Analysis of A Disintegrin and Metalloprotease 17 (ADAM17) Expression as a Prognostic Marker in Ovarian Cancer Patients Undergoing First-Line Treatment Plus Bevacizumab
Abstract
:1. Introduction
2. Materials and Methods
2.1. Immunohistochemistry
2.2. Evaluation of IHC Staining
2.3. Statistical Analysis
3. Results
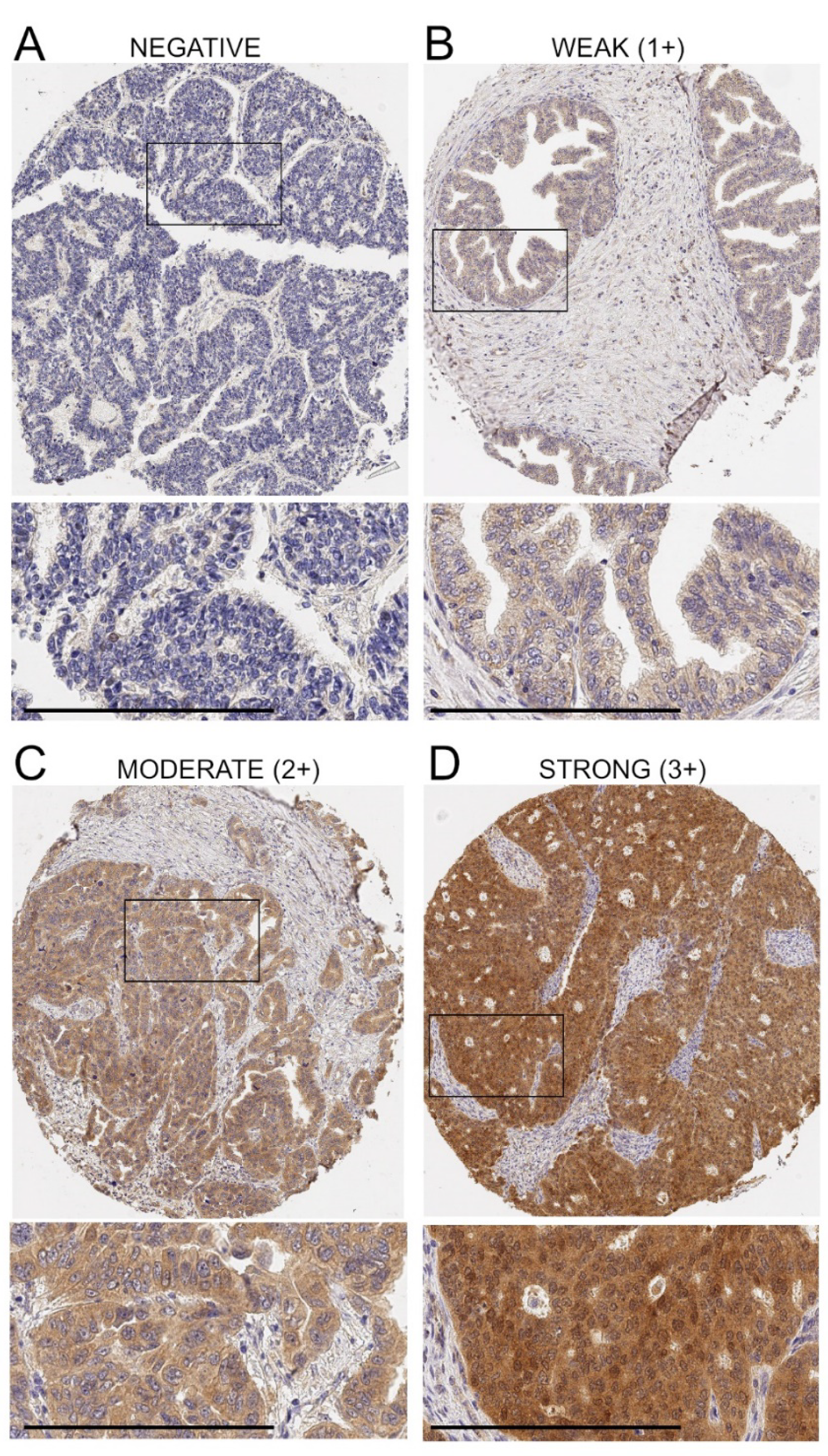
3.1. Expression of ADAM17 Protein in Ovarian Cancer Tissue Microarrays (TMAs)
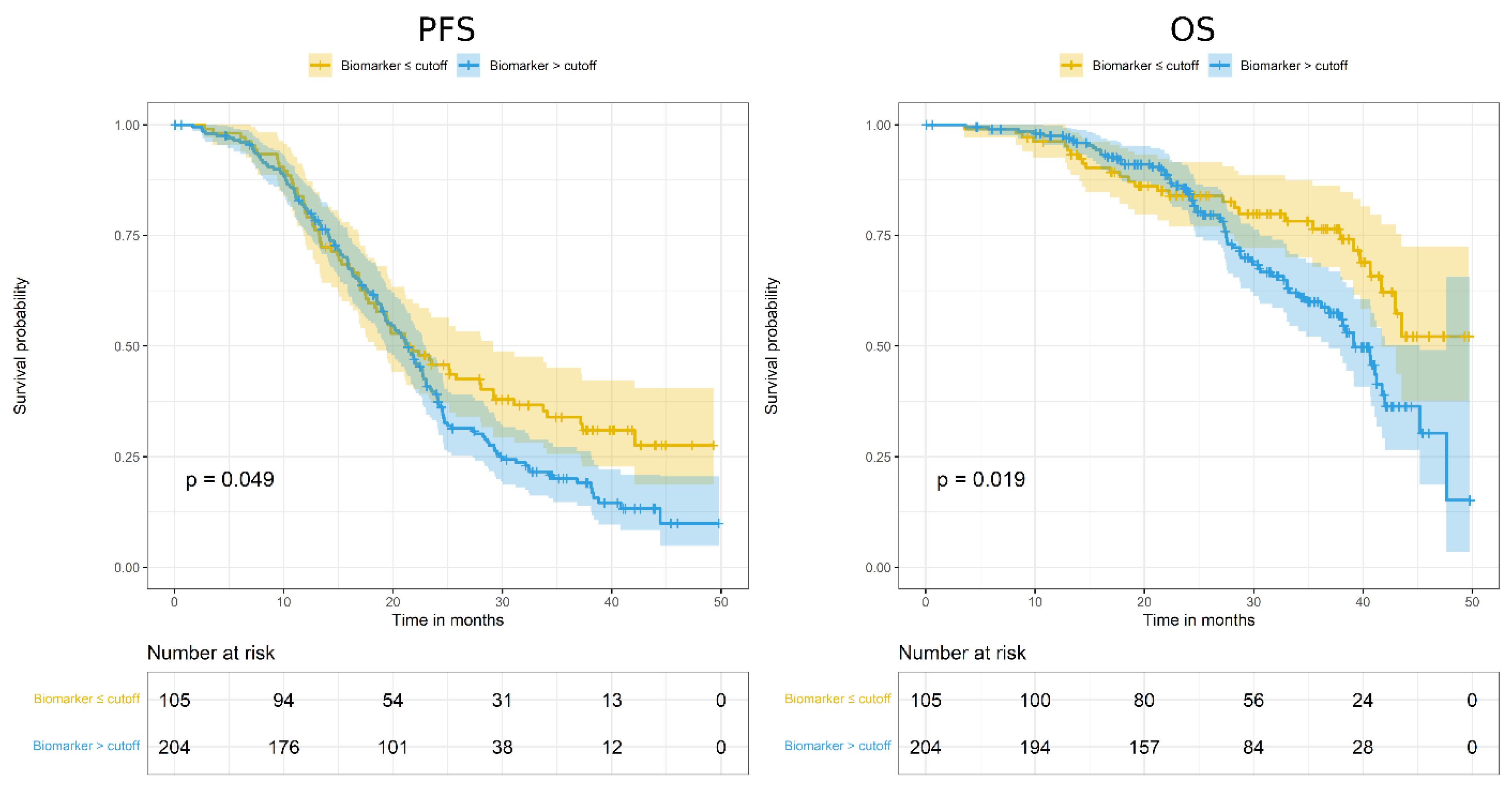
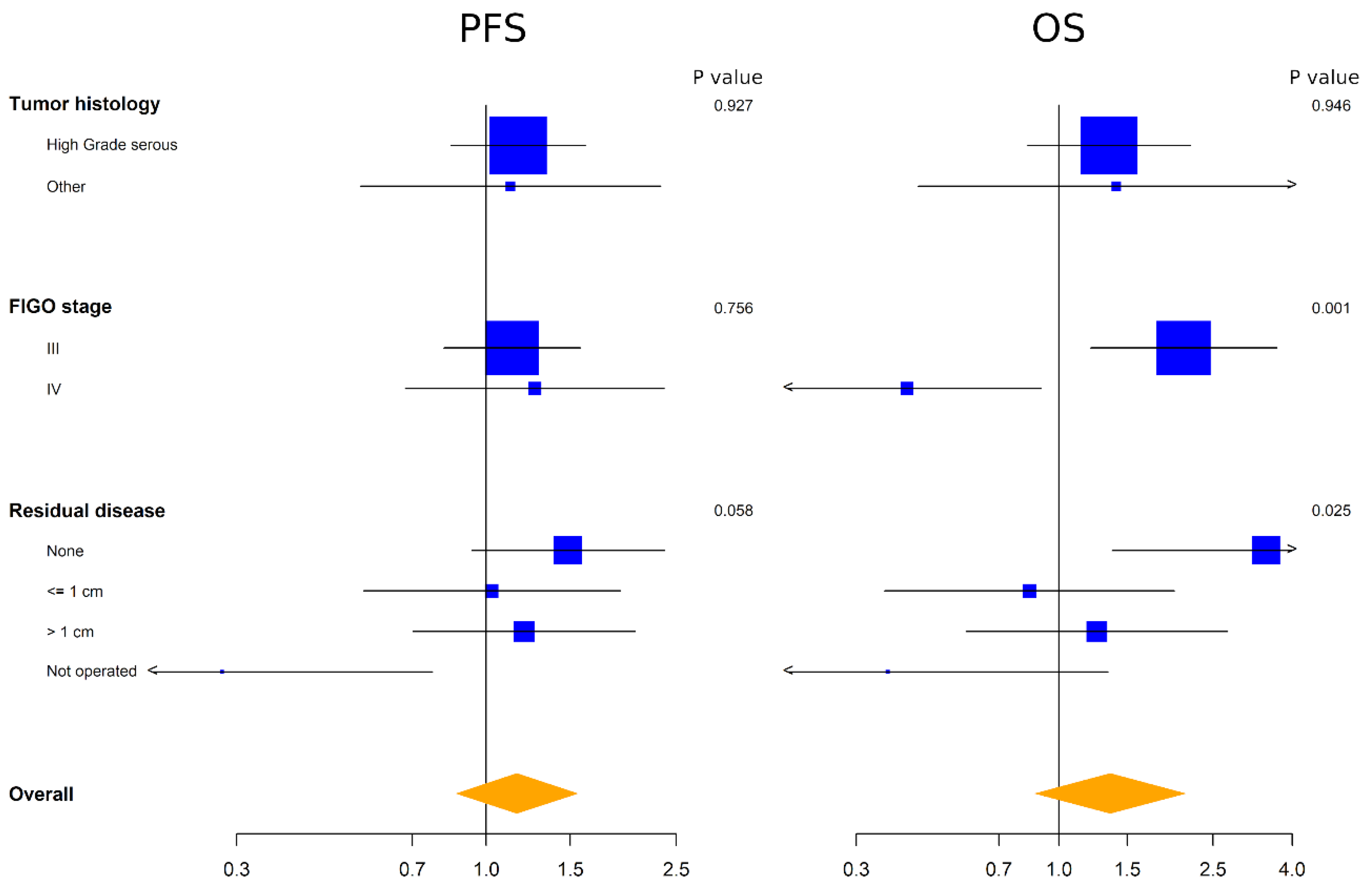
3.2. Evaluation of ADAM17 Expression as Prognostic Marker
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO Consensus Conference Recommendations on Ovarian Cancer: Pathology and Molecular Biology, Early and Advanced Stages, Borderline Tumours and Recurrent Disease. Int. J. Gynecol. Cancer 2019, 29, 728–760. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer Incidence and Mortality Patterns in Europe: Estimates for 40 Countries and 25 Major Cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of Bevacizumab in the Primary Treatment of Ovarian Cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Perren, T.J.; Swart, A.M.; Pfisterer, J.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C.; et al. A Phase 3 Trial of Bevacizumab in Ovarian Cancer. N. Engl. J. Med. 2011, 365, 2484–2496. [Google Scholar] [CrossRef] [PubMed]
- Oza, A.M.; Cook, A.D.; Pfisterer, J.; Embleton, A.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; et al. Standard Chemotherapy with or without Bevacizumab for Women with Newly Diagnosed Ovarian Cancer (ICON7): Overall Survival Results of a Phase 3 Randomised Trial. Lancet Oncol. 2015, 16, 928–936. [Google Scholar] [CrossRef]
- Tewari, K.S.; Burger, R.A.; Enserro, D.; Norquist, B.M.; Swisher, E.M.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Huang, H.; Homesley, H.D.; et al. Final Overall Survival of a Randomized Trial of Bevacizumab for Primary Treatment of Ovarian Cancer. J. Clin. Oncol. 2019, 37, 2317–2328. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef]
- Hennessy, B.T.J.; Timms, K.M.; Carey, M.S.; Gutin, A.; Meyer, L.A.; Flake, D.D.; Abkevich, V.; Potter, J.; Pruss, D.; Glenn, P.; et al. Somatic Mutations in BRCA1 and BRCA2 Could Expand the Number of Patients That Benefit from Poly (ADP Ribose) Polymerase Inhibitors in Ovarian Cancer. J. Clin. Oncol. 2010, 28, 3570–3576. [Google Scholar] [CrossRef]
