Trends in Infectious Keratitis in Taiwan: An Update on Predisposing Factors, Microbiological and Antibiotic Susceptibility Patterns
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
2.2. Patient Identification
2.3. Determination of Risk Factors
2.4. Isolates and Antibiotic Susceptibility
2.5. Statistical Analysis
3. Results
3.1. Demographics of the Study Population
3.2. Predisposing Factors for Infectious Keratitis
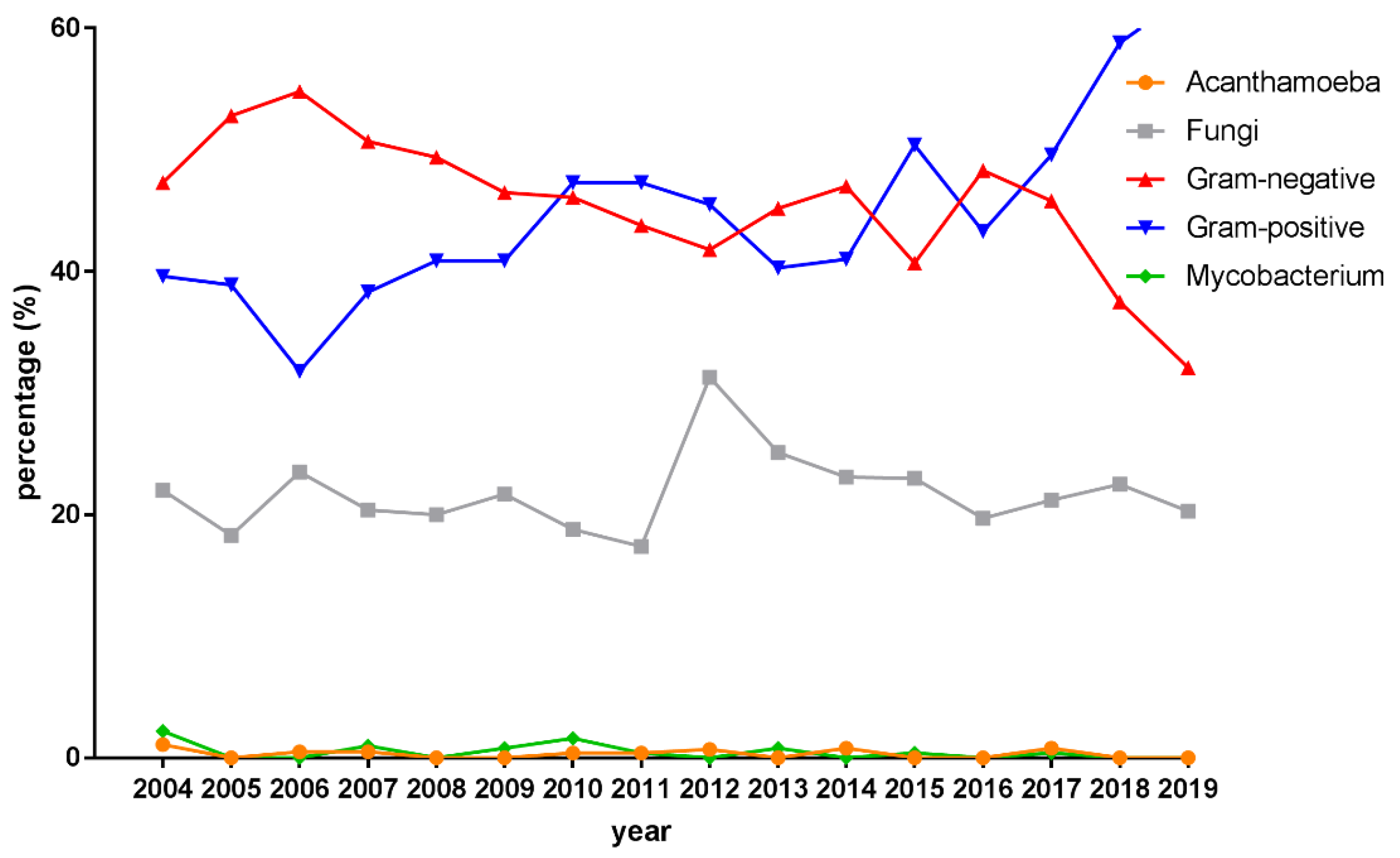
3.3. Microbiological Profiles
3.4. Antibiotic Susceptibility Patterns
3.4.1. Gram-Negative Isolates
3.4.2. Gram-Positive Isolates
3.4.3. Multidrug-Resistant Isolates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Conditions | ICD-9 Codes | ICD-10 Codes |
|---|---|---|
| Contact lens use (= Corneal disorder due to contact lens) | 71.82, 367.0, 367.1, 367.2, 367.9 | H18.82, H52.0X, H52.1, H52.2, H52.7 |
| Ocular trauma | 18, 918.0, 918.1, 918.2, 918.9 | S00.2, S00.01, S00.02, S05.00, S05.01, S05.9 |
| Dry eye | 375.15, 370.33, 710.2 | H04.12, H16.22, M35.00, M35.01, M35.09 |
| Trichiasis | 374.05 | H02.05 |
| Blepharitis | 372.20, 372.21, 372.22, 373.00, 373.01, 373.02, 373.31, 373.32, 373.33, 373.34 | H10.50, H10.52, H10.53, H01 |
| Exposure keratopathy, or lagophthalmos | 370.34, 374.2 | H16.21, H02.2 |
| Neurotrophic keratopathy | 370.35 | H16.23 |
| Corneal transplant status | V42.5 | Z94.7 |
| Diabetes mellitus | 250 | E11, E10, E09, E13, E08 |
| Sjögren syndrome | 710.2 | M35.0 |
| Autoimmune diseases (Non-Sjörgen) | 701.0, 710.1, 710.3, 710.4, 710.5, 710.8, 710.9, 714.0, 714.1, 714.2, 714.30, 714.31, 714.32, 714.33, 720, 725 | L90.0, L94.0, L94.1, L94.3, M32, M34, M35.1-9, M33, M05, M06, M08, M45, M46, M48, M49 |
| Atopic dermatitis | 691.8, 372.05 | L20.8, L20.9, H10.1 |
| Chronic kidney disease | 585 | N18.4-6, N18.9 |
| Human immunodeficiency virus infection | V08, 795.71, 042 | Z21, Z22.6, R75, B20, B21, B22, B23, B24 |
References
- Whitcher, J.P.; Srinivasan, M.; Upadhyay, M.P. Corneal blindness: A global perspective. Bull. World Health Organ. 2001, 79, 214–221. [Google Scholar] [PubMed]
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Global causes of blindness and distance vision impairment 1990-2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef]
- Koh, Y.Y.; Sun, C.C.; Hsiao, C.H. Epidemiology and the Estimated Burden of Microbial Keratitis on the Health Care System in Taiwan: A 14-Year Population-Based Study. Am. J. Ophthalmol. 2020, 220, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Collier, S.A.; Gronostaj, M.P.; MacGurn, A.K.; Cope, J.R.; Awsumb, K.L.; Yoder, J.S.; Beach, M.J. Estimated burden of keratitis—United States, 2010. MMWR Morb. Mortal Wkly. Rep. 2014, 63, 1027–1030. [Google Scholar]
