Predicting Placenta Accreta Spectrum Disorders in a Cohort of Pregnant Patients in the North-East Region of Romania—Diagnostic Accuracy of Ultrasound and Magnetic Resonance Imaging
Abstract
:1. Introduction
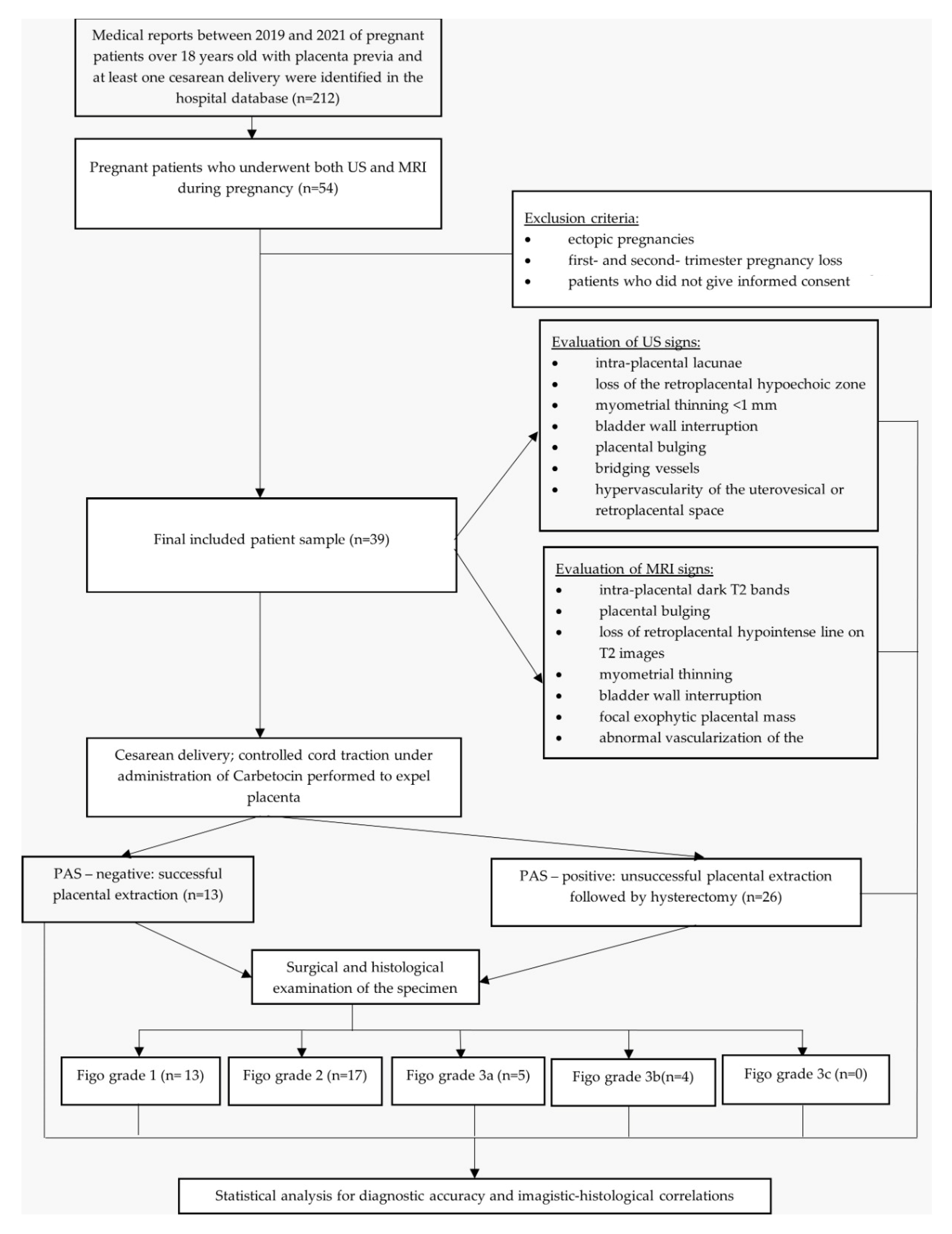
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jauniaux, E.; Collins, S.; Burton, G.J. Placenta accreta spectrum: Pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am. J. Obstet. Gynecol. 2018, 218, 75–87. [Google Scholar] [CrossRef]
- Luke, R.K.; Sharpe, J.W.; Greene, R.R. Placenta accreta: The adherent or invasive placenta. Am. J. Obstet. Gynecol. 1966, 95, 660–668. [Google Scholar] [CrossRef]
- Jauniaux, E.; Ayres-de-Campos, D.; Langhoff-Roos, J.; Fox, K.A.; Collins, S. FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int. J. Gynaecol. Obstet. 2019, 146, 20–24. [Google Scholar] [CrossRef]
- Li, H.T.; Hellerstein, S.; Zhou, Y.B.; Liu, J.M.; Blustein, J. Trends in Cesarean Delivery Rates in China, 2008-2018. JAMA 2020, 323, 89–91. [Google Scholar] [CrossRef]
- Jahnke, J.R.; Houck, K.M.; Bentley, M.E.; Thompson, A.L. Rising rates of cesarean delivery in Ecuador: Socioeconomic and institutional determinants over two decades. Birth 2019, 46, 335–343. [Google Scholar] [CrossRef]
- Plevani, C.; Incerti, M.; Del Sorbo, D.; Pintucci, A.; Vergani, P.; Merlino, L.; Locatelli, A. Cesarean delivery rates and obstetric culture—An Italian register-based study. Acta Obstet. Gynecol. Scand. 2017, 96, 359–365. [Google Scholar] [CrossRef]
- Betrán, A.P.; Ye, J.; Moller, A.B.; Zhang, J.; Gülmezoglu, A.M.; Torloni, M.R. The Increasing Trend in Caesarean Section Rates: Global, Regional and National Estimates: 1990–2014. PLoS ONE 2016, 11, e0148343. [Google Scholar] [CrossRef]
- Wu, S.; Kocherginsky, M.; Hibbard, J.U. Abnormal placentation: Twenty-year analysis. Am. J. Obstet. Gynecol. 2005, 192, 1458–1461. [Google Scholar] [CrossRef]
- Jauniaux, E.; Jurkovic, D. Placenta accreta: Pathogenesis of a 20th century iatrogenic uterine disease. Placenta 2012, 33, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, H.J.; Patterson, J.A.; Nippita, T.A.; Torvaldsen, S.; Ibiebele, I.; Simpson, J.M.; Ford, J.B. Antecedents of Abnormally Invasive Placenta in Primiparous Women: Risk Associated with Gynecologic Procedures. Obstet. Gynecol. 2018, 131, 227–233. [Google Scholar] [CrossRef]
- O’Brien, J.M.; Barton, J.R.; Donaldson, E.S. The management of placenta percreta: Conservative and operative strategies. Am. J. Obstet. Gynecol. 1996, 175, 1632–1638. [Google Scholar] [CrossRef]