- Gori, S.; Barberis, M.; Bella, M.A.; Buttitta, F.; Capoluongo, E.; Carrera, P.; Colombo, N.; Cortesi, L.; Genuardi, M.; Gion, M.; et al. Recommendations for the Implementation of BRCA Testing in Ovarian Cancer Patients and Their Relatives. Crit. Rev. Oncol. Hematol. 2019, 140, 67–72. [Google Scholar] [CrossRef]
- Longo, D.L. Personalized Medicine for Primary Treatment of Serous Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2471–2474. [Google Scholar] [CrossRef]
- Daniele, G.; Raspagliesi, F.; Scambia, G.; Pisano, C.; Colombo, N.; Frezzini, S.; Tognon, G.; Artioli, G.; Gadducci, A.; Lauria, R.; et al. Bevacizumab, Carboplatin, and Paclitaxel in the First Line Treatment of Advanced Ovarian Cancer Patients: The Phase IV MITO-16A/MaNGO-OV2A Study. Int. J. Gynecol. Cancer 2021, 31, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Kenny, P.A. TACE: A New Target in Epidermal Growth Factor Receptor Dependent Tumors. Differentiation 2007, 75, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Arribas, J.; Esselens, C. ADAM17 as a Therapeutic Target in Multiple Diseases. Curr. Pharm. Des. 2009, 15, 2319–2335. [Google Scholar] [CrossRef] [PubMed]
- Gooz, M. ADAM-17: The Enzyme That Does It All. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 146–169. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Chalaris, A.; Garbers, C.; Rose-John, S. ADAM17: A Molecular Switch to Control Inflammation and Tissue Regeneration. Trends Immunol. 2011, 32, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Rossello, A.; Nuti, E.; Ferrini, S.; Fabbi, M. Targeting ADAM17 Sheddase Activity in Cancer. Curr. Drug Targets 2016, 17, 1908–1927. [Google Scholar] [CrossRef] [PubMed]
- Düsterhöft, S.; Lokau, J.; Garbers, C. The Metalloprotease ADAM17 in Inflammation and Cancer. Pathol. Res. Pract. 2019, 215, 152410. [Google Scholar] [CrossRef]
- Prenzel, N.; Zwick, E.; Daub, H.; Leserer, M.; Abraham, R.; Wallasch, C.; Ullrich, A. EGF Receptor Transactivation by G-Protein-Coupled Receptors Requires Metalloproteinase Cleavage of ProHB-EGF. Nature 1999, 402, 884–888. [Google Scholar] [CrossRef]
- Borrell-Pagès, M.; Rojo, F.; Albanell, J.; Baselga, J.; Arribas, J. TACE Is Required for the Activation of the EGFR by TGF-Alpha in Tumors. EMBO J. 2003, 22, 1114–1124. [Google Scholar] [CrossRef]
