Assessment of Hearing and Vestibular Functions in a Post-COVID-19 Patient: A Clinical Case Study
Abstract
1. Introduction
2. Clinical Case Description
2.1. Patient’s Anamnesis
2.2. Results of Clinical and Laboratory Examinations
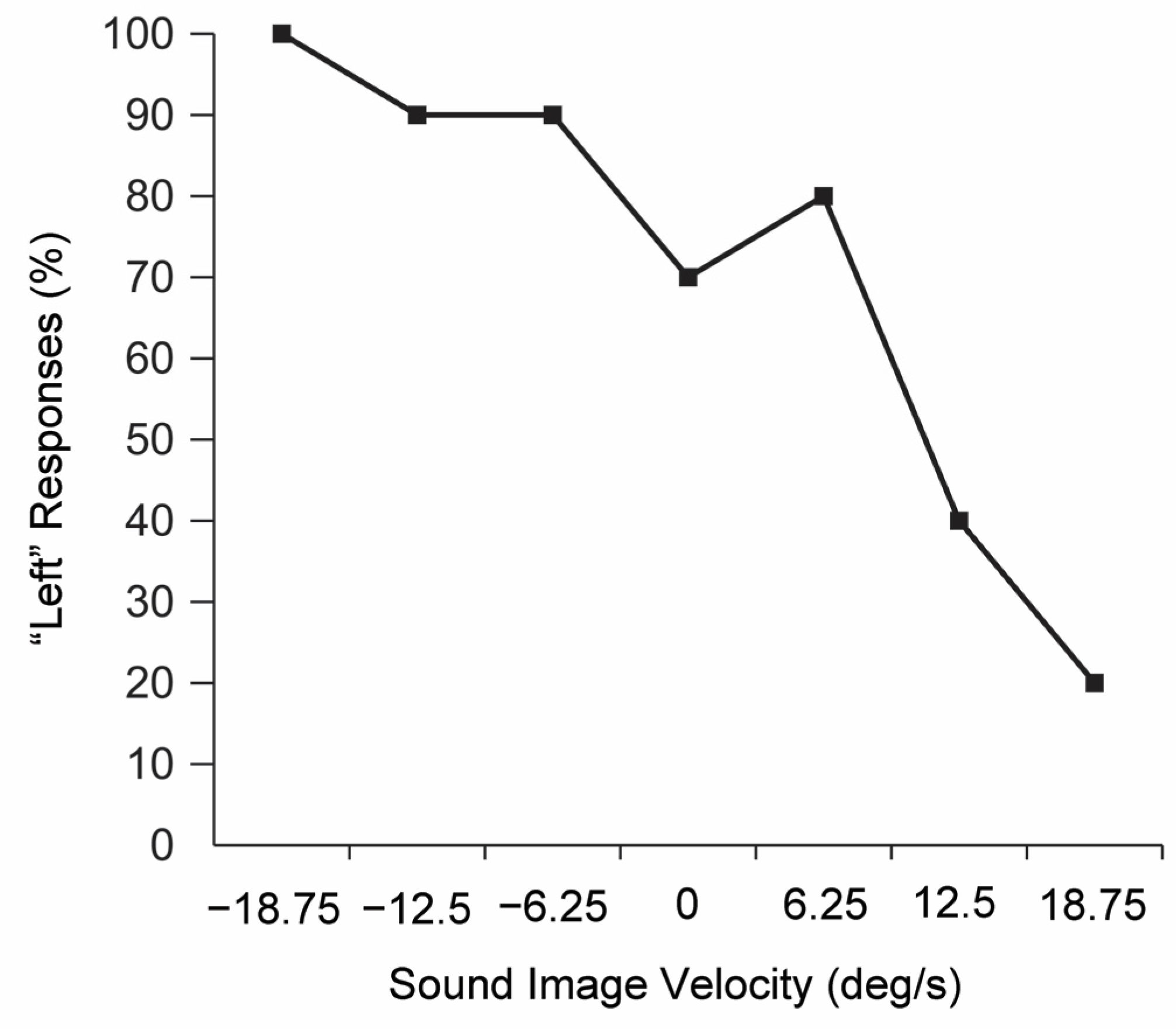
2.3. Vestibular Examination
2.4. Auditory Examination
2.5. Small Fiber Neuropathy Evaluation
3. Discussion
- -
- subjective (psychoacoustic) techniques did not allow clear identification of the nature of the dysfunction;
- -
- the data of subjective methods are unreliable because they depend on the attention, motivation, and level of cognitive development of the listener;
- -
- children’s age prevents the conduct of a full battery of psychoacoustic tests;
- -
- there are neurological disorders that do not allow the performance of the battery of psychoacoustic tests;
- -
- clarification of the localization of the disorder in the central parts of the auditory system is required if CAPD is detected on the basis of subjective techniques;
- -
- the inability to conduct psychoacoustic tests in the patient’s native language.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gavrilova, N.; Soprun, L.; Lukashenko, M.; Ryabkova, V.; Fedotkina, T.V.; Churilov, L.P.; Shoenfeld, Y. New Clinical Phenotype of the Post-Covid Syndrome: Fibromyalgia and Joint Hypermobility Condition. Pathophysiology 2022, 29, 24–29. [Google Scholar] [CrossRef]
- Ryabkova, V.A.; Churilov, L.P.; Shoenfeld, Y. Influenza infection, SARS, MERS and COVID-19: Cytokine storm—The common denominator and the lessons to be learned. Clin. Immunol. 2021, 223, 108652. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (accessed on 1 August 2022).
- Ehrenfeld, M.; Tincani, A.; Andreoli, L.; Cattalini, M.; Greenbaum, A.; Kanduc, D.; Alijotas-Reig, J.; Zinserling, V.; Semenova, N.; Amital, H.; et al. COVID-19 and autoimmunity. Autoimmun. Rev. 2020, 19, 102597. [Google Scholar] [CrossRef] [PubMed]
- Perrin, R.; Riste, L.; Hann, M.; Walther, A.; Mukherjee, A.; Heald, A. Into the looking glass: Post-viral syndrome post COVID-19. Med. Hypotheses 2020, 144, 110055. [Google Scholar] [CrossRef] [PubMed]
