sehlin, D. Functionalization of Radiolabeled Antibodies to Enhance Peripheral Clearance for High Contrast Brain Imaging. Mol. Pharm. 2022, 19, 4111–4122. [Google Scholar] [CrossRef] [PubMed]
- Koole, M.; Lohith, T.G.; Valentine, J.L.; Bennacef, I.; Declercq, R.; Reynders, T.; Riffel, K.; Celen, S.; Serdons, K.; Bormans, G.; et al. Preclinical Safety Evaluation and Human Dosimetry of [18F]MK-6240, a Novel PET Tracer for Imaging Neurofibrillary Tangles. Mol. Imaging Biol. 2020, 22, 173–180. [Google Scholar] [CrossRef]
- Iqbal, K.; Liu, F.; Gong, C.X. Tau and neurodegenerative disease: The story so far. Nat. Rev. Neurol. 2016, 12, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Kouri, N.; Ross, O.A.; Dombroski, B.; Younkin, C.S.; Serie, D.J.; Soto-Ortolaza, A.; Baker, M.; Finch, N.C.A.; Yoon, H.; Kim, J.; et al. Genome-wide association study of corticobasal degeneration identifies risk variants shared with progressive supranuclear palsy. Nat. Commun. 2015, 6, 7247. [Google Scholar] [CrossRef]
- Myers, A.J.; Pittman, A.M.; Zhao, A.S.; Rohrer, K.; Kaleem, M.; Marlowe, L.; Lees, A.; Leung, D.; McKeith, I.G.; Perry, R.H.; et al. The MAPT H1c risk haplotype is associated with increased expression of tau and especially of 4 repeat containing transcripts. Neurobiol. Dis. 2007, 25, 561–570. [Google Scholar] [CrossRef]
- Caillet-Boudin, M.L.; Buée, L.; Sergeant, N.; Lefebvre, B. Regulation of human MAPT gene expression. Mol. Neurodegener. 2015, 10, 28. [Google Scholar] [CrossRef]
- Chen, J.; Yu, J.T.; Wojta, K.; Wang, H.F.; Zetterberg, H.; Blennow, K.; Yokoyama, J.S.; Weiner, M.W.; Kramer, J.H.; Rosen, H.; et al. Genome-wide association study identifies MAPT locus influencing human plasma tau levels. Neurology 2017, 88, 669–676. [Google Scholar] [CrossRef]
- Laws, S.M.; Friedrich, P.; Diehl-Schmid, J.; Müller, J.; Eisele, T.; Bäuml, J.; Förstl, H.; Kurz, A.; Riemenschneider, M. Fine mapping of the MAPT locus using quantitative trait analysis identifies possible causal variants in Alzheimer’s disease. Mol. Psychiatry 2007, 12, 510–517. [Google Scholar] [CrossRef]