- Sahin, U.; Weskamp, G.; Kelly, K.; Zhou, H.-M.; Higashiyama, S.; Peschon, J.; Hartmann, D.; Saftig, P.; Blobel, C.P. Distinct Roles for ADAM10 and ADAM17 in Ectodomain Shedding of Six EGFR Ligands. J. Cell Biol. 2004, 164, 769–779. [Google Scholar] [CrossRef] [Green Version]
- Blobel, C.P. ADAMs: Key Components in EGFR Signalling and Development. Nat. Rev. Mol. Cell Biol. 2005, 6, 32–43. [Google Scholar] [CrossRef]
- Arteaga, C.L.; Hanauske, A.R.; Clark, G.M.; Osborne, C.K.; Hazarika, P.; Pardue, R.L.; Tio, F.; Von Hoff, D.D. Immunoreactive Alpha Transforming Growth Factor Activity in Effusions from Cancer Patients as a Marker of Tumor Burden and Patient Prognosis. Cancer Res. 1988, 48, 5023–5028. [Google Scholar]
- Carvalho, S.; Lindzen, M.; Lauriola, M.; Shirazi, N.; Sinha, S.; Abdul-Hai, A.; Levanon, K.; Korach, J.; Barshack, I.; Cohen, Y.; et al. An Antibody to Amphiregulin, an Abundant Growth Factor in Patients’ Fluids, Inhibits Ovarian Tumors. Oncogene 2016, 35, 438–447. [Google Scholar] [CrossRef]
- Richards, F.M.; Tape, C.J.; Jodrell, D.I.; Murphy, G. Anti-Tumour Effects of a Specific Anti-ADAM17 Antibody in an Ovarian Cancer Model in Vivo. PLoS ONE 2012, 7, e40597. [Google Scholar] [CrossRef]
- Hedemann, N.; Rogmans, C.; Sebens, S.; Wesch, D.; Reichert, M.; Schmidt-Arras, D.; Oberg, H.-H.; Pecks, U.; van Mackelenbergh, M.; Weimer, J.; et al. ADAM17 Inhibition Enhances Platinum Efficiency in Ovarian Cancer. Oncotarget 2018, 9, 16043–16058. [Google Scholar] [CrossRef]
- Rosso, O.; Piazza, T.; Bongarzone, I.; Rossello, A.; Mezzanzanica, D.; Canevari, S.; Orengo, A.M.; Puppo, A.; Ferrini, S.; Fabbi, M. The ALCAM Shedding by the Metalloprotease ADAM17/TACE Is Involved in Motility of Ovarian Carcinoma Cells. Mol Cancer Res. 2007, 5, 1246–1253. [Google Scholar] [CrossRef]
- Weskamp, G.; Mendelson, K.; Swendeman, S.; Le Gall, S.; Ma, Y.; Lyman, S.; Hinoki, A.; Eguchi, S.; Guaiquil, V.; Horiuchi, K.; et al. Pathological Neovascularization Is Reduced by Inactivation of ADAM17 in Endothelial Cells but Not in Pericytes. Circ. Res. 2010, 106, 932–940. [Google Scholar] [CrossRef]
- Das, S.; Czarnek, M.; Bzowska, M.; Mężyk-Kopeć, R.; Stalińska, K.; Wyroba, B.; Sroka, J.; Jucha, J.; Deneka, D.; Stokłosa, P.; et al. ADAM17 Silencing in Mouse Colon Carcinoma Cells: The Effect on Tumoricidal Cytokines and Angiogenesis. PLoS ONE 2012, 7, e50791. [Google Scholar] [CrossRef]
- Zheng, X.; Jiang, F.; Katakowski, M.; Lu, Y.; Chopp, M. ADAM17 Promotes Glioma Cell Malignant Phenotype. Mol. Carcinog. 2012, 51, 150–164. [Google Scholar] [CrossRef]