- Mohabbat, A.B.; Mohabbat, N.M.L.; Wight, E.C. Fibromyalgia and Chronic Fatigue Syndrome in the Age of COVID-19. Mayo Clin. Proc. Innov. Qual. Outcomes 2020, 4, 764–766. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.J.; Son, C.G. Review of case definitions for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J. Transl. Med. 2020, 18, 289. [Google Scholar] [CrossRef]
- Rasa, S.; Nora-Krukle, Z.; Henning, N.; Eliassen, E.; Shikova, E.; Harrer, T.; Scheibenbogen, C.; Murovska, M.; Prusty, B.K. European Network on ME/CFS (EUROMENE). Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J. Transl. Med. 2018, 16, 268. [Google Scholar] [CrossRef]
- Blomberg, J.; Gottfries, C.G.; Elfaitouri, A.; Rizwan, M.; Rosén, A. Infection Elicited Autoimmunity and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: An Explanatory Model. Front. Immunol. 2018, 9, 229. [Google Scholar] [CrossRef]
- Sotzny, F.; Blanco, J.; Capelli, E.; Castro-Marrero, J.; Steiner, S.; Murovska, M.; Scheibenbogen, C.; European Network on ME/CFS (EUROMENE). Myalgic Encephalomyelitis/Chronic Fatigue Syndrome—Evidence for an autoimmune disease. Autoimmun. Rev. 2018, 17, 601–609. [Google Scholar] [CrossRef]
- Ryabkova, V.A.; Churilov, L.P.; Shoenfeld, Y. Neuroimmunology: What Role for Autoimmunity, Neuroinflammation, and Small Fiber Neuropathy in Fibromyalgia, Chronic Fatigue Syndrome, and Adverse Events after Human Papillomavirus Vaccination? Int. J. Mol. Sci. 2019, 20, 5164. [Google Scholar] [CrossRef]
- Loebel, M.; Eckey, M.; Sotzny, F.; Hahn, E.; Bauer, S.; Grabowski, P.; Zerweck, J.; Holenya, P.; Hanitsch, L.G.; Wittke, K.; et al. Serological profiling of the EBV immune response in Chronic Fatigue Syndrome using a peptide microarray. PLoS ONE 2017, 12, e0179124. [Google Scholar] [CrossRef] [PubMed]
- Danilenko, O.V.; Gavrilova, N.Y.; Churilov, L.P. Chronic Fatigue Exhibits Heterogeneous Autoimmunity Characteristics Which Reflect Etiology. Pathophysiology 2022, 29, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Mandarano, A.H.; Maya, J.; Giloteaux, L.; Peterson, D.L.; Maynard, M.; Gottschalk, C.G.; Hanson, M.R. Myalgic encephalomyelitis/chronic fatigue syndrome patients exhibit altered T cell metabolism and cytokine associations. J. Clin. Investig. 2020, 130, 1491–1505. [Google Scholar] [CrossRef] [PubMed]
