The Influence of Eyelid Position and Environmental Conditions on the Corneal Changes in Early Postmortem Interval: A Prospective, Multicentric OCT Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
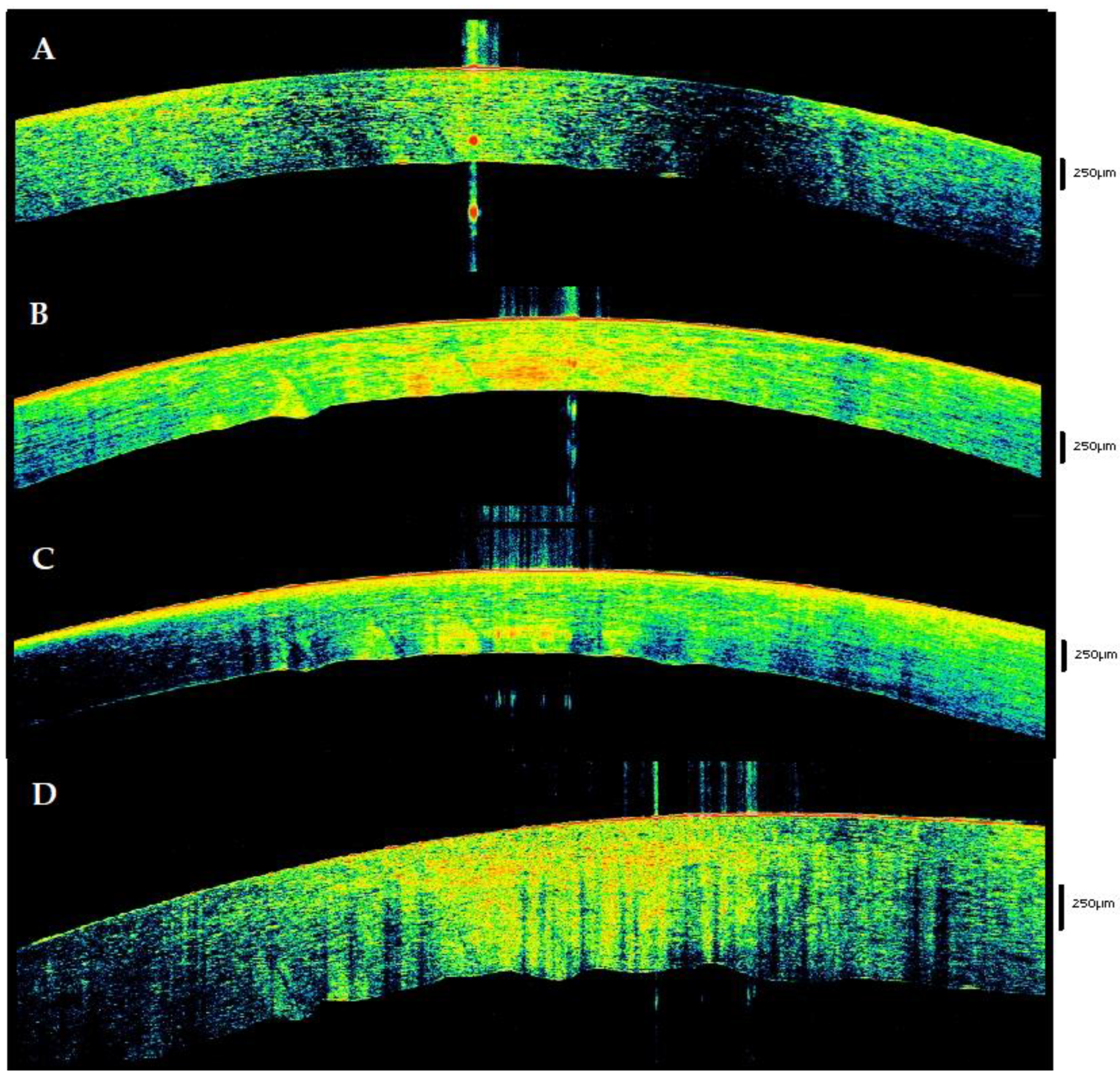
3.1. Baseline
- (a)
- Persistence of the tear film.
- (b)
- Preservation of the epithelial state.
- (c)
- Homogeneity and preservation of the physiological structure of the corneal stroma. The presence of alphabetic-shaped stromal striae (SS) was osservable in a variable (Figure 2) number of cases.
- (d)
- Morphological integrity of the endothelium.
