Understanding COVID Vaccination and Its Implication in Cancer Patients’ Imaging of Lymph Nodes by PET-CT
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. PET/CT Acquisition Protocol
2.3. Image Interpretation
2.4. Statistical Analysis
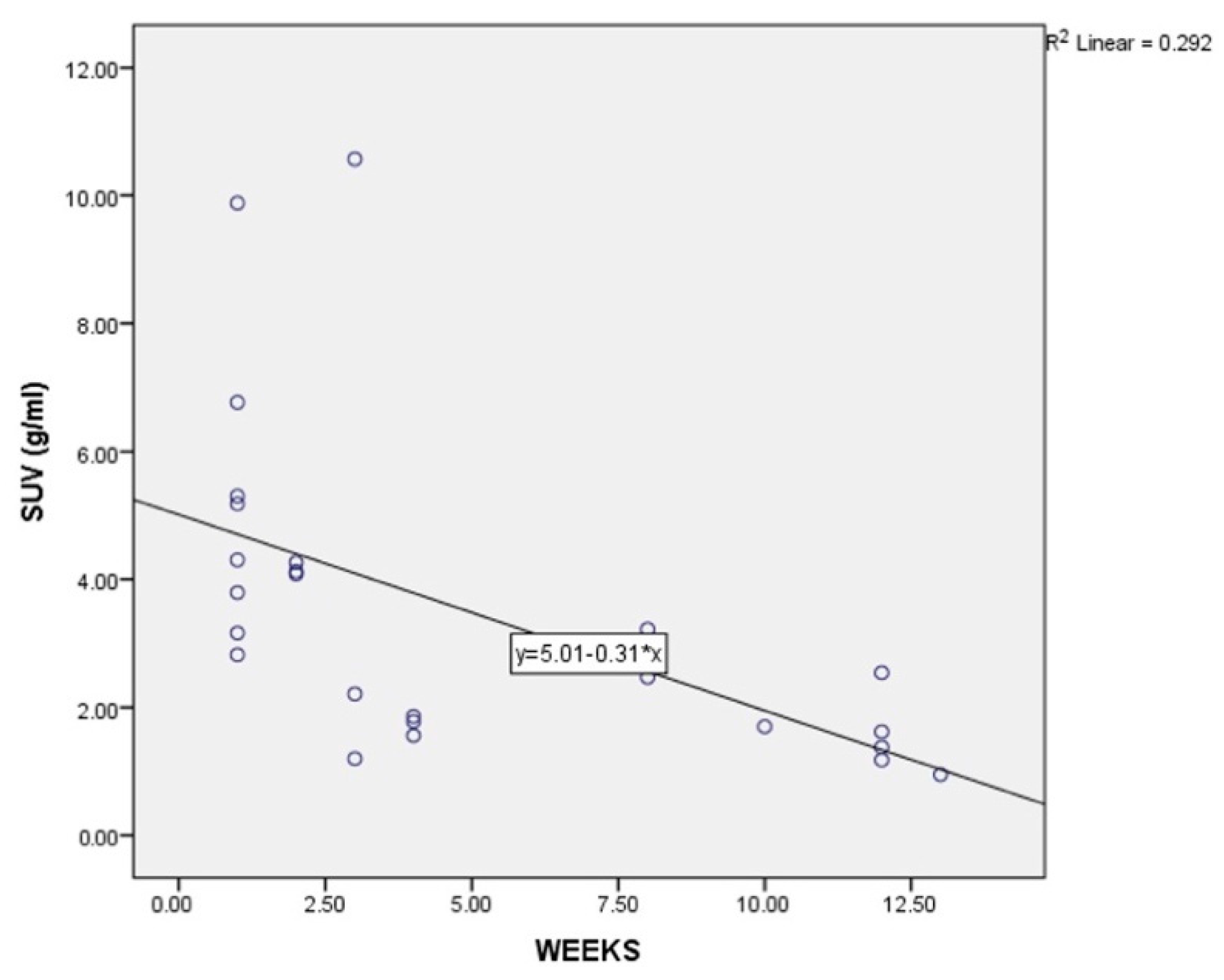
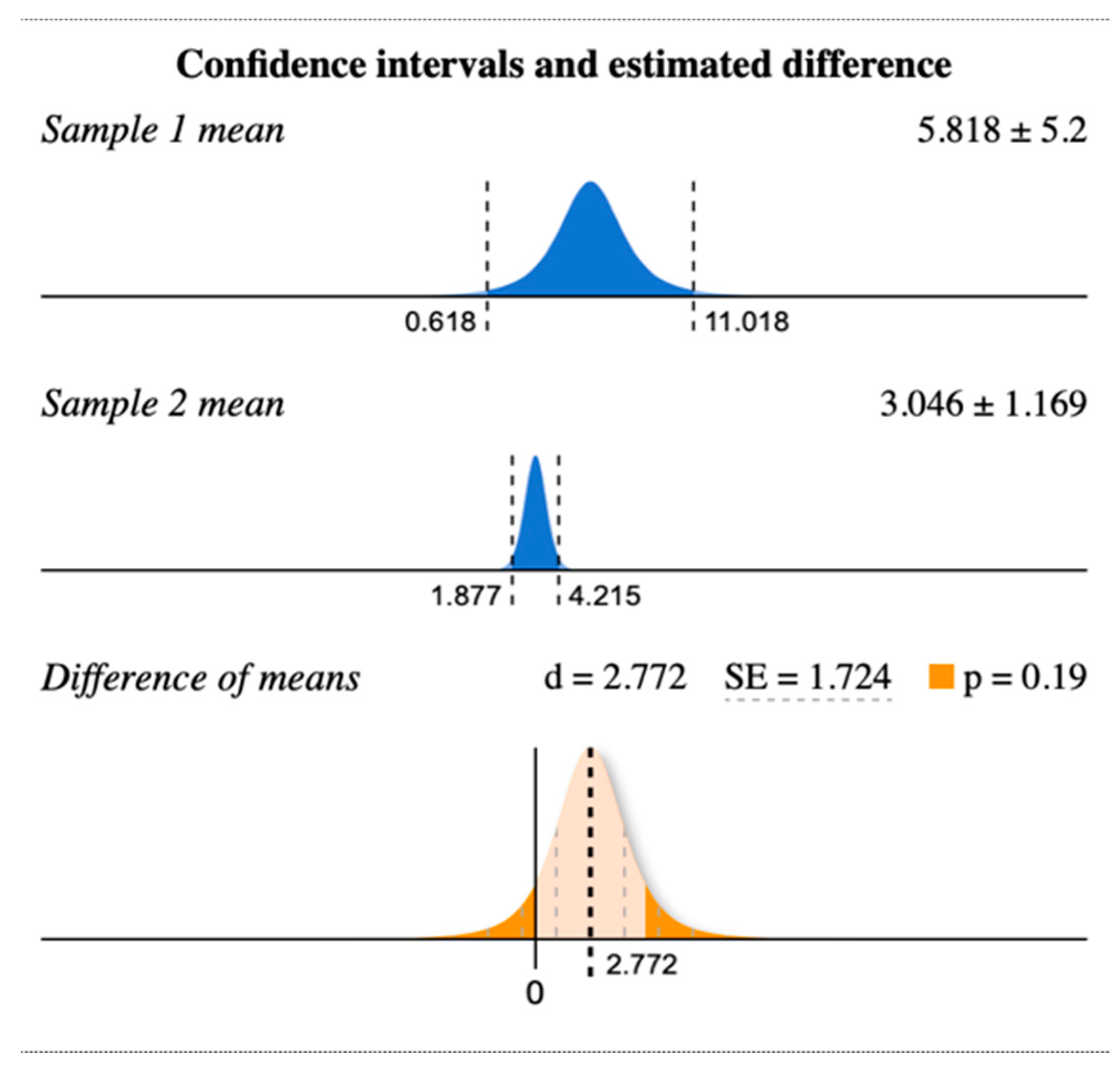
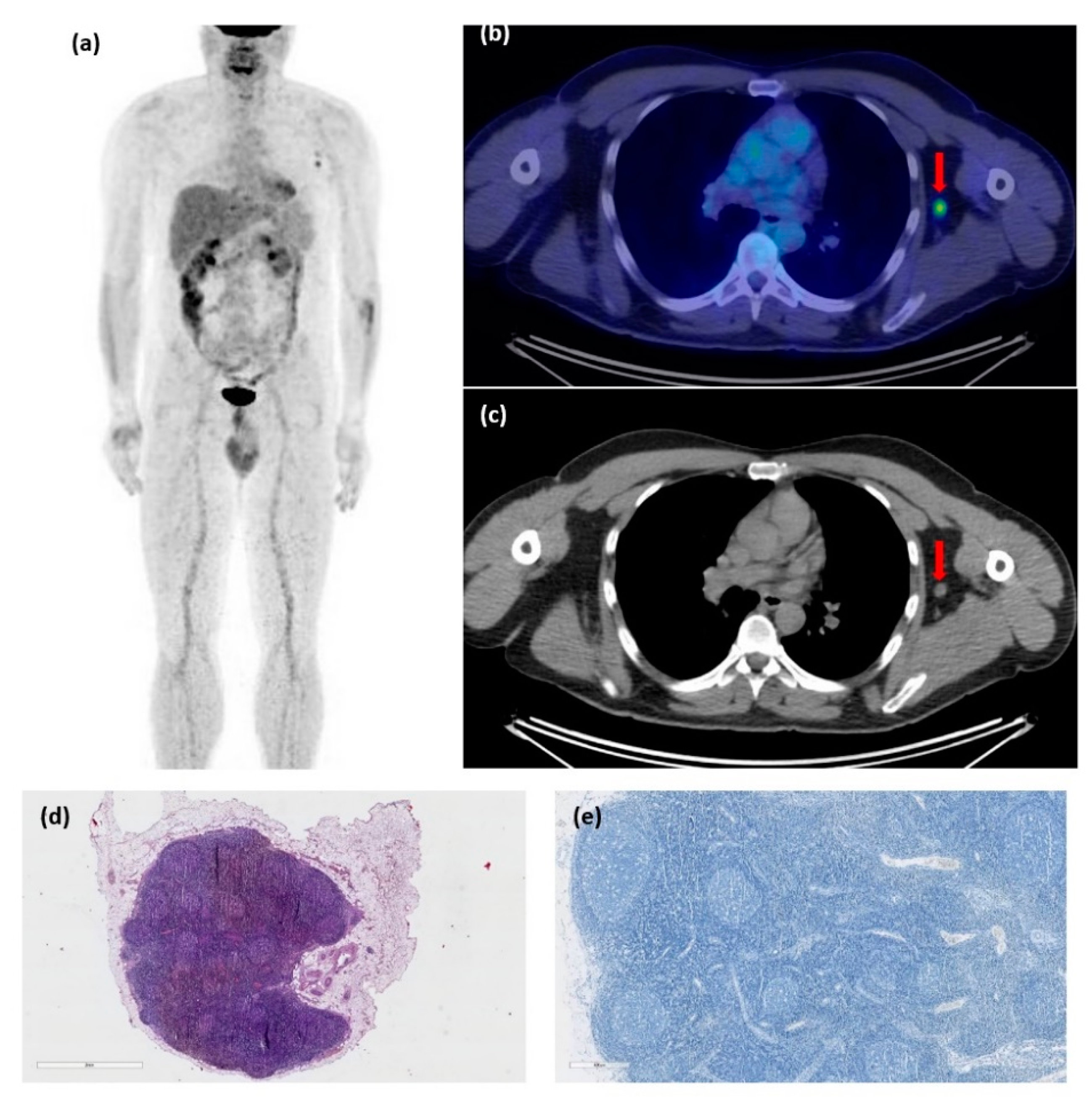
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Centre for Disease Prevention and Control COVID-19 Vaccine Tracker|European Centre for Disease Prevention and Control. Available online: https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#uptake-tab (accessed on 6 July 2022).
- Trifanescu, O.G.; Gales, L.; Bacinschi, X.; Serbanescu, L.; Georgescu, M.; Sandu, A.; Michire, A.; Anghel, R. Impact of the COVID-19 Pandemic on Treatment and Oncologic Outcomes for Cancer Patients in Romania. In Vivo 2022, 36, 934–941. [Google Scholar] [CrossRef]
- European Society for Medical Oncology ESMO 2021: Evidence Suggests That COVID Vaccines Do Protect Patients with Cancer-Ecancer. Available online: http://ecancer.org/en/news/20966-esmo-2021-evidence-suggests-that-covid-vaccines-do-protect-patients-with-cancer (accessed on 6 July 2022).