- Caolo, V.; Swennen, G.; Chalaris, A.; Wagenaar, A.; Verbruggen, S.; Rose-John, S.; Molin, D.G.M.; Vooijs, M.; Post, M.J. ADAM10 and ADAM17 Have Opposite Roles during Sprouting Angiogenesis. Angiogenesis 2015, 18, 13–22. [Google Scholar] [CrossRef]
- Mężyk-Kopeć, R.; Wyroba, B.; Stalińska, K.; Próchnicki, T.; Wiatrowska, K.; Kilarski, W.W.; Swartz, M.A.; Bereta, J. ADAM17 Promotes Motility, Invasion, and Sprouting of Lymphatic Endothelial Cells. PLoS ONE 2015, 10, e0132661. [Google Scholar] [CrossRef] [PubMed]
- Califano, D.; Russo, D.; Scognamiglio, G.; Losito, N.S.; Spina, A.; Bello, A.M.; Capiluongo, A.; Galdiero, F.; De Cecio, R.; Bevilacqua, S.; et al. Ovarian Cancer Translational Activity of the Multicenter Italian Trial in Ovarian Cancer (MITO) Group: Lessons Learned in 10 Years of Experience. Cells 2020, 9, 903. [Google Scholar] [CrossRef] [PubMed]
- Holländer, N.; Sauerbrei, W.; Schumacher, M. Confidence Intervals for the Effect of a Prognostic Factor after Selection of an “optimal” Cutpoint. Stat. Med. 2004, 23, 1701–1713. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Miyamoto, S.; Suzuki, S.O.; Oki, E.; Yagi, H.; Sonoda, K.; Yamazaki, A.; Mizushima, H.; Maehara, Y.; Mekada, E.; et al. Clinical Significance of Heparin-Binding Epidermal Growth Factor-like Growth Factor and a Disintegrin and Metalloprotease 17 Expression in Human Ovarian Cancer. Clin. Cancer Res. 2005, 11, 4783–4792. [Google Scholar] [CrossRef]
- Kirkegaard, T.; Naresh, A.; Sabine, V.S.; Tovey, S.M.; Edwards, J.; Dunne, B.; Cooke, T.G.; Jones, F.E.; Bartlett, J.M.S. Expression of Tumor Necrosis Factor Alpha Converting Enzyme in Endocrine Cancers. Am. J. Clin. Pathol. 2008, 129, 735–743. [Google Scholar] [CrossRef]
- Buchanan, P.C.; Boylan, K.L.M.; Walcheck, B.; Heinze, R.; Geller, M.A.; Argenta, P.A.; Skubitz, A.P.N. Ectodomain Shedding of the Cell Adhesion Molecule Nectin-4 in Ovarian Cancer Is Mediated by ADAM10 and ADAM17. J. Biol. Chem. 2017, 292, 6339–6351. [Google Scholar] [CrossRef]
- Cao, Y.; Shi, H.; Ren, F.; Jia, Y.; Zhang, R. Long Non-Coding RNA CCAT1 Promotes Metastasis and Poor Prognosis in Epithelial Ovarian Cancer. Exp. Cell Res. 2017, 359, 185–194. [Google Scholar] [CrossRef]
- Jia, D.; Underwood, J.; Xu, Q.; Xie, Q. NOTCH2/NOTCH3/DLL3/MAML1/ADAM17 Signaling Network Is Associated with Ovarian Cancer. Oncol. Lett. 2019, 17, 4914–4920. [Google Scholar] [CrossRef]