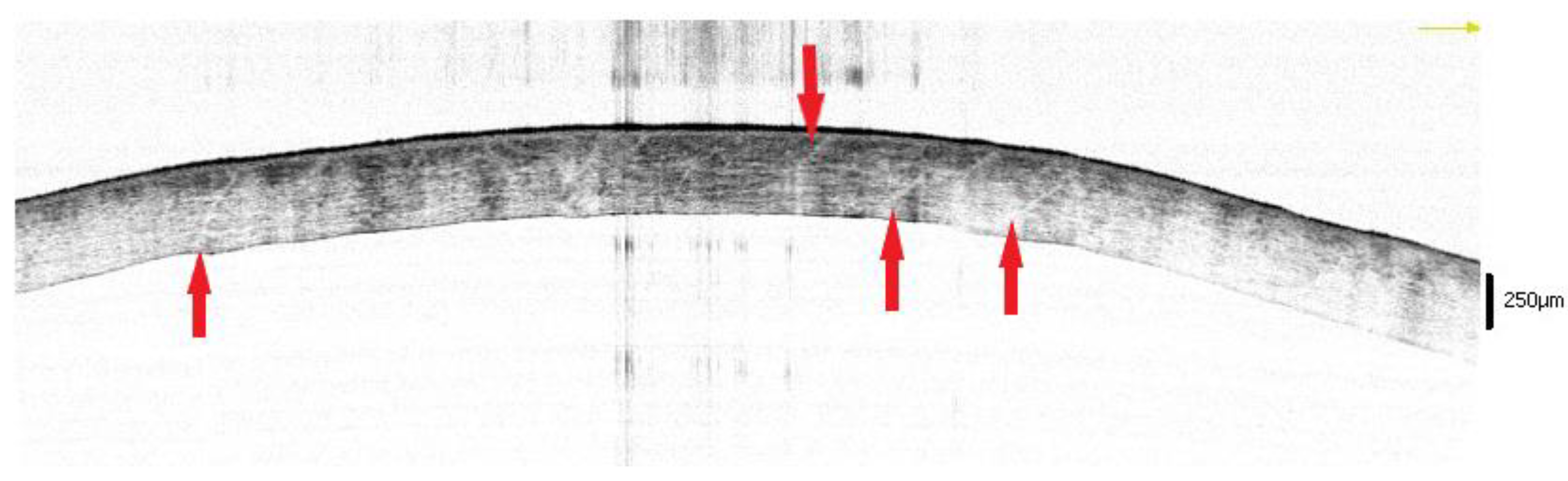
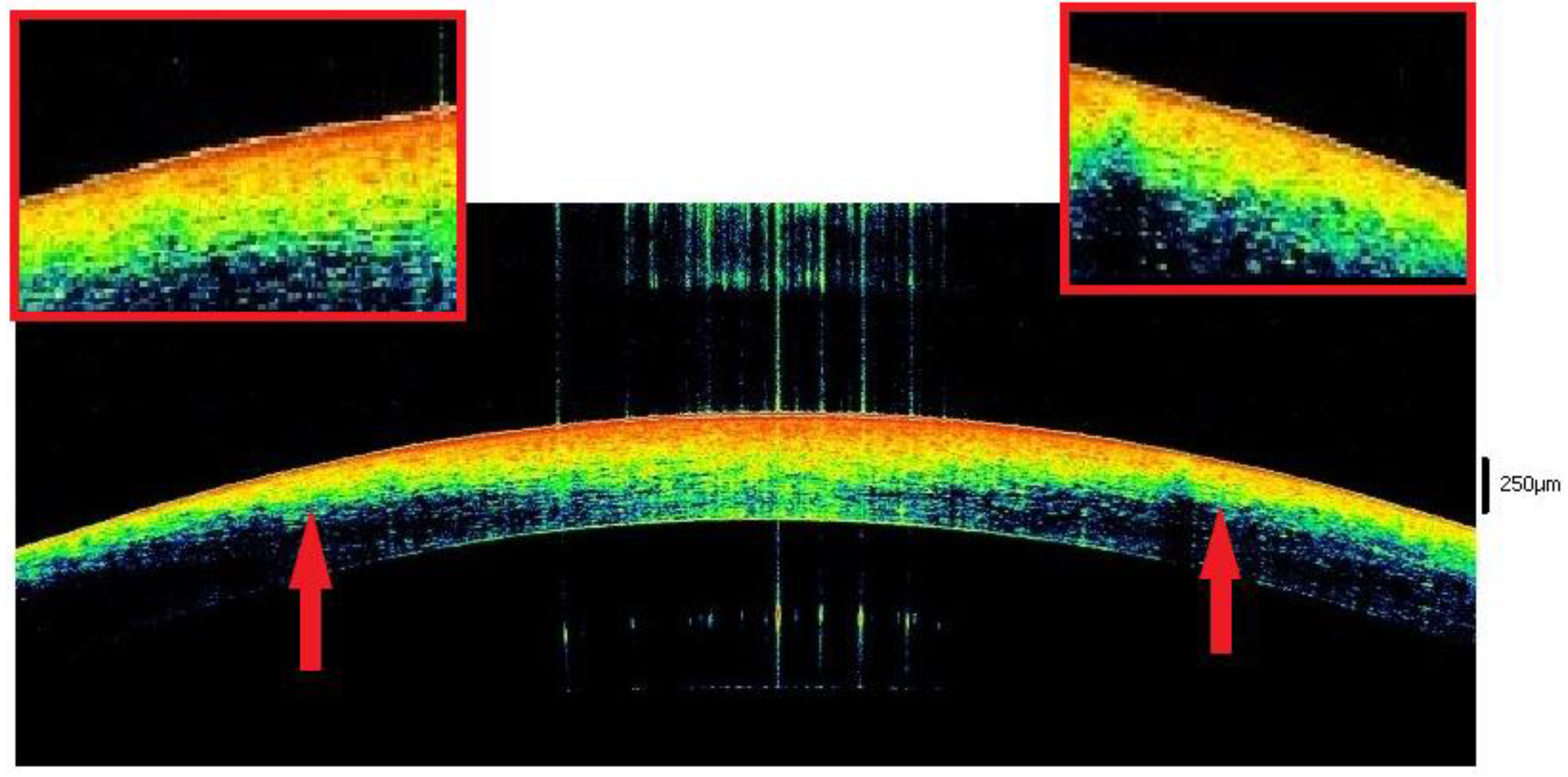
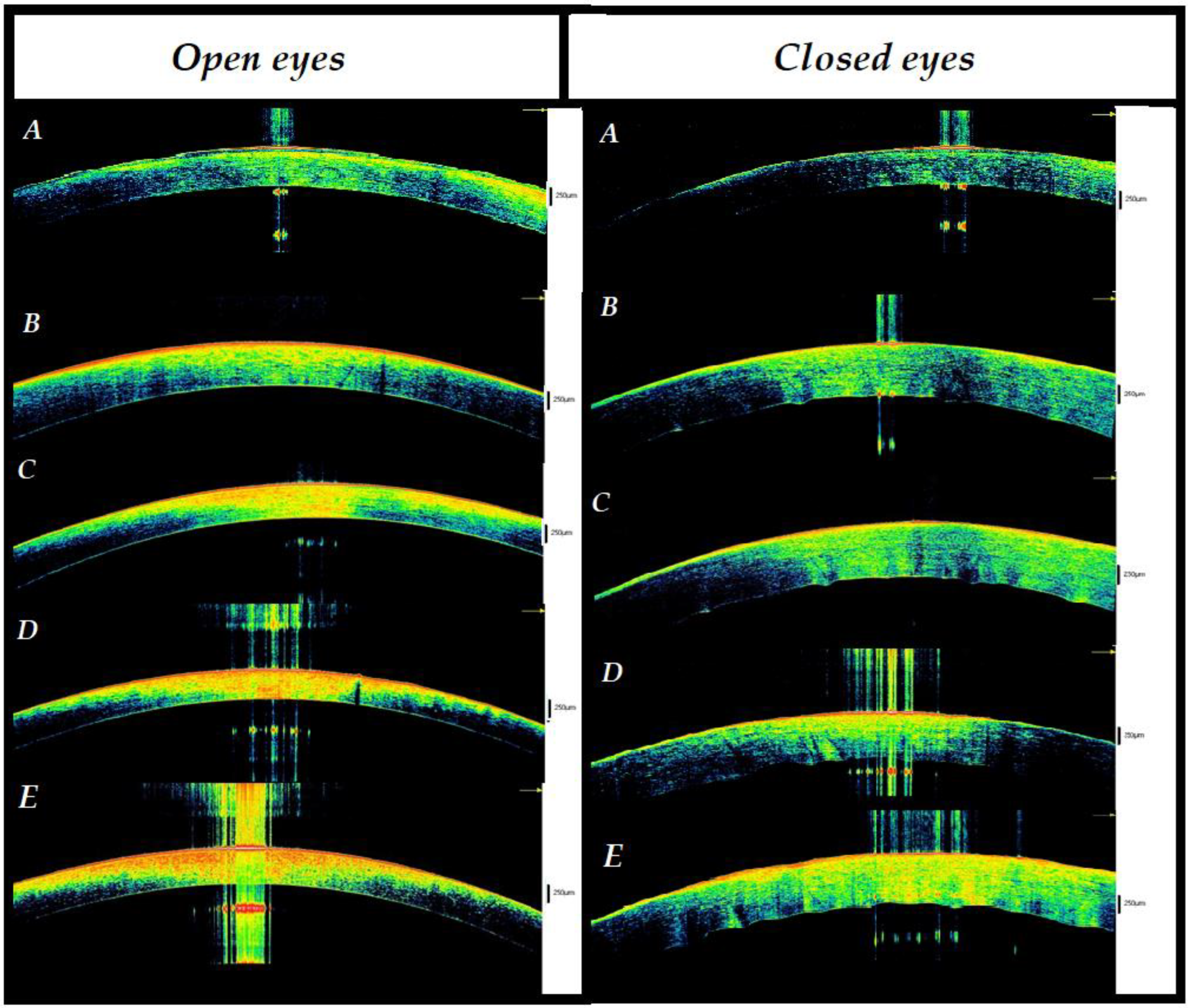
3.2. Open Eyes
3.2.1. The 3–6 h Interval
3.2.2. The 6–9 h Interval
3.2.3. The 9–12 h Interval
3.2.4. The 12–17 h Interval
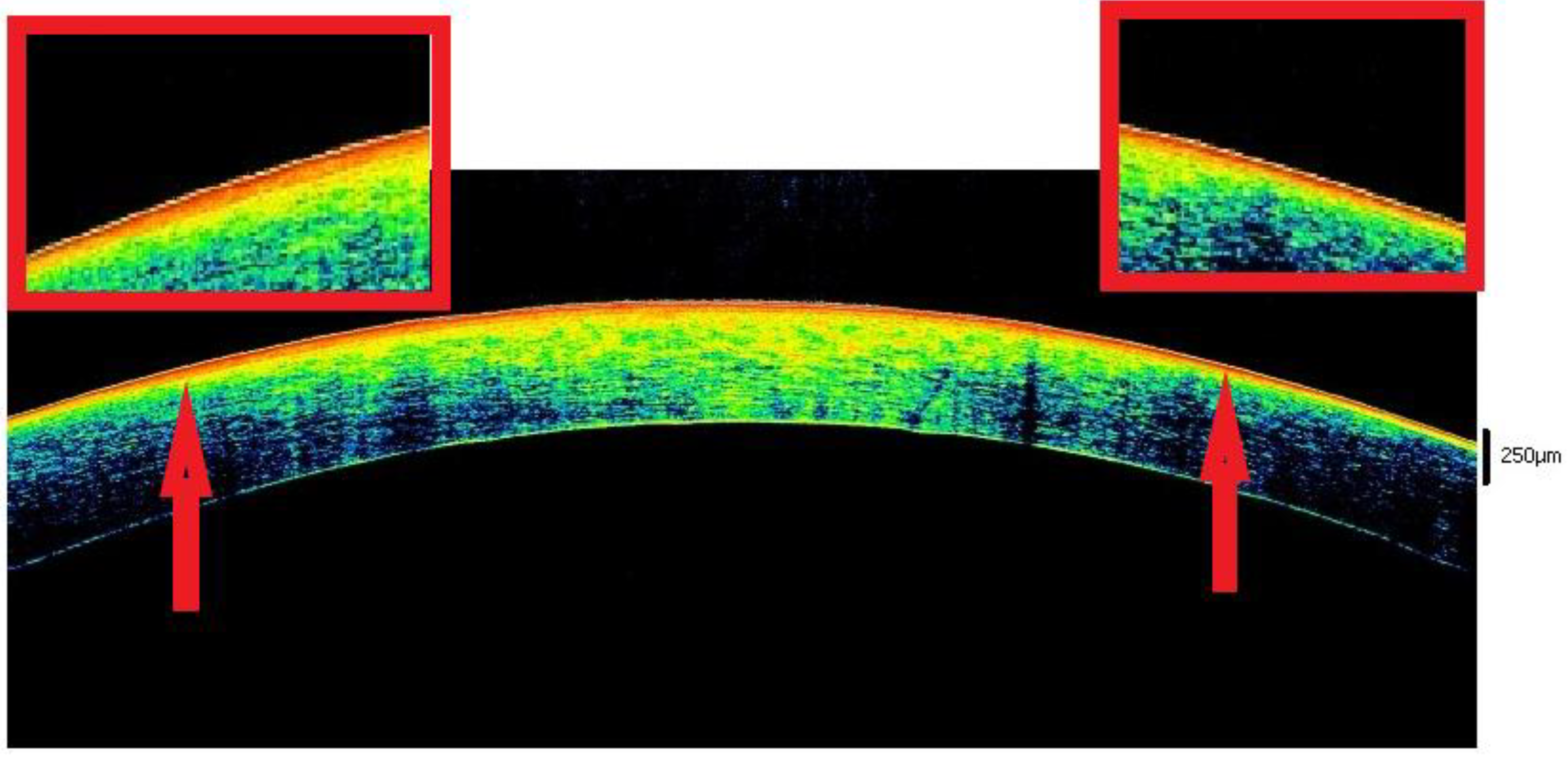
3.3. Closed Eyes
3.3.1. The 3–6 h Interval
3.3.2. The 6–9 h Interval
3.3.3. Interval 9–12 h
3.3.4. The 12–17 h Interval
3.4. Influence of Environmental Conditions
4. Discussion
4.1. Open Eye
4.2. Closed Eye
4.3. Influence of Environmental Conditions: Temperature and Humidity
4.4. Future Research and Practice Perspectives
4.5. Limits of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madea, B.; Henssge, C.; Hönig, W.; Gerbracht, A. References for determining the time of death by potassium in vitreous humor. Forensic Sci. Int. 1989, 40, 231–243. [Google Scholar] [CrossRef]
- Madea, B. Is there recent progress in the estimation of the postmortem interval by means of thanatochemistry? Forensic Sci. Int. 2005, 151, 139–149. [Google Scholar] [CrossRef]
- Stocchero, M.; Locci, E.; d’Aloja, E.; Nioi, M.; Baraldi, E.; Giordano, G. PLS2 in Metabolomics. Metabolites 2019, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Locci, E.; Stocchero, M.; Gottardo, R.; De-Giorgio, F.; Demontis, R.; Nioi, M.; Chighine, A.; Tagliaro, F.; d’Aloja, E. Comparative use of aqueous humour 1H NMR metabolomics and potassium concentration for PMI estimation in an animal model. Int. J. Legal. Med. 2021, 135, 845–852. [Google Scholar] [CrossRef]
- Zelentsova, E.A.; Yanshole, L.V.; Snytnikova, O.A.; Yanshole, V.V.; Tsentalovich, Y.P.; Sagdeev, R.Z. Post-mortem changes in themetabolomic compositions of rabbit blood, aqueous and vitreous humors. Metabolomics 2016, 12, 172. [Google Scholar] [CrossRef]
- Locci, E.; Stocchero, M.; Noto, A.; Chighine, A.; Natali, L.; Napoli, P.E.; Caria, R.; De-Giorgio, F.; Nioi, M.; d’Aloja, E. A 1 H NMR metabolomic approach for the estimation of the time since death using aqueous humour: An animal model. Metabolomics 2019, 15, 76. [Google Scholar] [CrossRef] [PubMed]