- Upadhyay, M.P.; Karmacharya, P.C.; Koirala, S.; Shah, D.N.; Shakya, S.; Shrestha, J.K.; Bajracharya, H.; Gurung, C.K.; Whitcher, J.P. The Bhaktapur eye study: Ocular trauma and antibiotic prophylaxis for the prevention of corneal ulceration in Nepal. Br. J. Ophthalmol. 2001, 85, 388–392. [Google Scholar] [CrossRef]
- Erie, J.C.; Nevitt, M.P.; Hodge, D.O.; Ballard, D.J. Incidence of ulcerative keratitis in a defined population from 1950 through 1988. Arch. Ophthalmol. 1993, 111, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Z.; Walkden, A.; Au, L.; Fullwood, C.; Hamilton, A.; Qamruddin, A.; Armstrong, M.; Brahma, A.K.; Carley, F. Twelve-year analysis of microbial keratitis trends at a UK tertiary hospital. Eye 2017, 31, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Khor, W.B.; Prajna, V.N.; Garg, P.; Mehta, J.S.; Xie, L.; Liu, Z.; Padilla, M.D.B.; Joo, C.K.; Inoue, Y.; Goseyarakwong, P.; et al. The Asia Cornea Society Infectious Keratitis Study: A Prospective Multicenter Study of Infectious Keratitis in Asia. Am. J. Ophthalmol. 2018, 195, 161–170. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Ho, C.S.; Cairns, J.; Elsahn, A.; Al-Aqaba, M.; Boswell, T.; Said, D.G.; Dua, H.S. 12-year analysis of incidence, microbiological profiles and in vitro antimicrobial susceptibility of infectious keratitis: The Nottingham Infectious Keratitis Study. Br. J. Ophthalmol. 2021, 105, 328–333. [Google Scholar] [CrossRef]
- Khoo, P.; Cabrera-Aguas, M.P.; Nguyen, V.; Lahra, M.M.; Watson, S.L. Microbial keratitis in Sydney, Australia: Risk factors, patient outcomes, and seasonal variation. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 1745–1755. [Google Scholar] [CrossRef]
- Green, M.; Apel, A.; Stapleton, F. Risk factors and causative organisms in microbial keratitis. Cornea 2008, 27, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Yeh, L.K.; Ma, D.H.; Chen, P.Y.; Lin, H.C.; Sun, C.C.; Tan, H.Y.; Chen, H.C.; Chen, S.Y.; Hsiao, C.H. Risk Factors and Microbiological Features of Patients Hospitalized for Microbial Keratitis: A 10-Year Study in a Referral Center in Taiwan. Medicine 2015, 94, e1905. [Google Scholar] [CrossRef] [PubMed]
- Ung, L.; Bispo, P.J.M.; Shanbhag, S.S.; Gilmore, M.S.; Chodosh, J. The persistent dilemma of microbial keratitis: Global burden, diagnosis, and antimicrobial resistance. Surv. Ophthalmol. 2019, 64, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Hanet, M.S.; Jamart, J.; Chaves, A.P. Fluoroquinolones or fortified antibiotics for treating bacterial keratitis: Systematic review and meta-analysis of comparative studies. Can. J. Ophthalmol. 2012, 47, 493–499. [Google Scholar] [CrossRef]