- Silver, R.M.; Landon, M.B.; Rouse, D.J.; Leveno, K.J.; Spong, C.Y.; Thom, E.A.; Moawad, A.H.; Caritis, S.; Harper, M.; Wapner, R.J.; et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet. Gynecol. 2006, 107, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Alfirevic, Z.; Bhide, A.G.; Belfort, M.A.; Burton, G.J.; Collins, S.L.; Dornan, S.; Jurkovic, D.; Kayem, G.; Kingdom, J.; et al. Placenta Praevia and Placenta Accreta: Diagnosis and Management: Green-top Guideline No. 27a. Bjog 2019, 126, e1–e48. [Google Scholar] [CrossRef]
- Cahill, A.G.; Beigi, R.; Heine, P.; Silver, R.; Wax, J.R. Obstetric Care Consensus No. 7 Summary: Placenta Accreta Spectrum. Obstet. Gynecol. 2018, 132, 1519–1521. [Google Scholar]
- Jauniaux, E.; Bhide, A.; Kennedy, A.; Woodward, P.; Hubinont, C.; Collins, S. FIGO consensus guidelines on placenta accreta spectrum disorders: Prenatal diagnosis and screening. Int. J. Gynaecol Obstet. 2018, 140, 274–280. [Google Scholar] [CrossRef]
- Hobson, S.R.; Kingdom, J.C.; Murji, A.; Windrim, R.C.; Carvalho, J.C.; Singh, S.S.; Ziegler, C.; Birch, C.; Frecker, E.; Lim, K.; et al. No. 383-Screening, Diagnosis, and Management of Placenta Accreta Spectrum Disorders. J Obstet. Gynaecol. Can. 2019, 41, 1035–1049. [Google Scholar] [CrossRef]
- Calì, G.; Timor-Trisch, I.E.; Palacios-Jaraquemada, J.; Monteaugudo, A.; Forlani, F.; Minneci, G.; Foti, F.; Buca, D.; Familiari, A.; Scambia, G.; et al. Changes in ultrasonography indicators of abnormally invasive placenta during pregnancy. Int. J. Gynaecol. Obstet. 2018, 140, 319–325. [Google Scholar] [CrossRef]
- Comstock, C.H.; Lee, W.; Vettraino, I.M.; Bronsteen, R.A. The early sonographic appearance of placenta accreta. J. Ultrasound Med. 2003, 22, 19–23; quiz 4–6. [Google Scholar] [CrossRef]
- D’Antonio, F.; Timor-Tritsch, I.E.; Palacios-Jaraquemada, J.; Monteagudo, A.; Buca, D.; Forlani, F.; Minneci, G.; Foti, F.; Manzoli, L.; Liberati, M.; et al. First-trimester detection of abnormally invasive placenta in high-risk women: Systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018, 51, 176–183. [Google Scholar] [CrossRef]
- Collins, S.L.; Ashcroft, A.; Braun, T.; Calda, P.; Langhoff-Roos, J.; Morel, O.; Stefanovic, V.; Tutschek, B.; Chantraine, F.; on behalf of the European Working Group on Abnormally Invasive Placenta (EW-AIP). Proposal for standardized ultrasound descriptors of abnormally invasive placenta (AIP). Ultrasound Obstet. Gynecol. 2016, 47, 271–275. [Google Scholar] [CrossRef]