- Wirth, K.; Scheibenbogen, C. A Unifying Hypothesis of the Pathophysiology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Recognitions from the finding of autoantibodies against ß2-adrenergic receptors. Autoimmun. Rev. 2020, 19, 102527. [Google Scholar] [CrossRef]
- Shoenfeld, Y.; Ryabkova, V.A.; Scheibenbogen, C.; Brinth, L.; Martinez-Lavin, M.; Ikeda, S.; Heidecke, H.; Watad, A.; Bragazzi, N.L.; Chapman, J.; et al. Complex syndromes of chronic pain, fatigue and cognitive impairment linked to autoimmune dysautonomia and small fiber neuropathy. Clin. Immunol. 2020, 214, 108384. [Google Scholar] [CrossRef]
- Lukashenko, M.V.; Gavrilova, N.Y.; Bregovskaya, A.V.; Soprun, L.A.; Churilov, L.P.; Petropoulos, I.N.; Malik, R.A.; Shoenfeld, Y. Corneal Confocal Microscopy in the Diagnosis of Small Fiber Neuropathy: Faster, Easier, and More Efficient than Skin Biopsy? Pathophysiology 2021, 29, 1–8. [Google Scholar] [CrossRef]
- Basantsova, N.Y.; Starshinova, A.A.; Dori, A.; Zinchenko, Y.S.; Yablonskiy, P.K.; Shoenfeld, Y. Small-fiber neuropathy definition, diagnosis, and treatment. Neurol. Sci. 2019, 40, 1343–1350. [Google Scholar] [CrossRef]
- Halpert, G.; Amital, H.; Shoenfeld, Y. Silicone Breast Implants—Historical Medical Error. Harefuah 2020, 159, 697–702. [Google Scholar]
- Chapman, J.; Rand, J.H.; Brey, R.L.; Levine, S.R.; Blatt, I.; Khamashta, M.A.; Shoenfeld, Y. Non-stroke neurological syndromes associated with antiphospholipid antibodies: Evaluation of clinical and experimental studies. Lupus 2003, 12, 514–517. [Google Scholar] [CrossRef]
- Moscavitch, S.D.; Szyper-Kravitz, M.; Shoenfeld, Y. Autoimmune pathology accounts for common manifestations in a wide range of neuro-psychiatric disorders: The olfactory and immune system interrelationship. Clin. Immunol. 2009, 130, 235–243. [Google Scholar] [CrossRef]
- Lee, J.; Biggs, K.; Muzaffar, J.; Bance, M.; Monksfield, P. Hearing loss in inner ear and systemic autoimmune disease: A systematic review of post-cochlear implantation outcomes. Laryngoscope Investig. Otolaryngol. 2021, 6, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.Y.; Tang, W.S.; Zakaria, Z. Unilateral sudden sensorineural hearing loss in post-COVID-19 patients: Case report. Malays. Fam. Physician 2022, 17, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Beukes, E.; Ulep, A.J.; Eubank, T.; Manchaiah, V. The Impact of COVID-19 and the Pandemic on Tinnitus: A Systematic Review. J. Clin. Med. 2021, 10, 2763. [Google Scholar] [CrossRef] [PubMed]