- Sharma, N.; Goel, M.; Bansal, S.; Agarwal, P.; Titiyal, J.S.; Upadhyaya, A.D.; Vajpayee, R.B. Evaluation of moxifloxacin 0.5% in treatment of nonperforated bacterial corneal ulcers: A randomized controlled trial. Ophthalmology 2013, 120, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Orlans, H.O.; Hornby, S.J.; Bowler, I.C. In vitro antibiotic susceptibility patterns of bacterial keratitis isolates in Oxford, UK: A 10-year review. Eye 2011, 25, 489–493. [Google Scholar] [CrossRef]
- Alexandrakis, G.; Alfonso, E.C.; Miller, D. Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. Ophthalmology 2000, 107, 1497–1502. [Google Scholar] [CrossRef]
- Brown, L. Resistance to ocular antibiotics: An overview. Clin. Exp. Optom. 2007, 90, 258–262. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, K.; Liu, J.; Wei, Z.; Xu, X.; Liang, Q. Pathogens and Antibiotic Susceptibilities of Global Bacterial Keratitis: A Meta-Analysis. Antibiotics 2022, 11, 238. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Ho, C.S.; Deshmukh, R.; Said, D.G.; Dua, H.S. Infectious keratitis: An update on epidemiology, causative microorganisms, risk factors, and antimicrobial resistance. Eye 2021, 35, 1084–1101. [Google Scholar] [CrossRef]
- Liu, H.Y.; Chu, H.S.; Wang, I.J.; Chen, W.L.; Hu, F.R. Microbial Keratitis in Taiwan: A 20-Year Update. Am. J. Ophthalmol. 2019, 205, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Y.W.; Boase, D.L.; Cree, I.A. Epidemiological characteristics, predisposing factors and microbiological profiles of infectious corneal ulcers: The Portsmouth corneal ulcer study. Br. J. Ophthalmol. 2009, 93, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.; Zhou, Q.; Zhai, H.; Cheng, J.; Wan, L.; Ge, C.; Xie, L. Clinical analysis of fungal keratitis in patients with and without diabetes. PLoS ONE 2018, 13, e0196741. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, S.; Zhai, H.L.; Zhang, Y.Y.; Cui, C.X.; Wang, J.Y.; Xie, L.X. A comparative study of risk factors for corneal infection in diabetic and non-diabetic patients. Int. J. Ophthalmol. 2018, 11, 43–47. [Google Scholar] [CrossRef]
- Li, S.; Yi, G.; Peng, H.; Li, Z.; Chen, S.; Zhong, H.; Chen, Y.; Wang, Z.; Deng, Q.; Fu, M. How Ocular Surface Microbiota Debuts in Type 2 Diabetes Mellitus. Front. Cell Infect. Microbiol. 2019, 9, 202. [Google Scholar] [CrossRef]
- Hine, J.L.; de Lusignan, S.; Burleigh, D.; Pathirannehelage, S.; McGovern, A.; Gatenby, P.; Jones, S.; Jiang, D.; Williams, J.; Elliot, A.J.; et al. Association between glycaemic control and common infections in people with Type 2 diabetes: A cohort study. Diabet. Med. 2017, 34, 551–557. [Google Scholar] [CrossRef]
- Zhu, L.; Titone, R.; Robertson, D.M. The impact of hyperglycemia on the corneal epithelium: Molecular mechanisms and insight. Ocul. Surf. 2019, 17, 644–654. [Google Scholar] [CrossRef]