- D’Antonio, F.; Iacovella, C.; Bhide, A. Prenatal identification of invasive placentation using ultrasound: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2013, 42, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Rac, M.W.; Dashe, J.S.; Wells, C.E.; Moschos, E.; McIntire, D.D.; Twickler, D.M. Ultrasound predictors of placental invasion: The Placenta Accreta Index. Am. J. Obstet. Gynecol. 2015, 212, 343.e1–343.e7. [Google Scholar] [CrossRef] [PubMed]
- Gilboa, Y.; Spira, M.; Mazaki-Tovi, S.; Schiff, E.; Sivan, E.; Achiron, R. A novel sonographic scoring system for antenatal risk assessment of obstetric complications in suspected morbidly adherent placenta. J. Ultrasound Med. 2015, 34, 561–567. [Google Scholar] [CrossRef]
- Cali, G.; Forlani, F.; Lees, C.; Timor-Tritsch, I.; Palacios-Jaraquemada, J.; Dall’Asta, A.; Bhide, A.; Flacco, M.E.; Manzoli, L.; Labate, F.; et al. Prenatal ultrasound staging system for placenta accreta spectrum disorders. Ultrasound Obstet. Gynecol. 2019, 53, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Pagani, G.; Cali, G.; Acharya, G.; Trisch, I.-T.; Palacios-Jaraquemada, J.; Familiari, A.; Buca, D.; Manzoli, L.; Flacco, M.E.; Fanfani, F.; et al. Diagnostic accuracy of ultrasound in detecting the severity of abnormally invasive placentation: A systematic review and meta-analysis. Acta Obstet. Gynecol. Scand. 2018, 97, 25–37. [Google Scholar] [CrossRef]
- Palacios-Jaraquemada, J.M.; Bruno, C.H.; Martín, E. MRI in the diagnosis and surgical management of abnormal placentation. Acta Obstet. Gynecol. Scand. 2013, 92, 392–397. [Google Scholar] [CrossRef]
- Morlando, M.; Collins, S. Placenta Accreta Spectrum Disorders: Challenges, Risks, and Management Strategies. Int. J. Womens Health 2020, 12, 1033–1045. [Google Scholar] [CrossRef]
- Karkhanis, P.; Ahmed, I.; Irani, S. Placenta accreta spectrum disorders—Detection rate and maternal outcomes following implementation of an institutional protocol. J. Obstet. Gynaecol. 2022, 42, 202–208. [Google Scholar] [CrossRef]
- Dwyer, B.K.; Belogolovkin, V.; Tran, L.; Rao, A.; Carroll, I.; Barth, R.; Chitkara, U. Prenatal diagnosis of placenta accreta: Sonography or magnetic resonance imaging? J. Ultrasound Med. 2008, 27, 1275–1281. [Google Scholar] [CrossRef]
- Silver, R.M.; Branch, D.W. Placenta accreta spectrum. New Engl. J. Med. 2018, 378, 1529–1536. [Google Scholar] [CrossRef]