- Rogmans, C.; Kuhlmann, J.D.; Hugendieck, G.; Link, T.; Arnold, N.; Weimer, J.P.; Flörkemeier, I.; Rambow, A.C.; Lieb, W.; Maass, N.; et al. ADAM17-A Potential Blood-Based Biomarker for Detection of Early-Stage Ovarian Cancer. Cancers 2021, 13, 5563. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, W.; Huang, M.; Fan, R.; Chen, X. Prognostic Value of ADAM17 in Human Gastric Cancer. Med. Oncol. 2012, 29, 2684–2690. [Google Scholar] [CrossRef]
- Shou, Z.-X.; Jin, X.; Zhao, Z.-S. Upregulated Expression of ADAM17 Is a Prognostic Marker for Patients with Gastric Cancer. Ann. Surg. 2012, 256, 1014–1022. [Google Scholar] [CrossRef]
- Aydin, D.; Bilici, A.; Yavuzer, D.; Kefeli, U.; Tan, A.; Ercelep, O.; Mert, A.; Yuksel, S.; Ozcelik, M.; Isik, D.; et al. Prognostic Significance of ADAM17 Expression in Patients with Gastric Cancer Who Underwent Curative Gastrectomy. Clin. Transl. Oncol. 2015, 17, 604–611. [Google Scholar] [CrossRef]
- Fang, W.; Qian, J.; Wu, Q.; Chen, Y.; Yu, G. ADAM-17 Expression Is Enhanced by FoxM1 and Is a Poor Prognostic Sign in Gastric Carcinoma. J. Surg. Res. 2017, 220, 223–233. [Google Scholar] [CrossRef]
- Jiao, X.; Yu, W.; Qian, J.; Chen, Y.; Wei, P.; Fang, W.; Yu, G. ADAM-17 Is a Poor Prognostic Indicator for Patients with Hilar Cholangiocarcinoma and Is Regulated by FoxM1. BMC Cancer 2018, 18, 570. [Google Scholar] [CrossRef]
- Xu, Q.; Ying, M.; Chen, G.; Lin, A.; Xie, Y.; Ohara, N.; Zhou, D. ADAM17 Is Associated with EMMPRIN and Predicts Poor Prognosis in Patients with Uterine Cervical Carcinoma. Tumour. Biol. 2014, 35, 7575–7586. [Google Scholar] [CrossRef]
- Liu, H.-B.; Yang, Q.-C.; Shen, Y.; Zhu, Y.; Zhang, X.-J.; Chen, H. A Disintegrin and Metalloproteinase 17 MRNA and Protein Expression in Esophageal Squamous Cell Carcinoma, as Well as Its Clinicopathological Factors and Prognosis. Mol. Med. Rep. 2015, 11, 961–967. [Google Scholar] [CrossRef]
- Ni, S.-S.; Zhang, J.; Zhao, W.-L.; Dong, X.-C.; Wang, J.-L. ADAM17 Is Overexpressed in Non-Small Cell Lung Cancer and Its Expression Correlates with Poor Patient Survival. Tumour. Biol. 2013, 34, 1813–1818. [Google Scholar] [CrossRef]
- Narita, D.; Seclaman, E.; Ursoniu, S.; Anghel, A. Increased Expression of ADAM12 and ADAM17 Genes in Laser-Capture Microdissected Breast Cancers and Correlations with Clinical and Pathological Characteristics. Acta Histochem. 2012, 114, 131–139. [Google Scholar] [CrossRef]