- Szalai, E.; Deak, E.; Modis, L., Jr.; Nemeth, G.; Berta, A.; Nagy, A.; Felszeghy, E.; Kaposzta, R.; Malik, R.A.; Csutak, A. Early Corneal Cellular and Nerve Fiber Pathology in Young Patients With Type 1 Diabetes Mellitus Identified Using Corneal Confocal Microscopy. Invest. Ophthalmol. Vis. Sci. 2016, 57, 853–858. [Google Scholar] [CrossRef]
- Jan, R.L.; Tai, M.C.; Weng, S.F.; Chang, C.; Wang, J.J.; Chang, Y.S. Risk of corneal ulcer in patients with end-stage renal disease: A retrospective large-scale cohort study. Br. J. Ophthalmol. 2018, 102, 868–872. [Google Scholar] [CrossRef]
- Weng, S.F.; Jan, R.L.; Chang, C.; Wang, J.J.; Su, S.B.; Huang, C.C.; Tseng, S.H.; Chang, Y.S. Risk of Band Keratopathy in Patients with End-Stage Renal Disease. Sci. Rep. 2016, 6, 28675. [Google Scholar] [CrossRef]
- Redfern, R.L.; McDermott, A.M. Toll-like receptors in ocular surface disease. Exp. Eye Res. 2010, 90, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Sagerfors, S.; Ejdervik-Lindblad, B.; Soderquist, B. Infectious keratitis: Isolated microbes and their antibiotic susceptibility pattern during 2004-2014 in Region Orebro County, Sweden. Acta Ophthalmol. 2020, 98, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.S.; Figueira, L.; Moreira-Goncalves, N.; Moreira, R.; Torrao, L.; Falcao-Reis, F. Clinical and Microbiological Profile of Bacterial Microbial Keratitis in a Portuguese Tertiary Referral Center-Where Are We in 2015? Eye Contact Lens 2018, 44, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Truong, D.T.; Bui, M.T.; Memon, P.; Cavanagh, H.D. Microbial Keratitis at an Urban Public Hospital: A 10-Year Update. J. Clin. Exp. Ophthalmol. 2015, 6, 498. [Google Scholar] [CrossRef]
- Fong, C.F.; Tseng, C.H.; Hu, F.R.; Wang, I.J.; Chen, W.L.; Hou, Y.C. Clinical characteristics of microbial keratitis in a university hospital in Taiwan. Am. J. Ophthalmol. 2004, 137, 329–336. [Google Scholar] [CrossRef]
- Green, M.; Sara, S.; Hughes, I.; Apel, A.; Stapleton, F. Trends in contact lens microbial keratitis 1999 to 2015: A retrospective clinical review. Clin. Exp. Ophthalmol. 2019, 47, 726–732. [Google Scholar] [CrossRef]
- Szczotka-Flynn, L.B.; Shovlin, J.P.; Schnider, C.M.; Caffery, B.E.; Alfonso, E.C.; Carnt, N.A.; Chalmers, R.L.; Collier, S.; Jacobs, D.S.; Joslin, C.E.; et al. American Academy of Optometry Microbial Keratitis Think Tank. Optom Vis. Sci. 2021, 98, 182–198. [Google Scholar] [CrossRef]
- Hsiao, C.H.; Sun, C.C.; Yeh, L.K.; Ma, D.H.; Chen, P.Y.; Lin, H.C.; Tan, H.Y.; Chen, H.C.; Chen, S.Y.; Huang, Y.C. Shifting Trends in Bacterial Keratitis in Taiwan: A 10-Year Review in a Tertiary-Care Hospital. Cornea 2016, 35, 313–317. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Settle, C.; Morgan, S.J.; Baylis, O.; Ghosh, S. A 10-year analysis of microbiological profiles of microbial keratitis: The North East England Study. Eye 2018, 32, 1416–1417. [Google Scholar] [CrossRef]