- Oosting, S.; der Veldt, A.A.M.V.; GeurtsvanKessel, C.H.; Fehrmann, R.S.N.; van Binnendijk, R.S.; Dingemans, A.-M.C.; Smit, E.F.F.; Hiltermann, T.J.N.; Den Hartog, G.; Jalving, M.; et al. LBA8 Vaccination against SARS-CoV-2 in Patients Receiving Chemotherapy, Immunotherapy, or Chemo-Immunotherapy for Solid Tumors. Ann. Oncol. 2021, 32, S1337. [Google Scholar] [CrossRef]
- Shepherd, S.T.C.; Fendler, A.; Au, L.; Byrne, F.; Wilkinson, K.; Wu, M.; Schmitt, A.M.; Joharatnam-Hogan, N.; Shum, B.; Del Rosario, L.; et al. 1557O Adaptive Immunity to SARS-CoV-2 Infection and Vaccination in Cancer Patients: The CAPTURE Study. Ann. Oncol. 2021, 32, S1129. [Google Scholar] [CrossRef]
- Megha, K.; Joseph, X.; Akhil, V.; Mohanan, P. Cascade of immune mechanism and consequences of inflammatory disorders. Phytomedicine 2021, 91, 153712. [Google Scholar] [CrossRef]
- Hervé, C.; Laupèze, B.; del Giudice, G.; Didierlaurent, A.M.; da Silva, F.T. The how’s and what’s of vaccine reactogenicity. npj Vaccines 2019, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhang, J.; Zhang, T. Immunomodulatory activities of polysaccharides from Ganoderma on immune effector cells. Food Chem. 2021, 340, 127933. [Google Scholar] [CrossRef]
- Minamimoto, R.; Kiyomatsu, T. Effects of COVID-19 Vaccination on FDG-PET/CT Imaging: A Literature Review. Glob. Health Med. 2021, 3, 129–133. [Google Scholar] [CrossRef]
- Gambhir, S.S. Molecular Imaging of Cancer with Positron Emission Tomography. Nat. Rev. Cancer 2002, 2, 683–693. [Google Scholar] [CrossRef]
- Indini, A.; Costa, S.; Ierardi, A.M.; Rijavec, E.; Passoni, E.; Grossi, F. COVID-19 Vaccination Mimicking Lymph-Node Progression in a Patient with Melanoma: A Case Report. Melanoma Res. 2021, 31, 490–493. [Google Scholar] [CrossRef]
- El-Sayed, M.S.; Wechie, G.N.; Low, C.S.; Adesanya, O.; Rao, N.; Leung, V.J. The Incidence and Duration of COVID-19 Vaccine-Related Reactive Lymphadenopathy on 18F-FDG PET-CT. Clin. Med. 2021, 21, e633–e638. [Google Scholar] [CrossRef]
- Treglia, G.; Cuzzocrea, M.; Giovanella, L.; Elzi, L.; Muoio, B. Prevalence and Significance of Hypermetabolic Lymph Nodes Detected by 2-[18F]FDG PET/CT after COVID-19 Vaccination: A Systematic Review and a Meta-Analysis. Pharmaceuticals 2021, 14, 762. [Google Scholar] [CrossRef] [PubMed]
- Orevi, M.; Chicheportiche, A.; Ben Haim, S. Lessons Learned from Post-COVID-19 Vaccination PET/CT Studies. J. Nucl. Med. 2022, 63, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.G.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM Procedure Guidelines for Tumour Imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef] [PubMed]
- Sarikaya, I.; Albatineh, A.N.; Sarikaya, A. Revisiting Weight-Normalized SUV and Lean-Body-Mass–Normalized SUV in PET Studies. J. Nucl. Med. Technol. 2020, 48, 163–167. [Google Scholar] [CrossRef]
- Kinahan, P.E.; Fletcher, J.W. Positron Emission Tomography-Computed Tomography Standardized Uptake Values in Clinical Practice and Assessing Response to Therapy. Semin. Ultrasound CT MRI 2010, 31, 496–505. [Google Scholar] [CrossRef]
- Cohen, D.; Krauthammer, S.H.; Wolf, I.; Even-Sapir, E. Hypermetabolic Lymphadenopathy following Administration of BNT162b2 MRNA COVID-19 Vaccine: Incidence Assessed by [18F]FDG PET-CT and Relevance to Study Interpretation. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1854–1863. [Google Scholar] [CrossRef]
- Home Page of Evan Miller. Available online: https://www.evanmiller.org/ (accessed on 6 July 2022).