- Pilloni, E.; Alemanno, M.G.; Gaglioti, P.; Sciarrone, A.; Garofalo, A.; Biolcati, M.; Botta, G.; Viora, E.; Todros, T. Accuracy of ultrasound in antenatal diagnosis of placental attachment disorders. Ultrasound Obstet. Gynecol. 2016, 47, 302–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jauniaux, E.; Collins, S.L.; Jurkovic, D.; Burton, G.J. Accreta placentation: A systematic review of prenatal ultrasound imaging and grading of villous invasiveness. Am. J. Obstet. Gynecol. 2016, 215, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.; Anand, R.; Aggarwal, K.; Agarwal, S.; Tomer, S. Ultrasound as a Sole Modality for Prenatal Diagnosis of Placenta Accreta Spectrum: Potentialities and Pitfalls. Indian J. Radiol. Imaging 2021, 31, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Thiravit, S.; Ma, K.; Goldman, I.; Chanprapaph, P.; Jha, P.; Hippe, D.S.; Dighe, M. Role of Ultrasound and MRI in Diagnosis of Severe Placenta Accreta Spectrum Disorder: An Intraindividual Assessment with Emphasis on Placental Bulge. Am. J. Roentgenol. 2021, 217, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, H.; Miyamoto, M.; Shinmoto, H.; Soga, S.; Matsuura, H.; Kakimoto, S.; Iwahashi, H.; Sakamoto, T.; Hada, T.; Suzuki, R.; et al. The use of magnetic resonance imaging to predict placenta previa with placenta accreta spectrum. Acta Obstet. Gynecol. Scand. 2020, 99, 1657–1665. [Google Scholar] [CrossRef]
- Jha, P.; Rabban, J.; Chen, L.M.; Goldstein, R.B.; Weinstein, S.; Morgan, T.A.; Shum, D.; Hills, N.; Ohliger, M.A.; Poder, L. Placenta accreta spectrum: Value of placental bulge as a sign of myometrial invasion on MR imaging. Abdom. Radiol. 2019, 44, 2572–2581. [Google Scholar] [CrossRef]
- Tinari, S.; Buca, D.; Cali, G.; Timor-Tritsch, I.; Palacios-Jaraquemada, J.; Rizzo, G.; Lucidi, A.; Di Mascio, D.; Liberati, M.; D’Antonio, F. Risk factors, histopathology and diagnostic accuracy in posterior placenta accreta spectrum disorders: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2021, 57, 903–909. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, X.; Cai, J.; Han, F.; Xu, G.; Zhang, X.; Zhang, T.; Yu, L. Prediction of placenta accreta spectrum by a scoring system based on maternal characteristics combined with ultrasonographic features. Taiwan. J. Obstet. Gynecol. 2021, 60, 1011–1017. [Google Scholar] [CrossRef]
- Romeo, V.; Verde, F.; Sarno, L.; Migliorini, S.; Petretta, M.; Mainenti, P.P.; D’Armiento, M.; Guida, M.; Brunetti, A.; Maurea, S. Prediction of placenta accreta spectrum in patients with placenta previa using clinical risk factors, ultrasound and magnetic resonance imaging findings. Radiol. Med. 2021, 126, 1216–1225. [Google Scholar] [CrossRef]
- Morel, O.; van Beekhuizen, H.J.; Braun, T.; Collins, S.; Pateisky, P.; Calda, P.; Henrich, W.; Al Naimi, A.; Norgaardt, L.N.; Chalubinski, K.M.; et al. Performance of antenatal imaging to predict placenta accreta spectrum degree of severity. Acta Obstet. Gynecol. Scand. 2021, 100, 21–28. [Google Scholar] [CrossRef]