- Al-Dhaheri, H.S.; Al-Tamimi, M.D.; Khandekar, R.B.; Khan, M.; Stone, D.U. Ocular Pathogens and Antibiotic Sensitivity in Bacterial Keratitis Isolates at King Khaled Eye Specialist Hospital, 2011 to 2014. Cornea 2016, 35, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Lichtinger, A.; Yeung, S.N.; Kim, P.; Amiran, M.D.; Iovieno, A.; Elbaz, U.; Ku, J.Y.; Wolff, R.; Rootman, D.S.; Slomovic, A.R. Shifting trends in bacterial keratitis in Toronto: An 11-year review. Ophthalmology 2012, 119, 1785–1790. [Google Scholar] [CrossRef] [PubMed]
- Green, M.; Carnt, N.; Apel, A.; Stapleton, F. Queensland Microbial Keratitis Database: 2005–2015. Br. J. Ophthalmol. 2019, 103, 1481–1486. [Google Scholar] [CrossRef]
- Pandita, A.; Murphy, C. Microbial keratitis in Waikato, New Zealand. Clin. Exp. Ophthalmol. 2011, 39, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Tam, A.L.C.; Cote, E.; Saldanha, M.; Lichtinger, A.; Slomovic, A.R. Bacterial Keratitis in Toronto: A 16-Year Review of the Microorganisms Isolated and the Resistance Patterns Observed. Cornea 2017, 36, 1528–1534. [Google Scholar] [CrossRef]
- Romanowski, J.E.; Nayyar, S.V.; Romanowski, E.G.; Jhanji, V.; Shanks, R.M.Q.; Kowalski, R.P. Speciation and Antibiotic Susceptibilities of Coagulase Negative Staphylococci Isolated from Ocular Infections. Antibiotics 2021, 10, 721. [Google Scholar] [CrossRef]
- Khoo, P.; Cabrera-Aguas, M.; Robaei, D.; Lahra, M.M.; Watson, S. Microbial Keratitis and Ocular Surface Disease: A 5-Year Study of the Microbiology, Risk Factors and Clinical Outcomes in Sydney, Australia. Curr. Eye Res. 2019, 44, 1195–1202. [Google Scholar] [CrossRef]
- Ovodenko, B.; Seedor, J.A.; Ritterband, D.C.; Shah, M.; Yang, R.; Koplin, R.S. The prevalence and pathogenicity of Propionibacterium acnes keratitis. Cornea 2009, 28, 36–39. [Google Scholar] [CrossRef]
- Shalchi, Z.; Gurbaxani, A.; Baker, M.; Nash, J. Antibiotic resistance in microbial keratitis: Ten-year experience of corneal scrapes in the United Kingdom. Ophthalmology 2011, 118, 2161–2165. [Google Scholar] [CrossRef]
- Soleimani, M.; Tabatabaei, S.A.; Masoumi, A.; Mirshahi, R.; Ghahvechian, H.; Tayebi, F.; Momenaei, B.; Mahdizad, Z.; Mohammadi, S.S. Infectious keratitis: Trends in microbiological and antibiotic sensitivity patterns. Eye 2021, 35, 3110–3115. [Google Scholar] [CrossRef]
- Asbell, P.A.; Colby, K.A.; Deng, S.; McDonnell, P.; Meisler, D.M.; Raizman, M.B.; Sheppard, J.D., Jr.; Sahm, D.F. Ocular TRUST: Nationwide antimicrobial susceptibility patterns in ocular isolates. Am. J. Ophthalmol. 2008, 145, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Haas, W.; Pillar, C.M.; Torres, M.; Morris, T.W.; Sahm, D.F. Monitoring antibiotic resistance in ocular microorganisms: Results from the Antibiotic Resistance Monitoring in Ocular micRorganisms (ARMOR) 2009 surveillance study. Am. J. Ophthalmol. 2011, 152, 567–574.e3. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef] [Green Version]