- Gerb, J.; Becker-Bense, S.; Zwergal, A.; Huppert, D. Vestibular syndromes after COVID-19 vaccination: A prospective cohort study. Eur. J. Neurol. 2022, 29, 3693–3700. [Google Scholar] [CrossRef]
- Garner, R.; Baraniuk, J.N. Orthostatic intolerance in chronic fatigue syndrome. J. Transl. Med. 2019, 17, 185. [Google Scholar] [CrossRef]
- Bellis, T. Assessment and Management of Central Auditory Processing Disorders in the Educational Setting from Science to Practice, 2nd ed.; Thomson Delmar Learning: New York, NY, USA, 2003; 533p. [Google Scholar]
- Chermak, G.D.; Musiek, F.E. Handbook of Central Auditory Processing Disorder. V.2. Comprehensive Intervention, 2nd ed.; Plural Publishing: San Diego, CA, USA, 2014; Volume 2, 769p. [Google Scholar]
- Nikravesh, M.; Jafari, Z.; Mehrpour, M.; Kazemi, R.; Amiri Shavaki, Y.; Hossienifar, S.; Azizi, M.P. The paced auditory serial addition test for working memory assessment: Psychometric properties. Med. J. Islam. Repub. Iran 2017, 31, 61. [Google Scholar] [CrossRef]
- Bryden, M.P. Correlates of the dichotic right-ear effect. Cortex 1988, 24, 313–319. [Google Scholar] [CrossRef]
- Iliadou, V.; Ptok, M.; Grech, H.; Pedersen, E.R.; Brechmann, A.; Deggouj, N.; Kiese-Himmel, C.; Sʹliwinʹska-Kowalska, M.; Nickisch, A.; Demanez, L.; et al. A European Perspective on Auditory Processing Disorder- Current Knowledge and Future Research Focus. Front. Neurol. 2017, 8, 622. [Google Scholar] [CrossRef]
- Bryden, M.P. An evaluation of some models of laterality in dichotic listening. Acta Oto-Laryngol. 1967, 63, 595–604. [Google Scholar] [CrossRef]
- Musiek, F.E.; Chermak, G.D. Handbook of Central Auditory Processing Disorder. Auditory Neuroscience and Diagnosis, 2nd ed.; Plural Publishing: San Diego, CA, USA, 2014; 745p. [Google Scholar]
- Collongues, N.; Samama, B.; Schmidt-Mutter, C.; Chamard-Witkowski, L.; Debouverie, M.; Chanson, J.B.; Antal, M.C.; Benardais, K.; de Seze, J.; Velten, M.; et al. Quantitative and qualitative normative dataset for intraepidermal nerve fibers using skin biopsy. PLoS ONE 2018, 13, e0191614. [Google Scholar] [CrossRef]