- Delli Pizzi, A.; Tavoletta, A.; Narciso, R.; Mastrodicasa, D.; Trebeschi, S.; Celentano, C.; Mastracchio, J.; Cianci, R.; Seccia, B.; Marrone, L.; et al. Prenatal planning of placenta previa: Diagnostic accuracy of a novel MRI-based prediction model for placenta accreta spectrum (PAS) and clinical outcome. Abdom. Radiol. 2019, 44, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xu, X.Q.; Shi, H.B.; Yang, Z.Q.; Zhou, X.; Pan, Y. Conventional MRI features for predicting the clinical outcome of patients with invasive placenta. Diagn. Interv. Radiol. 2017, 23, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Patient’s Characteristics | Pas Positive (n = 26) | Pas Negative (n = 13) | p Value |
|---|---|---|---|
| Age (years, mean ± SD) | 32 ± 3.7 | 31.08 ± 4.17 | 0.49 |
| BMI (kg/m2, mean ± SD) | 30.26 ± 3.49 | 28.13 ± 1.59 | 0.044 |
| Current smoker (%) | 13 (50%) | 4 (30.76%) | 0.25 |
| Number of gestations (mean ± SD) | 3.38 ± 2.17 | 2.54 ± 1.26 | 0.20 |
| Parity (mean ± SD) | 2.96 ± 2.14 | 2.23 ± 0.72 | 0.24 |
| Previous CS (mean ± SD) | 2.3 ± 1.54 | 1.31 ± 0.63 | 0.032 |
| Gestational age at delivery (weeks, mean ± SD) | 35.77 ± 2.1 | 36.15 ± 1.21 | 0.55 |
| Birth weight (grams, mean ± SD) | 2804.6 ± 482.4 | 2963 ± 307.3 | 0.219 |
| APGAR score (mean ± SD) | 6.9 ± 1.7 | 6.7 ± 1.1 | 0.868 |
| Hospitalization (days, mean ± SD) | 4.38 ± 1.75 | 7.77 ± 2.55 | <0.01 |
| Intraoperative Outcome | Characteristics | |
|---|---|---|
| Yes (No, %) | No (No, %) | |
| Total hysterectomy | 20 (76.9%) | 6 (23.1%) |
| Subtotal hysterectomy | 6 (23.1%) | 20 (76.9%) |
| Intraoperative urinary bladder injury | 14 (53.8%) | 12 (46.2%) |
| Intraoperative ureteral injury | 7 (26.9%) | 19 (73.1%) |
| Hypogastric artery ligation | 10 (38.5%) | 16 (61.5%) |
| Necessity of blood transfusion | 7 (26.9%) | 19 (73.1%) |
| Ultrasound Signs | Pas Positive (n = 26) | Pas Negative (n = 13) | Se | Sp | Accuracy | PPV | NPV | p Value |
|---|---|---|---|---|---|---|---|---|
| Placental lacunar spaces | 24 | 3 | 92.3% (24/26) | 76.9% (7/13) | 0.87 | 88.8% (24/27) | 83.3% (10/12) | <0.001 |
| Loss of retroplacental clear zone | 24 | 7 | 92.3% (24/26) | 46.1% (7/13) | 0.76 | 77.4% (24/31) | 75% (6/8) | 0.005 |
| Bladder wall interruption | 14 | 0 | 53.8% (14/26) | 100% (13/13) | 0.69 | 100% (14/14) | 52% (13/25) | 0.001 |
| Myometrial thinning < 1 mm | 24 | 3 | 92.3% (24/26) | 76.9% (10/13) | 0.87 | 88.8% (24/27) | 83.3% (10/12) | <0.001 |
| Placental bulging | 8 | 0 | 30.7% (8/26) | 100% (13/13) | 0.53 | 100% (8/8) | 41.9% (13/31) | 0.025 |