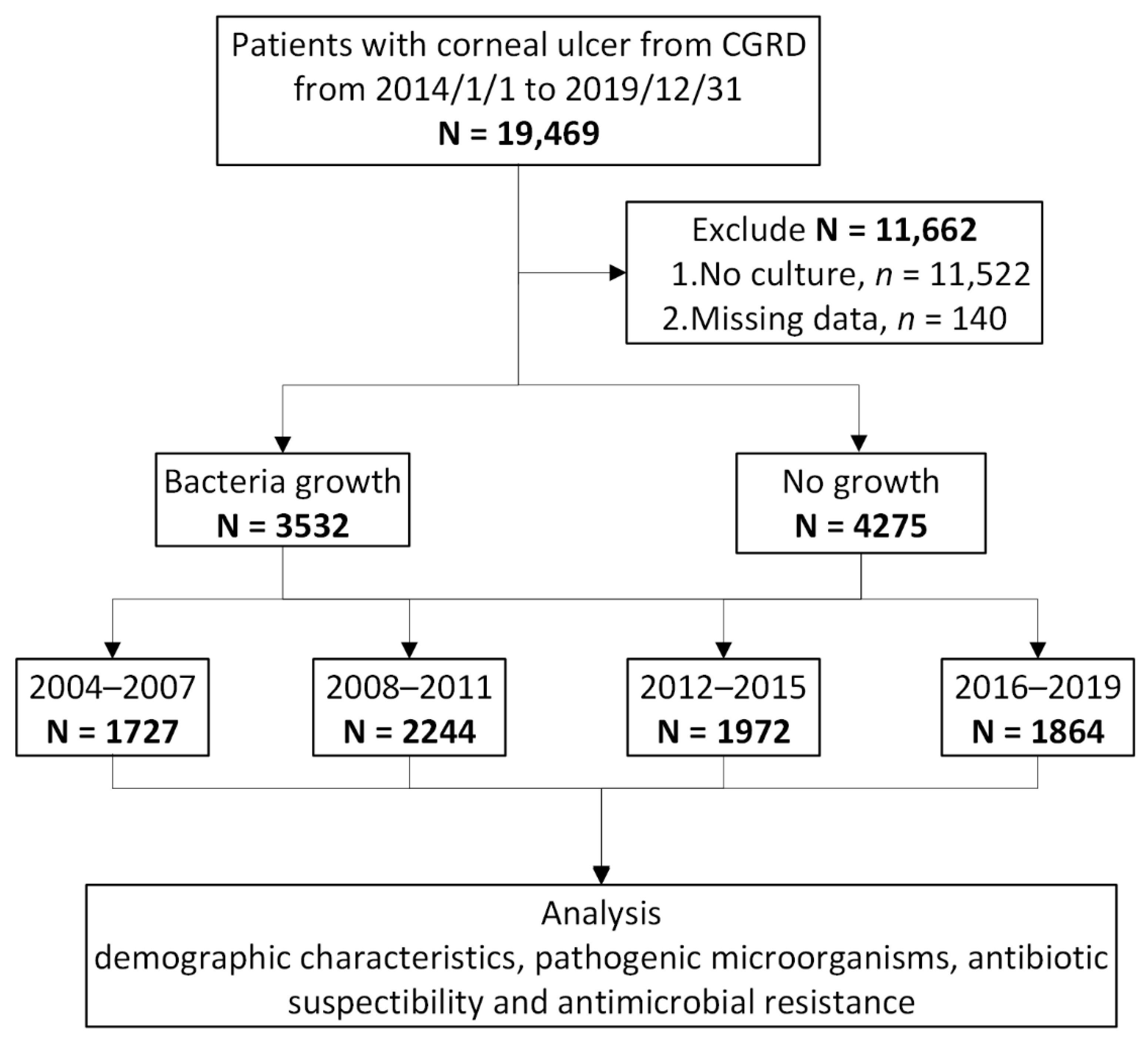
| 2004–2007 N = 1727 | 2008–2011 N = 2244 | 2012–2015 N = 1972 | 2016–2019 N = 1864 | ||
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | p-Value * | |
| Sex | 0.957 | ||||
| Male | 810 (46.9%) | 1146 (51.1%) | 955 (48.4%) | 898 (48.2%) | |
| Female | 917 (53.1%) | 1098 (48.9%) | 1017 (51.6%) | 966 (51.8%) | |
| Age (years) | 0.275 | ||||
| ≤65 | 1300 (75.3%) | 1698 (75.7%) | 1487 (75.4%) | 1375 (73.8%) | |
| >65 | 427 (24.7%) | 546 (24.3%) | 485 (24.6%) | 489 (26.2%) | |
| Mean ± SD | 45.5 ± 22.1 | 46.0 ± 22.1 | 47.5 ± 21.7 | 48.5 ± 22.5 | |
| Culture rate | <0.001 | ||||
| Number of bacterial growth | 689 (40%) | 992 (44%) | 874 (44%) | 977 (52%) | |
| No growth | 1038 (60%) | 1252 (56%) | 1098 (56%) | 887 (48%) |
| 2004–2007 N = 1727 | 2008–2011 N = 2244 | 2012–2015 N = 1972 | 2016–2019 N = 1864 | ||
|---|---|---|---|---|---|
| Variables | n (%) | n (%) | n (%) | n (%) | p-Value * |
| Contact lens wear | 105 (6.1%) | 254 (11.3%) | 238 (12.1%) | 181 (9.7%) | 0.001 |
| Ocular trauma | 138 (8.0%) | 235 (10.5%) | 174 (8.8%) | 141 (7.6%) | 0.236 |
| Recent ocular surgery | 82 (4.8%) | 83 (3.7%) | 42 (2.1%) | 43 (2.3%) | <0.001 |
| Ocular surface disease | 312 (18.1%) | 418 (18.6%) | 358 (18.2%) | 313 (16.8%) | 0.264 |
| Dry eye | 104 (6.0%) | 176 (7.8%) | 155 (7.9%) | 147 (7.9%) | 0.052 |
| Trichiasis | 26 (1.5%) | 37 (1.7%) | 30 (1.5%) | 16 (0.9%) | 0.083 |
| Blepharitis | 112 (6.5%) | 176 (7.8%) | 139 (7.1%) | 108 (5.8%) | 0.226 |
| Exposure keratopathy, or lagophthalmos | 21 (1.2%) | 29 (1.3%) | 24 (1.2%) | 7 (0.4%) | 0.012 |
| Neurotrophic keratopathy | 3 (0.2%) | 7 (0.3%) | 10 (0.5%) | 6 (0.3%) | 0.295 |
| Corneal transplantation status | 93 (5.4%) | 72 (3.2%) | 58 (2.9%) | 52 (2.8%) | <0.001 |
| Topical antiglaucoma agents | 60 (3.5%) | 57 (2.5%) | 57 (2.9%) | 65 (3.5%) | 0.744 |
| Topical steroid | 46 (2.7%) | 47 (2.1%) | 26 (1.3%) | 31 (1.7%) | 0.009 |
| Systemic disorder | 216 (12.5%) | 301 (13.4%) | 292 (14.8%) | 297 (15.9%) | 0.001 |
| Diabetes mellitus | 152 (8.8%) | 226 (10.1%) | 213 (10.8%) | 197 (10.6%) | 0.061 |
| Non-Sjörgen autoimmune dz (RA, SLE, AS, other CTDs…) | 51 (3.0%) | 63 (2.8%) | 47 (2.4%) | 70 (3.8%) | 0.255 |
| Sjögren syndrome | 44 (2.6%) | 40 (1.8%) | 25 (1.3%) | 27 (1.5%) | 0.006 |
| Atopy | 10 (0.6%) | 12 (0.5%) | 16 (0.8%) | 22 (1.2%) | 0.020 |
| Chronic kidney disease | 27 (1.6%) | 53 (2.4%) | 54 (2.7%) | 67 (3.6%) | <0.001 |
| HIV infection | 1 (0.1%) | 3 (0.1%) | 7 (0.4%) | 4 (0.2%) | 0.128 |
| 2004–2007 N = 689 | 2008–2011 N = 992 | 2012–2015 N = 874 | 2016–2019 N = 977 | Total N = 3532 | ||