- Tavakoli, M.; Ferdousi, M.; Petropoulos, I.N.; Morris, J.; Pritchard, N.; Zhivov, A.; Ziegler, D.; Pacaud, D.; Romanchuk, K.; Perkins, B.A.; et al. Normative values for corneal nerve morphology assessed using corneal confocal microscopy: A multinational normative data set. Diabetes Care 2015, 38, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Lüscher, E. The Difference Limen of Intensity Variations of Pure Tones and its Diagnostic Significance. J. Laryngol. Otol. 1951, 65, 486–510. [Google Scholar] [CrossRef] [PubMed]
- Keith, R.W. Random Gap Detection Test; Auditec: St. Louis, MO, USA, 2000; Volume 13. [Google Scholar]
- Heinrich, A.; Alain, C.; Schneider, B.A. Within- and between-channel gap detection in the human auditory cortex. NeuroReport 2004, 15, 2051–2056. [Google Scholar] [CrossRef]
- Robin, D.A.; Tranel, D.; Damasio, H. Auditory perception of temporal and spectral events in patients with focal left and right cerebral lesions. Brain Lang. 1990, 39, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Techentin, C.; Voyer, D. Word frequency, familiarity, and laterality effects in a dichotic listening task. Laterality 2011, 16, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Boissoneault, J.; Letzen, J.; Lai, S.; O’Shea, A.; Craggs, J.; Robinson, M.E.; Staud, R. Abnormal resting state functional connectivity in patients with chronic fatigue syndrome: An arterial spin-labeling fMRI study. Magn. Reson. Imaging 2016, 34, 603–608. [Google Scholar] [CrossRef]
- Hall, D.A.; Barrett, D.J.K.; Akeroyd, M.A.; Summerfield, A.Q. Cortical Representations of Temporal Structure in Sound. J. Neurophysiol. 2005, 94, 3181–3191. [Google Scholar] [CrossRef]
- Botvinick, M.; Nystrom, L.E.; Fissell, K.; Carter, C.S.; Cohen, J.D. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature 1999, 402, 179–181. [Google Scholar] [CrossRef]
- Falkenberg, L.E.; Specht, K.; Westerhausen, R. Attention and cognitive control networks assessed in a dichotic listening fMRI study. Brain Cogn. 2011, 76, 276–285. [Google Scholar] [CrossRef]