- Shen, X.N.; Miao, D.; Li, J.Q.; Tan, C.C.; Cao, X.P.; Tan, L.; Yu, J.T. MAPT rs242557 variant is associated with hippocampus tau uptake on 18F-AV-1451 PET in non-demented elders. Aging 2019, 11, 874–884. [Google Scholar] [CrossRef]
- Jones, D.T.; Knopman, D.S.; Graff-Radford, J.; Syrjanen, J.A.; Senjem, M.L.; Schwarz, C.G.; Dheel, C.; Wszolek, Z.; Rademakers, R.; Kantarci, K.; et al. In vivo 18F-AV-1451 tau PET signal in MAPT mutation carriers varies by expected tau isoforms. Neurology 2018, 90, e947–e954. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Okita, K.; Motoi, Y.; Mizuno, T.; Ikeda, M.; Sanjo, N.; Murakami, K.; Kambe, T.; Takayama, T.; Yamada, K.; et al. Clinical impact of amyloid PET using 18F-florbetapir in patients with cognitive impairment and suspected Alzheimer’s disease: A multicenter study. Ann. Nucl. Med. 2022, 36, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Lesman-Segev, O.H.; La Joie, R.; Iaccarino, L.; Lobach, I.; Rosen, H.J.; Seo, S.W.; Janabi, M.; Baker, S.L.; Edwards, L.; Pham, J.; et al. Diagnostic Accuracy of Amyloid versus 18F-Fluorodeoxyglucose Positron Emission Tomography in Autopsy-Confirmed Dementia. Ann. Neurol. 2021, 89, 389–401. [Google Scholar] [CrossRef]
- Gordon, B.A.; Blazey, T.M.; Christensen, J.; Dincer, A.; Flores, S.; Keefe, S.; Chen, C.; Su, Y.; McDade, E.M.; Wang, G.; et al. Tau PET in autosomal dominant Alzheimer’s disease: Relationship with cognition, dementia and other biomarkers. Brain 2019, 142, 1063–1076. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Haskali, M.B.; Liow, J.S.; Zoghbi, S.S.; Barth, V.N.; Kolodrubetz, M.C.; Bond, M.R.; Morse, C.L.; Gladding, R.L.; Frankland, M.P.; et al. Evaluation of a PET Radioligand to Image O-GlcNAcase in Brain and Periphery of Rhesus Monkey and Knock-Out Mouse. J. Nucl. Med. 2019, 60, 129–134. [Google Scholar] [CrossRef] [PubMed]