- Zapico, S.C.; Adserias-Garriga, J. Postmortem Interval Estimation: New Approaches by the Analysis of Human Tissues and Microbial Communities′ Changes. Forensic Sci. 2022, 2, 163–174. [Google Scholar] [CrossRef]
- Go, A.; Shim, G.; Park, J.; Hwang, J.; Nam, M.; Jeong, H.; Chung, H. Analysis of hypoxanthine and lactic acid levels in vitreous humor for the estimation of post-mortem interval (PMI) using LC–MS/MS. Forensic Sci. Int. 2019, 299, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.C.; Schandl, C.; Hyer, M.; Erin Presnell, S.J. Forensic Sci. Aqueous fluid as a viable substitute for vitreous fluid in postmortem chemistry analysis. J. Forensic sci. 2020, 65, 508–512. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Kenton, G.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178. [Google Scholar] [CrossRef]
- Napoli, P.E.; Nioi, M.; Mangoni, L.; Gentile, P.; Braghiroli, M.; d’Aloja, E.; Fossarello, M. Fourier-domain OCT imaging of the ocular surface and tear film dynamics: A review of the state of the art and an integrative model of the tear behavior during the inter-blink period and visual fixation. J Clin. Med. 2020, 9, 668. [Google Scholar] [CrossRef]
- Drexler, W.; Morgner, U.; Ghanta, R.K.; Kärtner, F.X.; Schuman, J.S.; Fujimoto, J.G. Ultrahigh-resolution ophthalmic optical coherence tomography. Nat Med. 2001, 7, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Nioi, M.; Napoli, P.E.; Mayerson, S.M.; Fossarello, M.; d’Aloja, E. Optical coherence tomography in forensic sciences: A review of the literature. Forensic Sci. Med. Pathol. 2019, 15, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Napoli, P.E.; Nioi, M.; d’Aloja, E.; Fossarello, M. Post-mortem corneal thickness measurements with a portable optical coherence tomography system: A reliability study. Sci. Rep. 2016, 6, 30428. [Google Scholar] [CrossRef]
- Napoli, P.E.; Nioi, M.; Gabiati, L.; Laurenzo, M.; De-Giorgio, F.; Scorcia, V.; Grassi, S.; d’Aloja, E.; Fossarello, M. Repeatability and reproducibility of post-mortem central corneal thickness measurements using a portable optical coherence tomography system in humans: A prospective multicenter study. Sci. Rep. 2020, 10, 14508. [Google Scholar] [CrossRef]