| Bridging vessels | 21 | 1 | 80.7% (21/26) | 92.3% (12/13) | 0.84 | 95.4% (21/22) | 70.5% (12/17) | <0.001 |
| Hypervascularity of the uterovesical or retroplacental space | 11 | 1 | 42.3% (11/26) | 92.3% (12/13) | 0.58 | 91.6% (11/12) | 44.4% (12/27) | 0.027 |
| ≥3 US signs present | 22 | 1 | 84.6% (22/26) | 92.3% (12/13) | 0.87 | 95.6% (22/23) | 75% (12/16) | <0.001 |
| Ultrasound Signs | Grade I | p Value | Grade II | p Value | Grade IIIa | p Value | Grade IIIb | p Value |
|---|---|---|---|---|---|---|---|---|
| Placental lacunar spaces (OR+ CI 95%) | 0.025 (0.004–0.173) | <0.001 | 6.250 (1.145–34.123) | 0.024 | 0.647 (0.505–0.829) | 0.110 | 0.657 (0.517–0.835) | 0.159 |
| Loss of retroplacental clear zone (OR+ CI 95%) | 0.097 (0.16–0.593) | 0.005 | 7.467 (0.819–68.1) | 0.047 | 1.037 (0.1–10.806) | 0.976 | 0.771 (0.644–0.924) | 0.284 |
| Myometrial thinning < 1 mm (OR+ CI 95%) | 0.58 (0.11–0.312) | <0.001 | 6.250 (1.145–34.123) | 0.024 | 1.913 (0.191–19.198) | 0.576 | 0.657 (0.517–0.835) | 0.159 |
| Bladder wall interruption (OR+ CI 95%) | 0.083 (0.009–0.737) | 0.009 | 0.602 (0.157–2.31) | 0.458 | 9.6 (0.951–96.922) | 0.028 | 0.286 (0.169–0.482) | 0.005 |
| Bridging vessels (OR+ CI 95%) | 0.20 (0.002–0.190) | <0.001 | 2.88 (0.755–10.987) | 0.117 | 0.5 (0.357–0.7) | 0.035 | 0.514 (0.373–0.71) | 0.063 |
| Placental bulging (OR+ CI 95%) | 0.692 (0.536–0.895) | 0.025 | 0.356 (0.062–2.043) | 0.234 | 3.111 (0.423–22.866) | 0.248 | 0.114 (0.45–0.287) | < 0.001 |
| Hypervascularity of the uterovesical or retroplacental space (OR+ CI 95%) | 0.114 (0.013–1.009) | 0.027 | 0.310 (0.068–1.4) | 0.119 | 13 (1.265–133.635) | 0.011 | 0.229 (0.124–0.42) | 0.002 |
| MRI Signs | Pas Positive (n = 26) | Pas Negative (n = 13) | Sp | Sp | Accuracy | PPV | NPV | p Value |
|---|---|---|---|---|---|---|---|---|
| Intraplacental dark T2 bands | 24 | 8 | 92.3% (24/26) | 38.4% (5/13) | 0.74 | 75.% (24/32) | 71.4% (5/7) | 0.018 |
| Placental bulging | 8 | 0 | 30.7% (8/26) | 100% (13/13) | 0.53 | 100% (8/8) | 41.9% (13/31) | 0.025 |
| Loss of retroplacental hypointense line on T2 images | 25 | 5 | 96.1% (25/26) | 61.5% (8/13) | 0.84 | 83.3% (25/30) | 88.8% (8/9) | <0.001 |
| Myometrial thinning < 1 mm | 26 | 12 | 100% (26/26) | 7.69% (1/13) | 0.69 | 68.4% (26/38) | 100% (1/1) | 0.152 |
| Bladder wall interruption | 11 | 0 | 42.3% (11/26) | 100% (13/13) | 0.61 | 100% (11/11) | 46.4% (13/28) | 0.006 |
| Focal exophytic placental mass | 10 | 0 | 38.4% (10/26) | 100% (13/13) | 0.58 | 100% (10/10) | 44.8% (13/29) | 0.010 |