| Study | Studied Tracer | Other Imaging Modalities Considered | Study Conducted on |
|---|---|---|---|
| Malpetti et al. [14] | 18F-AV-1451 | MRI | Humans |
| 11C-PK11195 | |||
| Rauchmann et al. [16] | [18F]GE-180 | MRI | Humans |
| Fairley et al. [17] | 11C-PBB3 18F-FEBMP | MRI | Mice |
| Bevan-Jones et al. [21] | 11C-PK-11195 18F-AV-1451 | / | Humans |
| Bevan-Jones et al. [22] | 18F-AV-1451 | / | Humans |
| Ji et al. [23] | 18F-FEBMP 11C-PK11195 11C-PBR28 11C-Ac5216 18F-FEDAA1106 | / | Mice |
| Horti et al. [25] | [11C]CPPC | / | Murine model |
| Ogata et al. [36] | [11C]NCGG401 | / | Rodents, Humans |
| Malpetti et al. [44] | [11C]PK11195, [18F]AV-1451 | / | Humans |
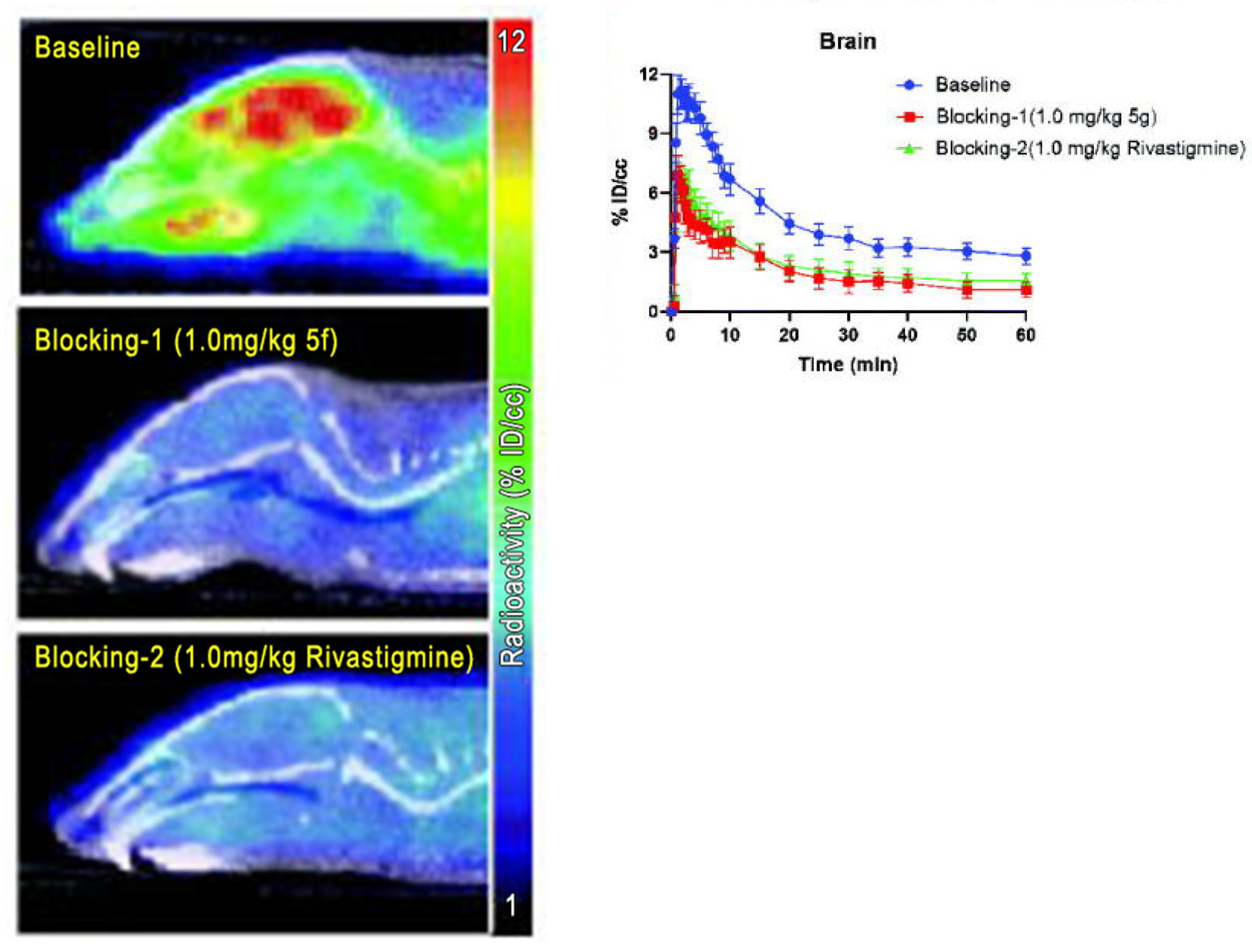
| Zhu et al. [45] | [11C]5f | / | Mice |
| Shi et al. [48] | 18F-APN-1607 | / | Humans |
| Schröter et al. [49] | 11C-PBB3 | / | Humans |
| Künze et al. [50] | [18F]PI-2620 | / | Simulation Workflow |
| Kumar et al. [51] | 11C-MPC-6827 | / | Mice |
| Meier et al. [53] | 124I-RmAb158-scFv158, 11C-PiB | / | Mice |
| Zeydan et al. [56] | 11C-PiB 18F-flutemetamol | MRI | Humans |
| Cho et al. [57] | 18F-florbetaben 18F-flutemetamol | / | Humans |
| Cho et al. [58] | 18F-florbetaben 18F-flutemetamol | / | Humans |
| Smith et al. [59] | [18F]flortaucipir [18F]RO948 | / | Humans |
| Mormino et al. [60] | 18F-PI-2620 | / | Humans |
| Gomar et al. [61] | [18F]-AV1451 | / | Humans |
| Palleis et al. [62] | [18F]PI-2620 [18F]Flutemetamol [18F]Florbetaben | / | Humans |
| Tsai et al. [63] | 18F-flortaucipir | / | Humans |
| Wren et al. [64] | [18F]flortaucipir [18F]T808 | / | Humans |
| Tiepold et al. [65] | (+)-[18F]Flubatine | / | Humans |
| Lemoine et al. [68] | 3H-MK6240 3H-THK5117 3H-PIB | / | Humans |
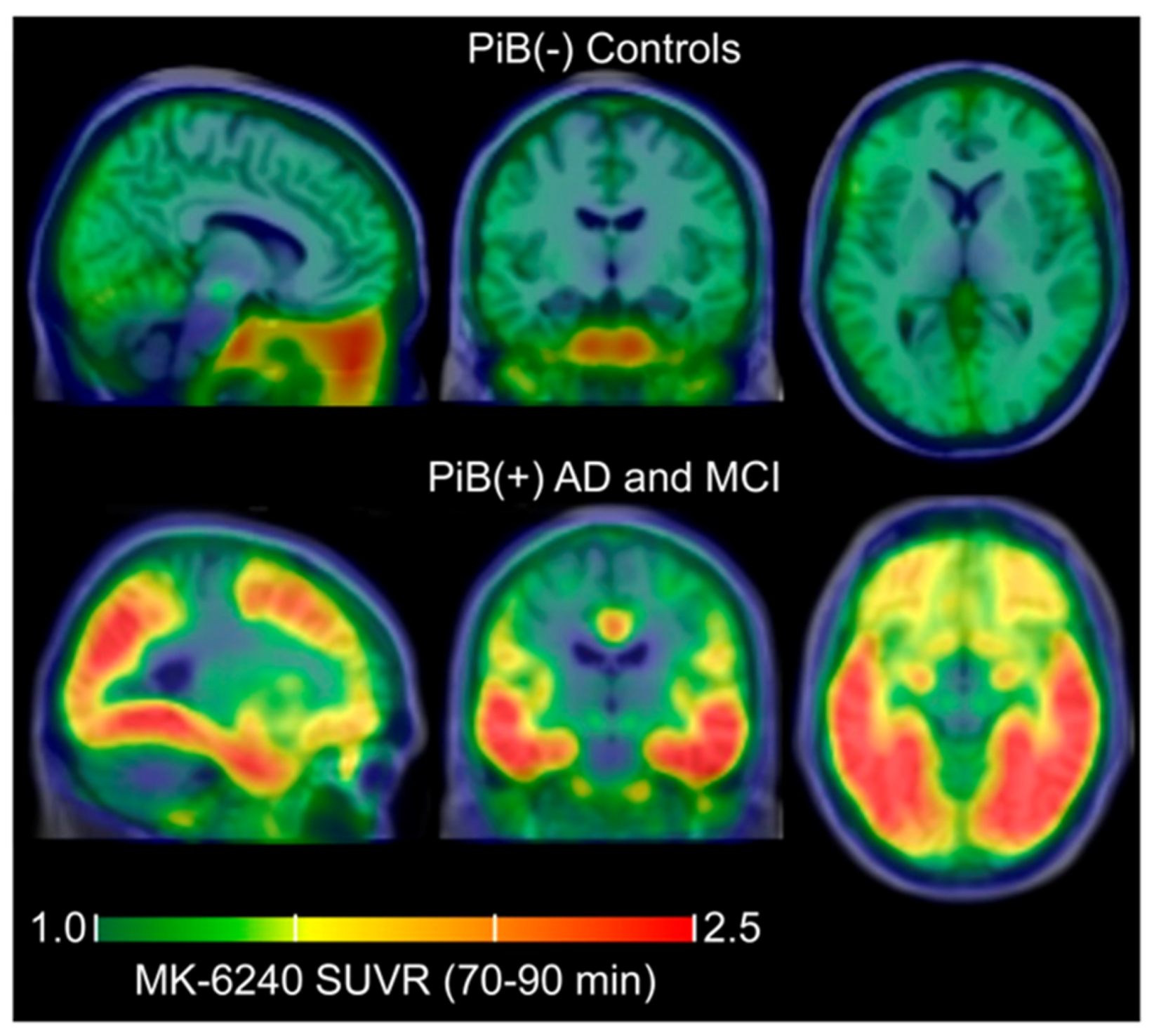
| Betthauser et al. [70] | 18F-MK-6240 | / | Humans |