- Centers for Disease Control and Prevention Moderna COVID-19 Vaccine’s Reactions and Adverse Events|CDC. Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html (accessed on 6 July 2022).
- McIntosh, L.J.; Bankier, A.A.; Vijayaraghavan, G.R.; Licho, R.; Rosen, M.P. COVID-19 Vaccination-Related Uptake on FDG PET/CT: An Emerging Dilemma and Suggestions for Management. AJR Am. J. Roentgenol. 2021, 217, 975–983. [Google Scholar] [CrossRef]
- Shalata, W.; Levin, D.; Fridman, J.; Makarov, V.; Iraqi, M.; Golosky, M.; Rouvinov, K.; Kian, W.; Yakobson, A. Metabolic Activity Assessment by 18F-Fluorodeoxyglucose Positron Emission Tomography in Patients after COVID-19 Vaccination. Curr. Oncol. 2022, 29, 989–1000. [Google Scholar] [CrossRef]
- Quak, E.; Morel, A.; Faudemer, J.; Levy, C.; Ciappuccini, R. Subdiaphragmatic Lymph Nodes Uptake on 18F-FDG PET/CT After COVID-19 Vaccination in the Thigh. Clin. Nucl. Med. 2022, 47, 275–276. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Gierada, D.S.; Joe, B.N. COVID-19 Vaccination-Related Lymphadenopathy: What to Be Aware of. Radiol. Imaging Cancer 2021, 3, e210038. [Google Scholar] [CrossRef]
- Shirone, N.; Shinkai, T.; Yamane, T.; Uto, F.; Yoshimura, H.; Tamai, H.; Imai, T.; Inoue, M.; Kitano, S.; Kichikawa, K.; et al. Axillary Lymph Node Accumulation on FDG-PET/CT after Influenza Vaccination. Ann. Nucl. Med. 2012, 26, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Coates, E.E.; Costner, P.J.; Nason, M.C.; Herrin, D.M.; Conant, S.; Herscovitch, P.; Sarwar, U.N.; Holman, L.; Mitchell, J.; Yamshchikov, G.; et al. Lymph Node Activation by PET/CT Following Vaccination with Licensed Vaccines for Human Papillomaviruses. Clin. Nucl. Med. 2017, 42, 329–334. [Google Scholar] [CrossRef]
- Becker, A.S.; Perez-Johnston, R.; Chikarmane, S.A.; Chen, M.M.; El Homsi, M.; Feigin, K.N.; Gallagher, K.M.; Hanna, E.Y.; Hicks, M.; Ilica, A.T.; et al. Multidisciplinary Recommendations Regarding Post-Vaccine Adenopathy and Radiologic Imaging: Radiology Scientific Expert Panel. Radiology 2021, 300, E323–E327. [Google Scholar] [CrossRef] [PubMed]
- Voinea, S.; Sandru, A.; Gherghe, M. Pitfalls in Cutaneous Melanoma Lymphatic Drainage. Chirurgia 2016, 111, 87–89. [Google Scholar] [PubMed]
- Bordea, C.; Gherghe, M.; Capsa, C.; Noditi, A.; Ianovici, C.; Caragheorghe, G.; Blidaru, A. Sentinel Lymph Node Biopsy after Neoadjuvant Treatment for Breast Cancer. The Validation Protocol. Chirurgia 2021, 116, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Voinea, S.; Sandru, A.; Gherghe, M.; Blidaru, A. Peculiarities of Lymphatic Drainage in Cutaneous Malignant Melanoma: Clinical Experience in 75 Cases. Chirurgia 2014, 109, 26–33. [Google Scholar]
- Gherghe, M.; Bordea, C.; Blidaru, A. Clinical Significance of the Lymphoscintigraphy in the Evaluation of Non-Axillary Sentinel Lymph Node Localization in Breast Cancer. Chirurgia 2015, 110, 26–32. [Google Scholar]
- Grimm, L.; Destounis, S.; Dogan, B.; Nicholson, B.; Dontchos, B.; Sonnenblick, E.; Milch, H.; Pushkin, J.; Benson, J.; Dodelzon, K.; et al. Revised SBI Recommendations for the Management of Axillary Adenopathy in Patients with Recent COVID-19 Vaccination. Available online: https://www.sbi-online.org/Portals/0/Position%20Statements/2022/SBI-recommendations-for-managing-axillary-adenopathy-post-COVID-vaccination_updatedFeb2022.pdf (accessed on 6 July 2022).