|---|---|---|---|---|---|---|
| Bacteria | n (%) | n (%) | n (%) | n (%) | n (%) | p-Value * |
| Staphylococcus spp. | 140 (20.3) | 248 (25) | 193 (22.1) | 320 (32.8) | 901 (25.5) | <0.001 |
| S. aureus | 53 (7.7) | 68 (6.9) | 55 (6.3) | 81 (8.3) | 257 (7.3) | 0.619 |
| S. epidermis | 26 (3.8) | 50 (5.0) | 67 (7.7) | 169 (17.3) | 312 (8.8) | <0.001 |
| Other CNS | 63 (9.1) | 138 (13.9) | 67 (7.7) | 51 (5.2) | 319 (9) | <0.001 |
| Streptococcus spp. | 55 (8) | 49 (4.9) | 36 (4.1) | 52 (5.3) | 192 (5.4) | 0.033 |
| S. pneumoniae | 34 (4.9) | 27 (2.7) | 13 (1.5) | 16 (1.6) | 90 (2.5) | <0.001 |
| Propionebacterium spp. | 14 (2.0) | 90 (9.1) | 71 (8.1) | 89 (9.1) | 264 (7.5) | <0.001 |
| Pseudomonas spp. | 229 (33.2) | 283 (28.5) | 280 (32) | 254 (26) | 1046 (29.6) | 0.012 |
| P. aureuginosa | 225 (32.7) | 277 (27.9) | 252 (28.8) | 248 (25.4) | 1002 (28.4) | 0.004 |
| Serratia spp. | 43 (6.2) | 49 (4.9) | 29 (3.3) | 29 (3) | 150 (4.3) | <0.001 |
| 2004–2007 | 2008–2011 | 2012–2015 | 2016–2019 | Total | ||
|---|---|---|---|---|---|---|
| Antibiotics | n (%) | n (%) | n (%) | n (%) | n (%) | p-Value * |
| Piperacillin | 298 (94.6) | 346 (94.8) | 302 (97.0) | 69 (94.2) | 1015 (95.4) | 0.365 |
| Ceftazidime | 324 (96.3) | 417 (94.7) | 355 (96.6) | 349 (96.6) | 1445 (96.0) | 0.517 |
| Cefepime | 260 (95.8) | 329 (93.9) | 311 (96.1) | 278 (96.8) | 1178 (95.6) | 0.305 |
| Imipenem | 313 (97.4) | 338 (95.3) | 311 (94.9) | 278 (95.0) | 1240 (95.7) | 0.132 |
| Meropenem | 220 (95.5) | 332 (94.0) | 309 (95.8) | 270 (95.9) | 1131 (95.2) | 0.506 |
| Gemamicin | 323 (90.1) | 415 (88.9) | 355 (92.1) | 338 (91.7) | 1431 (90.6) | 0.231 |
| Amikacin | 325 (92.0) | 415 (94.0) | 355 (94.9) | 338 (96.5) | 1433 (94.4) | 0.012 |
| Ciprofloxacin | 326 (95.1) | 414 (94.2) | 357 (96.6) | 338 (95.6) | 1435 (95.4) | 0.449 |
| Levofloxacin | 105 (96.2) | 50 (98.0) | 235 (97.5) | 338 (97.0) | 728 (97.1) | 0.792 |
| 2004–2007 | 2008–2011 | 2012–2015 | 2016–2019 | Total | ||
|---|---|---|---|---|---|---|
| Antibiotics | n (%) | n (%) | n (%) | n (%) | n (%) | p-Value * |
| Piperacillin/tazobacatm | 126 (98.4) | 268 (98.1) | 245 (98.4) | 234 (99.6) | 873 (98.7) | 0.247 |
| Ceftazidime | 214 (99.5) | 268 (99.6) | 246 (99.6) | 235 (99.6) | 963 (99.6) | 0.969 |
| Cefepime | 214 (99.5) | 262 (99.2) | 246 (99.2) | 235 (99.6) | 957 (99.4) | 0.956 |
| Amikacin | 214 (99.1) | 268 (98.9) | 246 (99.6) | 235 (100.0) | 963 (99.4) | 0.120 |
| Gentamycin | 214 (95.8) | 268 (94.8) | 246 (97.2) | 235 (96.6) | 963 (96.1) | 0.377 |
| Ciprofloxacin | 214 (97.7) | 268 (97.8) | 246 (98.8) | 235 (97.9) | 963 (98.0) | 0.685 |
| Levofloxacin | 80 (96.3) | N/A | 142 (98.6) | 235 (97.9) | 457 (97.8) | 0.438 |
| 2004–2007 | 2008–2011 | 2012–2015 | 2016–2019 | Total | ||
|---|---|---|---|---|---|---|