| Lepelletier et al. [71] | 18F-IAM6067 | / | Mice Humans |
| Zhong et al. [72] | [18F]10a-d | / | Rodents |
| Wang et al. [75] | [18F]15 | / | Rats |
| Bonvicini et al. [79] | [125I]I-OX265-F(ab’)2-Bapi [124I]I-OX265-F(ab’)2-Bapi | / | Rats |
| Schlein et al. [82] | TCO-[125I]I-RmAb158 | / | Mice |
| Koole et al. [83] | [18F]MK-6240 | / | Rats Humans |
| Shen et al. [90] | 18F-AV-1451 | / | Humans |
| Jones et al. [91] | 18F-AV-1451 | / | Humans |
| Matsuda et al. [92] | 18F-florbetapir | / | Humans |
| Lesman-Segev et al. [93] | 11C-PiB 18F-fluorodeoxyglucose | / | Humans |
| Gordon et al. [94] | 11C PiB 18F-AV-45 | / | Humans |
| Paul et al. [95] | 18F-LSN3316612 | / | Monkeys Mice |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conte, M.; De Feo, M.S.; Sidrak, M.M.A.; Corica, F.; Gorica, J.; Granese, G.M.; Filippi, L.; De Vincentis, G.; Frantellizzi, V. Imaging of Tauopathies with PET Ligands: State of the Art and Future Outlook. Diagnostics 2023, 13, 1682. https://doi.org/10.3390/diagnostics13101682
Conte M, De Feo MS, Sidrak MMA, Corica F, Gorica J, Granese GM, Filippi L, De Vincentis G, Frantellizzi V. Imaging of Tauopathies with PET Ligands: State of the Art and Future Outlook. Diagnostics. 2023; 13(10):1682. https://doi.org/10.3390/diagnostics13101682
Chicago/Turabian StyleConte, Miriam, Maria Silvia De Feo, Marko Magdi Abdou Sidrak, Ferdinando Corica, Joana Gorica, Giorgia Maria Granese, Luca Filippi, Giuseppe De Vincentis, and Viviana Frantellizzi. 2023. "Imaging of Tauopathies with PET Ligands: State of the Art and Future Outlook" Diagnostics 13, no. 10: 1682. https://doi.org/10.3390/diagnostics13101682
APA StyleConte, M., De Feo, M. S., Sidrak, M. M. A., Corica, F., Gorica, J., Granese, G. M., Filippi, L., De Vincentis, G., & Frantellizzi, V. (2023). Imaging of Tauopathies with PET Ligands: State of the Art and Future Outlook. Diagnostics, 13(10), 1682. https://doi.org/10.3390/diagnostics13101682