- Skawran, S.; Gennari, A.G.; Dittli, M.; Treyer, V.; Muehlematter, U.J.; Maurer, A.; Burger, I.A.; Mader, C.; Messerli, O.; Grünig, H.; et al. [18F]FDG Uptake of Axillary Lymph Nodes after COVID-19 Vaccination in Oncological PET/CT: Frequency, Intensity, and Potential Clinical Impact. Eur. Radiol. 2022, 32, 508–516. [Google Scholar] [CrossRef]
| Characteristic | Number | Percentage |
|---|---|---|
| Gender | ||
| Female | 21 | 84% |
| Male | 4 | 16% |
| Age | ||
| <65 | 21 | 84% |
| >65 | 4 | 16% |
| Vaccine type | ||
| Moderna | 4 | 16% |
| Pfizer | 15 | 60% |
| Unknown | 6 | 24% |
| Diagnosis | ||
| Breast cancer | 6 | 24% |
| Lung cancer | 2 | 8% |
| Colo-rectal cancer | 8 | 32% |
| Melanoma | 3 | 12% |
| CUP | 1 | 4% |
| Head and Neck | 1 | 4% |
| Ovarian cancer | 2 | 8% |
| Sarcoma | 1 | 4% |
| Pancreatic cancer | 1 | 4% |
| Lymphatic Region | Number | Percentage |
|---|---|---|
| Ipsilateral axillar adenopathies | 24 | 96% |
| Ipsilateral cervical adenopathies | 5 | 20% |
| Ipsilateral retropectoral adenopathies | 5 | 20% |
| Pulmonary hilum adenopathies | 2 | 8% |
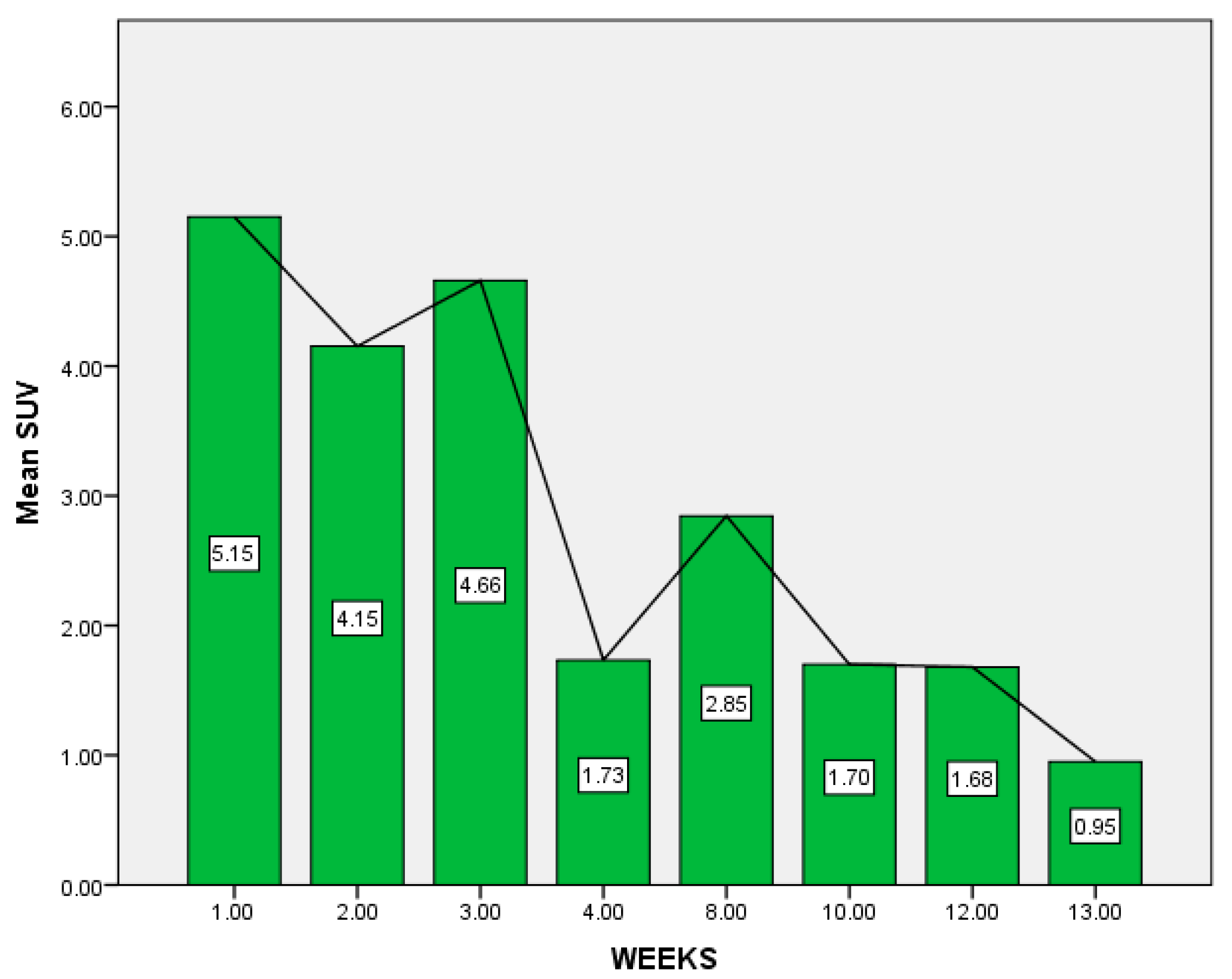
| No. Weeks Between Vaccination and PET-CT | No. Patients | Maximum Value of SUV Max (lbm) |
|---|---|---|
| 1 | 8 | 9.88 |
| 2 | 3 | 4.26 |