- Nioi, M.; Napoli, P.E.; Iovino, C.; Paribello, F.; Fossarello, M.; d’Aloja, E. Corneal findings after death: A preliminary OCT study on an animal model. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4873. [Google Scholar]
- Nioi, M.; Napoli, P.E.; Demontis, R.; Locci, E.; Fossarello, M.; d’Aloja, E. Morphological analysis of corneal findings modifications after death: A preliminary OCT study on an animal model. Exp. Eye Res. 2018, 169, 20–27. [Google Scholar] [CrossRef]
- Nioi, M.; Napoli, P.E.; Paribello, F.; Demontis, R.; De-Giorgio, F.; Porru, E.; Fossarello, M.; D′Aloja, E. Use of Optical Coherence Tomography (OCT) on detection of postmortem Ocular Findings: Pilot data from two cases. J. Integr. OMICS 2017, 7, 9–10. [Google Scholar]
- Nioi, M.; Napoli, P.E.; Demontis, R.; Locci, E.; Fossarello, M.; d’Aloja, E. Postmortem Ocular Findings in the Optical Coherence Tomography Era: A Proof of Concept Study Based on Six Forensic Cases. Diagnostics 2021, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- Napoli, P.E.; Coronella, F.; Satta, G.M.; Zucca, I.A.; Fossarello, M. A novel OCT technique to measure in vivo the corneal adhesiveness for sodium carboxymethylcellulose in humans and its validity in the diagnosis of dry eye. IOVS 2014, 55, 3179–3185. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Del Monte, D.W.; Kim, T. Anatomy and physiology of the cornea. J. Cataract Refract Surg. 2011, 37, 588–598. [Google Scholar] [CrossRef]
- Nishida, T.; Saika, S.; Morishige, N. Cornea and sclera: Anatomy and physiology. Cornea 2011, 1, 1–22. [Google Scholar]
- Gandhi, S.; Jain, S. The Anatomy and Physiology of cornea. In Keratoprostheses and Artificial Corneas; Springer: Berlin/Heidelberg, Germany, 2015; pp. 19–25. [Google Scholar]
- Leung, B.K.; Bonanno, J.A.; Radke, C.J. Oxygen-deficient metabolism and corneal edema. Prog. Retin. Eye Res. 2011, 30, 471–492. [Google Scholar] [CrossRef]
- Takatori, S.C.; de la Jara, P.L.; Holden, B.; Ehrmann, K.; Ho, A.; Radke, C.J. In vivo oxygen uptake into the human cornea. IOVS 2012, 53, 6331–6337. [Google Scholar] [CrossRef]