- Chermak, G.D.; Bamiou, D.E.; Iliadou, V.; Musiek, F.E. Practical guidelines to minimise language and cognitive confounds in the diagnosis of CAPD: A brief tutorial. Int. J. Audiol. 2017, 56, 499–506. [Google Scholar] [CrossRef]
- Kamali, B.; Khavarghazalani, B.; Hosseini Dastgerdi, Z. Auditory processing disorder in elderly. Hear. Balance Commun. 2022, 20, 240–246. [Google Scholar] [CrossRef]
- Ryndina, A.M.; Berdnikova, I.P.; Tsvyleva, I.D. Audiometriya chereduyushchimisya rechevymi signalami v diagnostike tsentral’nykh porazheniy slukhovogo analizatora. Vestn. Otorinolaringol. 1998, 6, 13–14. (In Russia) [Google Scholar]
- Lange, G.; Steffener, J.; Cook, D.B.; Bly, B.M.; Christodoulou, C.; Liu, W.-C.; DeLuca, J.; Natelsonb, B.H. Objective evidence of cognitive complaints in chronic fatigue syndrome: A BOLD fMRI study of verbal working memory. Neuroimage 2005, 26, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Mancini, P.; Atturo, F.; Di Mario, A.; Portanova, G.; Ralli, M.; De Virgilio, A.; de Vincentiis, M.; Greco, A. Hearing loss in autoimmune disorders: Prevalence and therapeutic options. Autoimmun Rev. 2018, 17, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Yehudai, D.; Shoenfeld, Y.; Toubi, E. The autoimmune characteristics of progressive or sudden sensorineural hearing loss. Autoimmunity 2006, 39, 153–158. [Google Scholar] [CrossRef]
- Chawki, S.; Aouizerate, J.; Trad, S.; Prinseau, J.; Hanslik, T. Bilateral sudden sensorineural hearing loss as a presenting feature of systemic lupus erythematosus: Case report and brief review of other published cases. Medicine 2016, 95, e4345. [Google Scholar] [CrossRef]
- Umashankar, A.; Prakash, P.; Prabhu, P. Sudden Sensorineural Hearing Loss Post Coronavirus Disease: A Systematic Review of Case Reports. Indian J. Otolaryngol. Head Neck Surg. 2021, 74, 3028–3035. [Google Scholar] [CrossRef]
- Koumpa, F.S.; Forde, C.T.; Manjaly, J.G. Sudden irreversible hearing loss post COVID-19. BMJ Case Rep. 2020, 13, e238419. [Google Scholar] [CrossRef]
- Fancello, V.; Fancello, G.; Hatzopoulos, S.; Bianchini, C.; Stomeo, F.; Pelucchi, S.; Ciorba, A. Sensorineural Hearing Loss Post-COVID-19 Infection: An Update. Audiol. Res. 2022, 12, 307–315. [Google Scholar] [CrossRef]