| 3 | 3 | 10.57 |
| 4 | 3 | 1.86 |
| 8 | 2 | 3.22 |
| 10 | 1 | 1.7 |
| 12 | 4 | 2.54 |
| 13 | 1 | 0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gales, L.N.; Brotea-Mosoiu, S.; Trifanescu, O.G.; Lazar, A.M.; Gherghe, M. Understanding COVID Vaccination and Its Implication in Cancer Patients’ Imaging of Lymph Nodes by PET-CT. Diagnostics 2022, 12, 2163. https://doi.org/10.3390/diagnostics12092163
Gales LN, Brotea-Mosoiu S, Trifanescu OG, Lazar AM, Gherghe M. Understanding COVID Vaccination and Its Implication in Cancer Patients’ Imaging of Lymph Nodes by PET-CT. Diagnostics. 2022; 12(9):2163. https://doi.org/10.3390/diagnostics12092163
Chicago/Turabian StyleGales, Laurentia Nicoleta, Silvia Brotea-Mosoiu, Oana Gabriela Trifanescu, Alexandra Maria Lazar, and Mirela Gherghe. 2022. "Understanding COVID Vaccination and Its Implication in Cancer Patients’ Imaging of Lymph Nodes by PET-CT" Diagnostics 12, no. 9: 2163. https://doi.org/10.3390/diagnostics12092163
APA StyleGales, L. N., Brotea-Mosoiu, S., Trifanescu, O. G., Lazar, A. M., & Gherghe, M. (2022). Understanding COVID Vaccination and Its Implication in Cancer Patients’ Imaging of Lymph Nodes by PET-CT. Diagnostics, 12(9), 2163. https://doi.org/10.3390/diagnostics12092163