- Smelser, G.K.; Ozanics, V. Importance of atmospheric oxygen for maintenance of the optical properties of the human cornea. Science 1952, 115, 140. [Google Scholar] [CrossRef]
- Larrea, X.; BuÜchler, P. A transient diffusion model of the cornea for the assessment of oxygen diffusivity and consumption. IOVS 2009, 50, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Saukko, P.; Knight, B. Knight’s Forensic Pathology; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Rosa, M.F.; Scano, P.; Noto, A.; Nioi, M.; Sanna, R.; Paribello, F.; De-Giorgio, F.; Locci, E.; d’Aloja, E. Monitoring the modifications of the vitreous humor metabolite profile after death: An animal model. BioMd Res. Int. 2015, 2015, 627201. [Google Scholar] [CrossRef] [PubMed]
- Coburn, C.; Allman, E.; Mahanti, P.; Benedetto, A.; Cabreiro, F.; Pincus, Z.; Matthijssens, F.; Araiz, C.; Mandel, A.; Vlachos, M.; et al. Anthranilate fluorescence marks a calcium-propagated necrotic wave that promotes organismal death in C. elegans. PLoS Biol. 2013, 11, e1001613. [Google Scholar] [CrossRef]
- Zelentsova, E.A.; Yanshole, L.V.; Melnikov, A.D.; Kudryavtsev, I.S.; Novoselov, V.P.; Tsentalovich, Y.P. Post-mortem changes in metabolomic profiles of human serum, aqueous humor and vitreous humor. Metabolomics 2020, 16, 80. [Google Scholar] [CrossRef]
- Polse, K.A.; Brand, R.J.; Cohen, S.R.; Guillon, M. Hypoxic effects on corneal morphology and function. IOVS 1990, 31, 1542–1554. [Google Scholar]
- Pang, K.; Lennikov, A.; Yang, M. Hypoxia adaptation in the cornea: Current animal models and underlying mechanisms. Anim. Models Exp. Med. 2021, 4, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Karamichos, D.; Onochie, O.E.; Hutcheon, A.E.; Rich, C.B.; Zieske, J.D.; Trinkaus-Randall, V. Hypoxia modulates the development of a corneal stromal matrix model. Exp. Eye Res. 2018, 170, 127–137. [Google Scholar] [CrossRef]
- Nguyen, T.; Soni, P.S.; Brizendine, E.; Bonanno, J.A. Variability in hypoxia-induced corneal swelling is associated with variability in corneal metabolism and endothelial function. Eye Contact Lens 2003, 29, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, S.P.; Murugan, S.; Rachapalle, S.; Padmanabhan, P. Functions of the corneal endothelium as measured by swelling and deswelling dynamics in response to contact lens-induced transient hypoxia. IOVS 2018, 59, 2914. [Google Scholar]