- Merchant, N.B.; Voskresensky, I.; Rogers, C.M.; Lafleur, B.; Dempsey, P.J.; Graves-Deal, R.; Revetta, F.; Foutch, A.C.; Rothenberg, M.L.; Washington, M.K.; et al. TACE/ADAM-17: A Component of the Epidermal Growth Factor Receptor Axis and a Promising Therapeutic Target in Colorectal Cancer. Clin. Cancer Res. 2008, 14, 1182–1191. [Google Scholar] [CrossRef]
- Lin, H.-M.; Chatterjee, A.; Lin, Y.-H.; Anjomshoaa, A.; Fukuzawa, R.; McCall, J.L.; Reeve, A.E. Genome Wide Expression Profiling Identifies Genes Associated with Colorectal Liver Metastasis. Oncol. Rep. 2007, 17, 1541–1549. [Google Scholar] [CrossRef]
- Li, Y.; Ren, Z.; Wang, Y.; Dang, Y.-Z.; Meng, B.-X.; Wang, G.-D.; Zhang, J.; Wu, J.; Wen, N. ADAM17 Promotes Cell Migration and Invasion through the Integrin Β1 Pathway in Hepatocellular Carcinoma. Exp. Cell Res. 2018, 370, 373–382. [Google Scholar] [CrossRef]
- McGowan, P.M.; McKiernan, E.; Bolster, F.; Ryan, B.M.; Hill, A.D.K.; McDermott, E.W.; Evoy, D.; O’Higgins, N.; Crown, J.; Duffy, M.J. ADAM-17 Predicts Adverse Outcome in Patients with Breast Cancer. Ann. Oncol. 2008, 19, 1075–1081. [Google Scholar] [CrossRef]
- Li, G.; Forest, F.; Feng, G.; Gentil-Perret, A.; Péoc’h, M.; Cottier, M.; Mottet, N. A Novel Marker ADAM17 for Clear Cell Renal Cell Carcinomas: Implication for Patients’ Prognosis. Urol. Oncol. 2014, 32, 1272–1276. [Google Scholar] [CrossRef]
- Guo, Y.; He, X.; Zhang, M.; Qu, Y.; Gu, C.; Ren, M.; Wang, H.; Ning, W.; Li, J.; Yu, C.; et al. Reciprocal Control of ADAM17/EGFR/Akt Signaling and MiR-145 Drives GBM Invasiveness. J. Neurooncol. 2020, 147, 327–337. [Google Scholar] [CrossRef]
- Liu, H.B.; Zhu, Y.; Yang, Q.C.; Shen, Y.; Zhang, X.J.; Chen, H. Expression and Clinical Significance of ADAM17 Protein in Esophageal Squamous Cell Carcinoma. Genet. Mol. Res. 2015, 14, 4391–4398. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, Y.; Xu, D.; Wang, J.; Yu, G. Differential Expression of ANXA1 in Benign Human Gastrointestinal Tissues and Cancers. BMC Cancer 2014, 14, 520. [Google Scholar] [CrossRef] [PubMed]