| Abnormal vascularization of the placental bed | 17 | 2 | 65.3% (17/26) | 84.6% (11/13) | 0.71 | 89.4% (17/19) | 63.6% (7/11) | 0.003 |
| ≥3 MRI signs present | 24 | 5 | 92.3% (24/26) | 61.5% (8/13) | 0.82 | 82.7% (24/29) | 80% (8/10) | <0.001 |
| MRI Signs | Grade I | p Value | Grade II | p Value | Grade IIIa | p Value | Grade IIIb | p Value |
|---|---|---|---|---|---|---|---|---|
| Placental bulging (OR+ CI 95%) | 0.692 (0.536–0.895) | 0.025 | 0.134 (0.15–1.221) | 0.47 | 8.7 (1.148–65.934) | 0.019 | 0.114 (0.045–0.287) | <0.001 |
| Intraplacental dark T2 bands (OR+ CI 95%) | 2.16 (1.2–3.89) | <0.001 | 0.682 (0.513–0.907) | 0.010 | 0.794 (0.669–0.942) | 0.263 | 0.8 (0.678–0.944) | 0.323 |
| Loss of retroplacental hypointense line on T2 images (OR+ CI 95%) | 0.25 (0.003–0.247) | <0.001 | 9.143 (1.014–82.442) | 0.025 | 0.735 (0.601–0.9) | 0.190 | 0.743 (0.611–0.903) | 0.248 |
| Bladder wall interruption (OR+ CI 95%) | 0.133 (0.15–1.189) | 0.044 | 0.375 (0.82–1.714) | 0.198 | 15.429 (1.481–160.76) | 0.006 | 10.125 (0.922–111.247) | 0.028 |
| Focal exophytic placental mass (OR+ CI 95%) | 0.615 (0.454–0.834) | 0.010 | 0.821 (0.190–3.539) | 0.791 | 18.667 (1.759–198.103) | 0.003 | 3.375 (0.408–27.916) | 0.239 |
| Abnormal vascularization of the placental bed (OR+ CI 95%) | 0.188 (0.041–0.851) | 0.023 | 3.208 (0.857–12.018) | 0.079 | 0.667 (0.099–4.508) | 0.676 | 3.563 (0.337–37.687) | 0.267 |
| Sign | Relative Incidence | True Predictive Value | |||
|---|---|---|---|---|---|
| PAS Positive | PAS Negative | Total | |||
| Ultrasonography signs | Placental lacunar spaces | 92.3% (24/26) | 23% (3/13) | 69.2% (27/39) | 88.8% (24/27) |
| Loss of retroplacental clear zone | 92.3% (24/26) | 53.8% (7/13) | 79.4% (31/39) | 77.4% (24/31) | |
| Myometrial thinning < 1 mm | 92.3% (24/26) | 23% (3/13) | 69.2% (27/39) | 88.8% (24/27) | |
| Bladder wall interruption | 53.8% (14/26) | 0% (0/13) | 35.9% (14/39) | 100% (14/14) | |
| Bridging vessels | 80.7% (21/26) | 7.7% (1/13) | 56.4% (22/39) | 95.4% (21/22) | |
| Placental bulging | 30.7% (8/26) | 0% (0/13) | 20.5% (8/39) | 100% (8/8) | |
| Hypervascularity of the uterovesical or retroplacental space | 42.3% (11/26) | 7.7% (1/13) | 30.7% (12/39) | 91.6% (11/12) | |
| MRI signs | Intraplacental dark T2 bands | 92.3% (24/26) | 61.5% (8/13) | 82% (32/39) | 75% (24/32) |
| Placental bulging | 30.7 % (8/26) | 0% (0/13) | 20.5% (8/39) | 100% (8/8) | |
| Loss of retroplacental hypointense line on T2 images | 96.1% (25/26) | 38.5% (5/13) | 77% (30/39) | 83.3% (25/30) | |
| Myometrial thinning < 1 mm | 100% (26/26) | 92.3% (12/13) | 97.4% (38/39) | 68.4% (26/38) | |