- Peng, C.C.; Cerretani, C.; Braun, R.J.; Radke, C.J. Evaporation-driven instability of the precorneal tear film. Adv. Colloid Interface Sci. 2014, 206, 250–264. [Google Scholar] [CrossRef]
- Napoli, P.E.; Nioi, M.; d’Aloja, E.; Loy, F.; Fossarello, M. The architecture of corneal stromal striae on optical coherence tomography and histology in an animal model and in humans. Sci. Rep. 2020, 10, 19861. [Google Scholar] [CrossRef]
- Grieve, K.; Ghoubay, D.; Georgeon, C.; Latour, G.; Nahas, A.; Plamann, K.; Crotti, C.; Bocheux, R.; Borderie, M.; Nguyen, T.M.; et al. Stromal striae: A new insight into corneal physiology and mechanics. Sci. Rep. 2017, 7, 13584. [Google Scholar] [CrossRef] [PubMed]
- Napoli, P.E.; Nioi, M.; d’Aloja, E.; Fossarello, M. The bull’s eye pattern of the tear film in humans during visual fixation on en-face optical coherence tomography. Sci. Rep. 2019, 9, 1413. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, J.A. Molecular mechanisms underlying the corneal endothelial pump. Exp. Eye Res. 2012, 95, 2–7. [Google Scholar] [CrossRef]
- Freund, D.E.; McCally, R.L.; Farrell, R.A.; Cristol, S.M.; L′Hernault, N.L.; Edelhauser, H.F. Ultrastructure in anterior and posterior stroma of perfused human and rabbit corneas. Relation to transparency. IOVS 1995, 36, 1508–1523. [Google Scholar]
- Müller, L.J.; Pels, E.; Vrensen, G.F. The specific architecture of the anterior stroma accounts for maintenance of corneal curvature. Br. J. Ophthamol. 2001, 85, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.M.; Ziebarth, N.M. Anterior and posterior corneal stroma elasticity assessed using nanoindentation. Exp. Eye Res. 2013, 115, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Wilson, G. Non-uniform swelling properties of the corneal stroma. Cureye Res. 1981, 1, 457–461. [Google Scholar] [CrossRef] [PubMed]

| Epithelium | Stroma | Endothelium | Other Signs | |