- Hedemann, N.; Herz, A.; Schiepanski, J.H.; Dittrich, J.; Sebens, S.; Dempfle, A.; Feuerborn, J.; Rogmans, C.; Tribian, N.; Flörkemeier, I.; et al. ADAM17 Inhibition Increases the Impact of Cisplatin Treatment in Ovarian Cancer Spheroids. Cancers 2021, 13, 2039. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, T.; Petry, A.; Kračun, D.; Rieß, F.; Pike, L.; Harris, A.L.; Görlach, A. The Unfolded Protein Response Controls Induction and Activation of ADAM17/TACE by Severe Hypoxia and ER Stress. Oncogene 2012, 31, 3621–3634. [Google Scholar] [CrossRef]
- Califano, D.; Gallo, D.; Rampioni Vinciguerra, G.L.; De Cecio, R.; Arenare, L.; Signoriello, S.; Russo, D.; Ferrandina, G.; Citron, F.; Losito, N.S.; et al. Evaluation of Angiogenesis-Related Genes as Prognostic Biomarkers of Bevacizumab Treated Ovarian Cancer Patients: Results from the Phase IV MITO16A/ManGO OV-2 Translational Study. Cancers 2021, 13, 5152. [Google Scholar] [CrossRef] [PubMed]
| Patients in Analysis | MITO16A Population | |
|---|---|---|
| (N = 309) | (N = 398) | |
| Median age (IQR) | 58.9 (49.8;66.2) | 59.2 (49.8;66.5) |
| Age category | ||
| <65 | 220 (71.2%) | 278 (69.8%) |
| ≥65 | 89 (28.8%) | 120 (30.2%) |
| ECOG performance status | ||
| 0 | 248 (80.3%) | 315 (79.1%) |
| 1 | 53 (17.2%) | 69 (17.3%) |
| 2 | 8 (2.6%) | 14 (3.5%) |
| Residual disease | ||
| None | 130 (42.1%) | 153 (38.4%) |
| ≤1 cm | 63 (20.4%) | 72 (18.1%) |
| >1 cm | 96 (31.1%) | 120 (30.2%) |
| not operated | 20 (6.5%) | 53 (13.3%) |
| FIGO stage | ||
| IIIb | 29 (9.4%) | 36 (9.0%) |
| IIIc | 220 (71.2%) | 275 (69.1%) |
| IV | 60 (19.4%) | 87 (21.9%) |
| Tumor histology | ||
| High-grade serous | 265 (85.8%) | 333 (83.7%) |
| Low-grade serous | 11 (3.6%) | 13 (3.3%) |
| Endometrioid | 9 (2.9%) | 9 (2.3%) |
| Clear cell | 8 (2.6%) | 11 (2.8%) |
| Mucinous | 2 (0.6%) | 3 (0.8%) |
| Mixed | 2 (0.6%) | 4 (1.0%) |
| Other | 12 (3.9%) | 25 (6.3%) |
| Median (IQR) | P | |
|---|---|---|
| Age category | 0.3417 | |
| <65 | 75.8 (31.7–123.3) | |
| ≥65 | 83.3 (50.0–140.0) | |
| ECOG performance status | 0.3169 | |
| 0 | 78.3 (30.0–123.3) | |
| 1–2 | 88.3 (50.0–133.3) | |
| Residual disease | 0.2962 | |
| None | 66.7 (26.7–123.3) | |
| ≤1 cm | 86.7 (45.0–125.0) | |
| >1 cm | 93.3 (42.5–133.3) | |
| not operated | 81.6 (35.0–131.7) | |
| FIGO stage | 0.4144 | |
| IIIb | 83.3 (33.3–125) | |
| IV | 66.7 (28.3–131.7) | |
| Tumor histology | 0.0884 | |
| High-grade serous | 83.3 (33.3–133.3) | |
| Other | 65.0 (13.3–103.3) |
| Progression-Free Survival | ||||||||
| Univariate Analysis | Multivariate Analysis | |||||||
| Original Coefficients | Shrunken Coefficients | Original Coefficients | Shrunken Coefficients | |||||