| Bladder wall interruption | 42.3% (11/26) | 0% (0/13) | 28.2% (11/39) | 100% (11/11) | |
| Focal exophytic placental mass | 38.4% (10/26) | 0% (0/13) | 25.6% (10/39) | 100% (10/10) | |
| Abnormal vascularization of the placental bed | 65.3% (17/26) | 15.4% (2/13) | 48.7% (19/39) | 89.4% (17/19) | |
| Surgical Outcomes | ≥3 US Signs Present | ≥3 MRI Signs Present | ||
|---|---|---|---|---|
| r | p | r | p | |
| Total hysterectomy | 0.54 | <0.001 | 0.60 | <0.001 |
| Subtotal hysterectomy | 0.21 | 0.19 | −0.07 | 0.64 |
| Intraoperative urinary bladder injury | 0.29 | 0.06 | 0.31 | 0.04 |
| Intraoperative ureteral injury | 0.25 | 0.11 | 0.12 | 0.46 |
| Hypogastric artery ligation | 0.25 | 0.12 | 0.34 | 0.03 |
| Necessity of blood transfusion | 0.11 | 0.47 | 0.27 | 0.091 |
| Days of hospitalization | 0.33 | 0.03 | 0.4 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haba, R.M.; Pristavu, A.I.; Cobzeanu, M.-L.; Carauleanu, A.; Sadiye Scripcariu, I.; Vasilache, I.A.; Minciuna, D.A.; Negru, D.; Socolov, D.G. Predicting Placenta Accreta Spectrum Disorders in a Cohort of Pregnant Patients in the North-East Region of Romania—Diagnostic Accuracy of Ultrasound and Magnetic Resonance Imaging. Diagnostics 2022, 12, 2130. https://doi.org/10.3390/diagnostics12092130
Haba RM, Pristavu AI, Cobzeanu M-L, Carauleanu A, Sadiye Scripcariu I, Vasilache IA, Minciuna DA, Negru D, Socolov DG. Predicting Placenta Accreta Spectrum Disorders in a Cohort of Pregnant Patients in the North-East Region of Romania—Diagnostic Accuracy of Ultrasound and Magnetic Resonance Imaging. Diagnostics. 2022; 12(9):2130. https://doi.org/10.3390/diagnostics12092130
Chicago/Turabian StyleHaba, Raluca Maria, Anda Ioana Pristavu, Maria-Luiza Cobzeanu, Alexandru Carauleanu, Ioana Sadiye Scripcariu, Ingrid Andrada Vasilache, Dorina Adelina Minciuna, Dragos Negru, and Demetra Gabriela Socolov. 2022. "Predicting Placenta Accreta Spectrum Disorders in a Cohort of Pregnant Patients in the North-East Region of Romania—Diagnostic Accuracy of Ultrasound and Magnetic Resonance Imaging" Diagnostics 12, no. 9: 2130. https://doi.org/10.3390/diagnostics12092130
APA StyleHaba, R. M., Pristavu, A. I., Cobzeanu, M.-L., Carauleanu, A., Sadiye Scripcariu, I., Vasilache, I. A., Minciuna, D. A., Negru, D., & Socolov, D. G. (2022). Predicting Placenta Accreta Spectrum Disorders in a Cohort of Pregnant Patients in the North-East Region of Romania—Diagnostic Accuracy of Ultrasound and Magnetic Resonance Imaging. Diagnostics, 12(9), 2130. https://doi.org/10.3390/diagnostics12092130