| 0–3 | Presence of lacrimal tear | Physiological thickness. | Preserved | Nothing. |
| 3–6 | Lacrimal tear progressively disappears. Hyperreflectivity with a “binary” pattern. | Initial thinning with differentiation between the anterior (hyper-reflective) and posterior (hypo-reflective) parts. | Hyper-reflective whit spots. | Initial sawtooth and stromal striae are detectable in the stroma. |
| 6–9 | Single, hyperreflective layer. | Decrease in stromal thickness. Hyperreflectivity in ¾ of anterior tissue in 68.5% of cases. | Unchanged. | Unchanged |
| 9–12 | Unchanged | Hyperreflectivity reaches the endothelium and takes a trapezoid form. | Enhancing of hyperreflectivity. | Stromal striae (65.8%) Sawtooth sign (78,2%) Initial loosing of the spheric form of the tissue in 21.7% of cases |
| 12–17 | Unchanged | Rising of posterior stroma hypo-reflectivity that in some cases reaches the middle of the tissue. | Are evident areas of hypo-reflectivity. | Stromal striae (100%) Sawtooth sign (100%) Initial loosing of the spheric form of the tissue in 34.7% of cases |
| Epithelium | Stroma | Endothelium | Other Signs | |
| 0–3 | Presence of lacrimal tear | Physiological thickness. | Preserved | Nothing |
| 3–6 | Lacrimal tear disappears. Binary sign with hyperreflectivity of the outer and inner layer and hyperreflectivity in the middle. | Posterior waves (Nioi Napoli sign grade 0). Thickening of the stroma with initial differentiation between posterior and anterior tissue. In 69,5%, the posterior stroma assumes a wavy morphology. | Hyperreflective respect to the posterior stroma. Presence of Endothelial spots | V-shapes in 56.5% of cases. Nioi–Napoli I (21.7%) |
| 6–9 | Appears like a single, hyperreflective layer in 50% of cases. In 50% ‘binary morphology’. | Differentiation between anterior hyperreflective and posterior hypo-reflective stroma in 56.5% of cases. Presence of one tissue peak that protrudes in the anterior chamber (Nioi-Napoli 1) | Hyper-reflectivity with the presence of multiple spots. | Nioi–Napoli II (21.7%). Initial loosing of the spheric form of the tissue.in 34.7% of cases |