| ADAM17 >50 vs. ≤50 | HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p |
| 1.33 (1.00–1.77) | 0.050 | 1.24 (0.67–2.29) | 0.501 | 1.16 (0.86–1.56) | 0.329 | 0.99 (0.42–2.35) | 0.986 | |
| Overall Survival | ||||||||
| Univariate Analysis | Multivariate Analysis | |||||||
| Original Coefficients | Shrunken Coefficients | Original Coefficients | Shrunken Coefficients | |||||
| ADAM17 >50 vs. ≤50 | HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p |
| 1.68 (1.08–2.60) | 0.020 | 1.52 (0.47–4.94) | 0.483 | 1.36 (0.86–2.13) | 0.186 | 1.14 (0.25–5.15) | 0.868 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabbi, M.; Costa, D.; Russo, D.; Arenare, L.; Gaggero, G.; Signoriello, S.; Scambia, G.; Pisano, C.; Colombo, N.; Losito, N.S.; et al. Analysis of A Disintegrin and Metalloprotease 17 (ADAM17) Expression as a Prognostic Marker in Ovarian Cancer Patients Undergoing First-Line Treatment Plus Bevacizumab. Diagnostics 2022, 12, 2118. https://doi.org/10.3390/diagnostics12092118
Fabbi M, Costa D, Russo D, Arenare L, Gaggero G, Signoriello S, Scambia G, Pisano C, Colombo N, Losito NS, et al. Analysis of A Disintegrin and Metalloprotease 17 (ADAM17) Expression as a Prognostic Marker in Ovarian Cancer Patients Undergoing First-Line Treatment Plus Bevacizumab. Diagnostics. 2022; 12(9):2118. https://doi.org/10.3390/diagnostics12092118
Chicago/Turabian StyleFabbi, Marina, Delfina Costa, Daniela Russo, Laura Arenare, Gabriele Gaggero, Simona Signoriello, Giovanni Scambia, Carmela Pisano, Nicoletta Colombo, Nunzia Simona Losito, and et al. 2022. "Analysis of A Disintegrin and Metalloprotease 17 (ADAM17) Expression as a Prognostic Marker in Ovarian Cancer Patients Undergoing First-Line Treatment Plus Bevacizumab" Diagnostics 12, no. 9: 2118. https://doi.org/10.3390/diagnostics12092118
APA StyleFabbi, M., Costa, D., Russo, D., Arenare, L., Gaggero, G., Signoriello, S., Scambia, G., Pisano, C., Colombo, N., Losito, N. S., Filaci, G., Spina, A., Califano, D., Scognamiglio, G., Gadducci, A., Mezzanzanica, D., Bagnoli, M., Ferrini, S., Canzonieri, V., ... Pignata, S. (2022). Analysis of A Disintegrin and Metalloprotease 17 (ADAM17) Expression as a Prognostic Marker in Ovarian Cancer Patients Undergoing First-Line Treatment Plus Bevacizumab. Diagnostics, 12(9), 2118. https://doi.org/10.3390/diagnostics12092118