- Gallus, R.; Melis, A.; Rizzo, D.; Piras, A.; De Luca, L.M.; Tramaloni, P.; Serra, A.; Longoni, E.; Soro, G.M.; Bussu, F. Audiovestibular symptoms and sequelae in COVID-19 patients. J. Vestib. Res. 2021, 31, 381–387. [Google Scholar] [CrossRef]
- Di Mauro, P.; La Mantia, I.; Cocuzza, S.; Sciancalepore, P.I.; Rasà, D.; Maniaci, A.; Ferlito, S.; Tundo, I.; Anzivino, R. Acute Vertigo After COVID-19 Vaccination: Case Series and Literature Review. Front. Med. 2022, 8, 790931. [Google Scholar] [CrossRef] [PubMed]


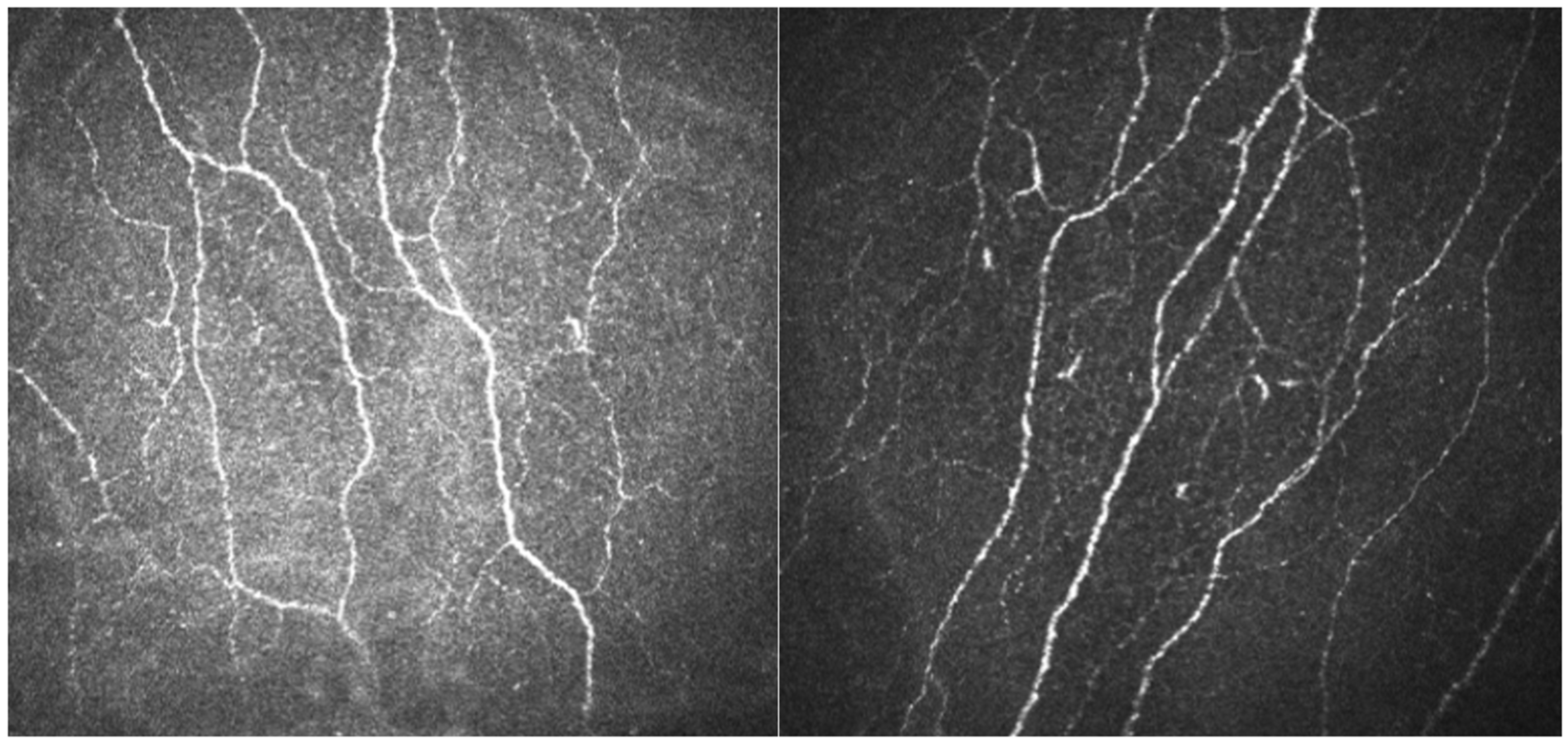
| CNFD, Corneal Nerve Fiber Density | CNBD, Corneal Nerve Branch Density | CNFL, Corneal Nerve Fiber Length | |
|---|---|---|---|
| Patient’s results | 29.86 | 49.30 | 18.79 |
| Normal median values for 36–45 years, female [35] | 28.56 | 63.27 | 23.28 |
| Normal 5th quartile for 36–45 years, female [35] | 14.79 | 18.19 | 12.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreeva, I.G.; Gvozdeva, A.; Pimenova, V.; Ryabkova, V.; Lukashenko, M.; Kamaeva, E.; Shapkina, V.; Soprun, L.; Gavrilova, N.; Fedotkina, T.V.; et al. Assessment of Hearing and Vestibular Functions in a Post-COVID-19 Patient: A Clinical Case Study. Diagnostics 2023, 13, 122. https://doi.org/10.3390/diagnostics13010122
Andreeva IG, Gvozdeva A, Pimenova V, Ryabkova V, Lukashenko M, Kamaeva E, Shapkina V, Soprun L, Gavrilova N, Fedotkina TV, et al. Assessment of Hearing and Vestibular Functions in a Post-COVID-19 Patient: A Clinical Case Study. Diagnostics. 2023; 13(1):122. https://doi.org/10.3390/diagnostics13010122
Chicago/Turabian StyleAndreeva, Irina Germanovna, Alisa Gvozdeva, Vera Pimenova, Varvara Ryabkova, Maria Lukashenko, Evelina Kamaeva, Valeria Shapkina, Lidia Soprun, Natalia Gavrilova, Tamara Viktorovna Fedotkina, and et al. 2023. "Assessment of Hearing and Vestibular Functions in a Post-COVID-19 Patient: A Clinical Case Study" Diagnostics 13, no. 1: 122. https://doi.org/10.3390/diagnostics13010122
APA StyleAndreeva, I. G., Gvozdeva, A., Pimenova, V., Ryabkova, V., Lukashenko, M., Kamaeva, E., Shapkina, V., Soprun, L., Gavrilova, N., Fedotkina, T. V., Churilov, L. P., & Shoenfeld, Y. (2023). Assessment of Hearing and Vestibular Functions in a Post-COVID-19 Patient: A Clinical Case Study. Diagnostics, 13(1), 122. https://doi.org/10.3390/diagnostics13010122