| 9–12 | Single hyperreflective layer in 91.3% of cases. In the 8.7% initial flaking of the epithelium. | Thickening of the stoma. Anterior-posterior differentiation in 100% of cases. Waving with one of two protruding peaks in 14 cases (60.8%). | Unchanged | Nioi–Napoli III (60.8%) |
| 12–17 | Single hyperreflective layer in 100%. Epithelium flacking in 34.7% of cases. | Important thickening, structural inhomogeneity with anterior-posterior differentiation and multiple hyporeflective spots. Posterior waving with more than two peaks in 100% of cases. | Hyperreflective. | Nioi–Napoli IV in 100% of cases. Stromal Striae in 60.8% of cases. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nioi, M.; Napoli, P.E.; Demontis, R.; Chighine, A.; De-Giorgio, F.; Grassi, S.; Scorcia, V.; Fossarello, M.; d’Aloja, E. The Influence of Eyelid Position and Environmental Conditions on the Corneal Changes in Early Postmortem Interval: A Prospective, Multicentric OCT Study. Diagnostics 2022, 12, 2169. https://doi.org/10.3390/diagnostics12092169
Nioi M, Napoli PE, Demontis R, Chighine A, De-Giorgio F, Grassi S, Scorcia V, Fossarello M, d’Aloja E. The Influence of Eyelid Position and Environmental Conditions on the Corneal Changes in Early Postmortem Interval: A Prospective, Multicentric OCT Study. Diagnostics. 2022; 12(9):2169. https://doi.org/10.3390/diagnostics12092169
Chicago/Turabian StyleNioi, Matteo, Pietro Emanuele Napoli, Roberto Demontis, Alberto Chighine, Fabio De-Giorgio, Simone Grassi, Vincenzo Scorcia, Maurizio Fossarello, and Ernesto d’Aloja. 2022. "The Influence of Eyelid Position and Environmental Conditions on the Corneal Changes in Early Postmortem Interval: A Prospective, Multicentric OCT Study" Diagnostics 12, no. 9: 2169. https://doi.org/10.3390/diagnostics12092169
APA StyleNioi, M., Napoli, P. E., Demontis, R., Chighine, A., De-Giorgio, F., Grassi, S., Scorcia, V., Fossarello, M., & d’Aloja, E. (2022). The Influence of Eyelid Position and Environmental Conditions on the Corneal Changes in Early Postmortem Interval: A Prospective, Multicentric OCT Study. Diagnostics, 12(9), 2169. https://doi.org/10.3390/diagnostics12092169