| Antibiotics | n (%) | n (%) | n (%) | n (%) | n (%) | p-Value * |
| Penicillin | N/A | 10 (90.0) | 11 (90.9) | 15 (73.3) | 36 (83.3) | 0.238 |
| Oxacillin | 133 (57.9) | 229 (63.8) | 190 (60.0) | 325 (68.0) | 877 (63.6) | 0.062 |
| Teicoplanin | 170 (100.0) | 257 (99.6) | 222 (99.6) | 361 (99.7) | 1010 (99.7) | 0.715 |
| Vancomycin | 168 (99.4) | 278 (100.0) | 232 (100.0) | 374 (99.2) | 1052 (99.6) | 0.383 |
| Linezolid | 10 (100.0) | 40 (100.0) | 39 (100.0) | 63 (100.0) | 152 (100.0) | NA |
| Erythromycin | 158 (51.9) | 248 (52.0) | 214 (52.8) | 350 (53.7) | 970 (52.8) | 0.643 |
| Clindamycin | 172 (71.5) | 335 (75.5) | 286 (74.8) | 434 (76.0) | 1227 (75.0) | 0.366 |
| TMP-SMX | 145 (82.8) | 233 (84.6) | 190 (82.1) | 325 (90.2) | 893 (85.8) | 0.025 |
| Fusidic acid | NA | 51 (96.1) | 51 (98.0) | 81 (97.5) | 183 (97.3) | 0.656 |
| 2004–2007 | 2008–2011 | 2012–2015 | 2016–2019 | Total | ||
|---|---|---|---|---|---|---|
| Antibiotics | n (%) | n (%) | n (%) | n (%) | n (%) | p-Value * |
| Penicillin | 129 (14.0) | 229 (20.1) | 187 (13.4) | 312 (22.4) | 857 (18.6) | 0.099 |
| Oxacillin | 133 (57.9) | 229 (63.8) | 187 (59.4) | 312 (66.4) | 861 (62.8) | 0.157 |
| Vancomycin | 126 (100.0) | 229 (100.0) | 187 (100.0) | 311 (100.0) | 853 (100.0) | NA |
| Erythromycin | 133 (51.9) | 229 (50.7) | 187 (52.9) | 312 (55.1) | 861 (53.0) | 0.353 |
| Clindamycin | 133 (69.9) | 229 (68.1) | 187 (67.9) | 312 (72.1) | 861 (69.8) | 0.467 |
| TMP-SMX | 133 (85.0) | 229 (84.7) | 187 (81.8) | 312 (90.1) | 861 (86.1) | 0.097 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.-J.; Lai, C.-H.; Chen, C.-Y.; Liu, C.-Y.; Lin, M.-H.; Yang, Y.-H.; Wu, P.-L. Trends in Infectious Keratitis in Taiwan: An Update on Predisposing Factors, Microbiological and Antibiotic Susceptibility Patterns. Diagnostics 2022, 12, 2095. https://doi.org/10.3390/diagnostics12092095
Wang J-J, Lai C-H, Chen C-Y, Liu C-Y, Lin M-H, Yang Y-H, Wu P-L. Trends in Infectious Keratitis in Taiwan: An Update on Predisposing Factors, Microbiological and Antibiotic Susceptibility Patterns. Diagnostics. 2022; 12(9):2095. https://doi.org/10.3390/diagnostics12092095
Chicago/Turabian StyleWang, Jin-Jhe, Chien-Hsiung Lai, Chau-Yin Chen, Chia-Yen Liu, Meng-Hung Lin, Yao-Hsu Yang, and Pei-Lun Wu. 2022. "Trends in Infectious Keratitis in Taiwan: An Update on Predisposing Factors, Microbiological and Antibiotic Susceptibility Patterns" Diagnostics 12, no. 9: 2095. https://doi.org/10.3390/diagnostics12092095
APA StyleWang, J.-J., Lai, C.-H., Chen, C.-Y., Liu, C.-Y., Lin, M.-H., Yang, Y.-H., & Wu, P.-L. (2022). Trends in Infectious Keratitis in Taiwan: An Update on Predisposing Factors, Microbiological and Antibiotic Susceptibility Patterns. Diagnostics, 12(9), 2095. https://doi.org/10.3390/diagnostics12092095






