Cell Toxicity Study of Antiseptic Solutions Containing Povidone–Iodine and Hydrogen Peroxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antiseptics Solutions
2.2. Cell Culture
2.3. Cytotoxicity Analysis
2.4. Statistical Analysis
3. Results
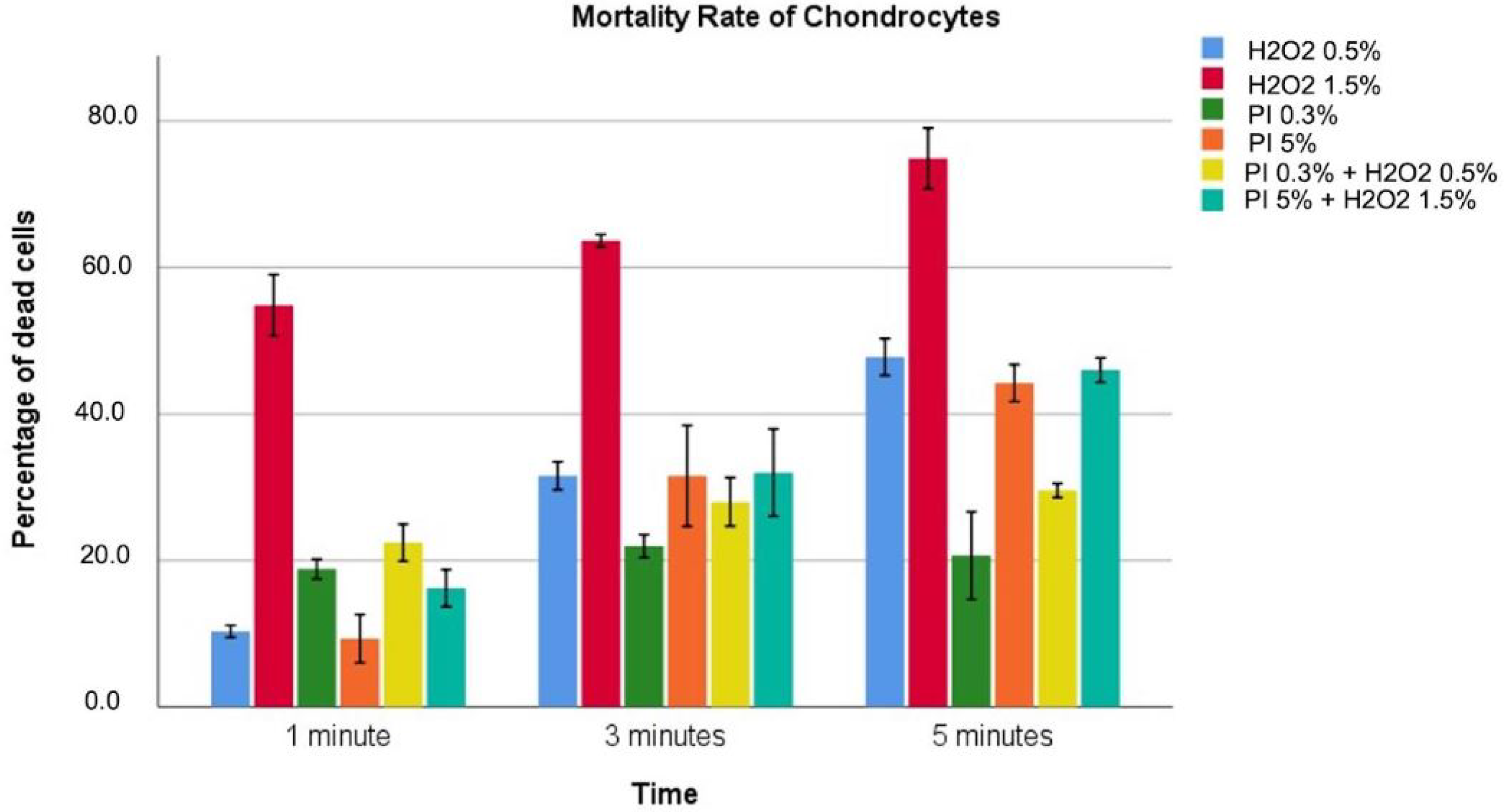
3.1. Chondrocytes
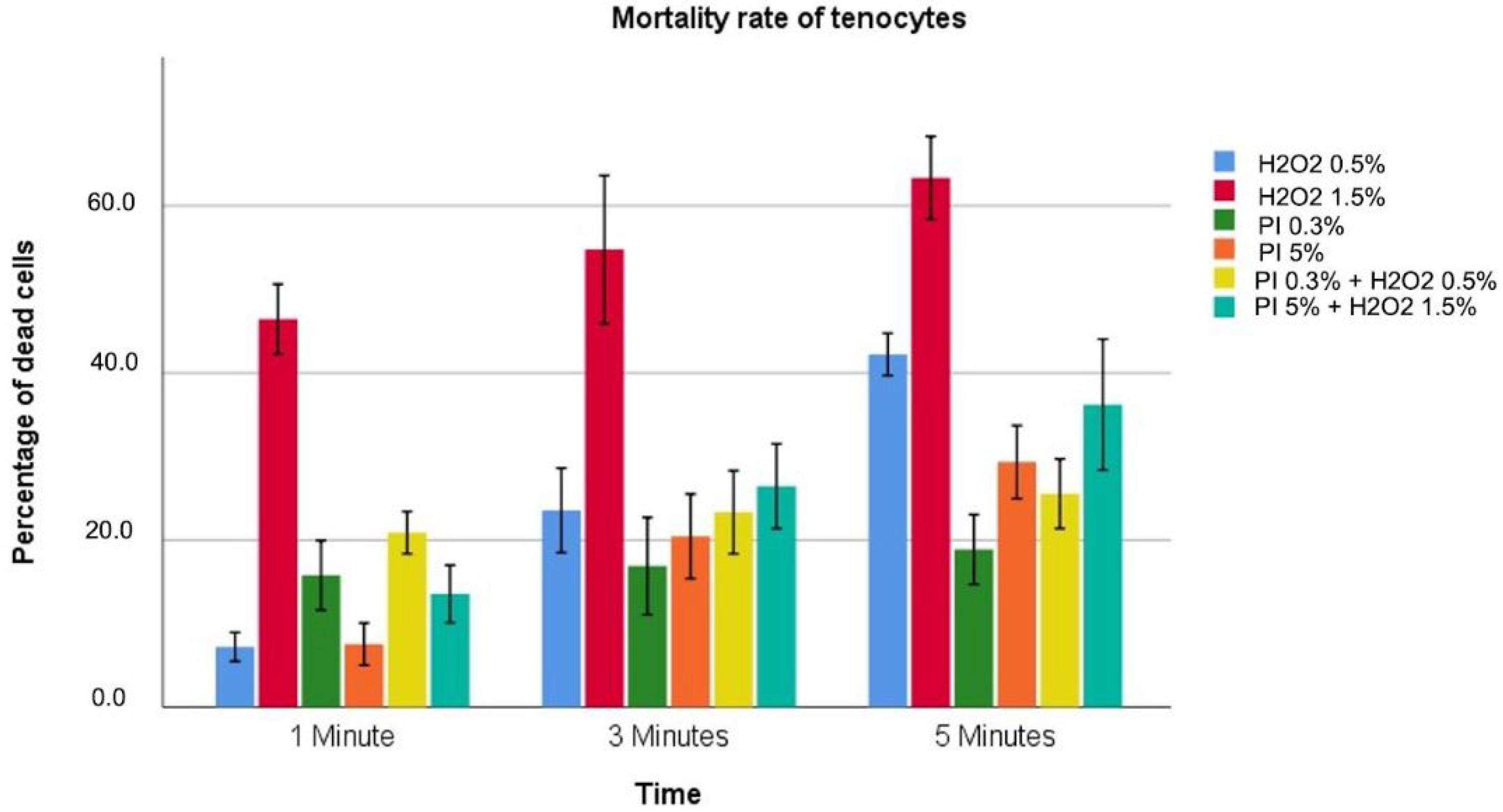
3.2. Tenocytes
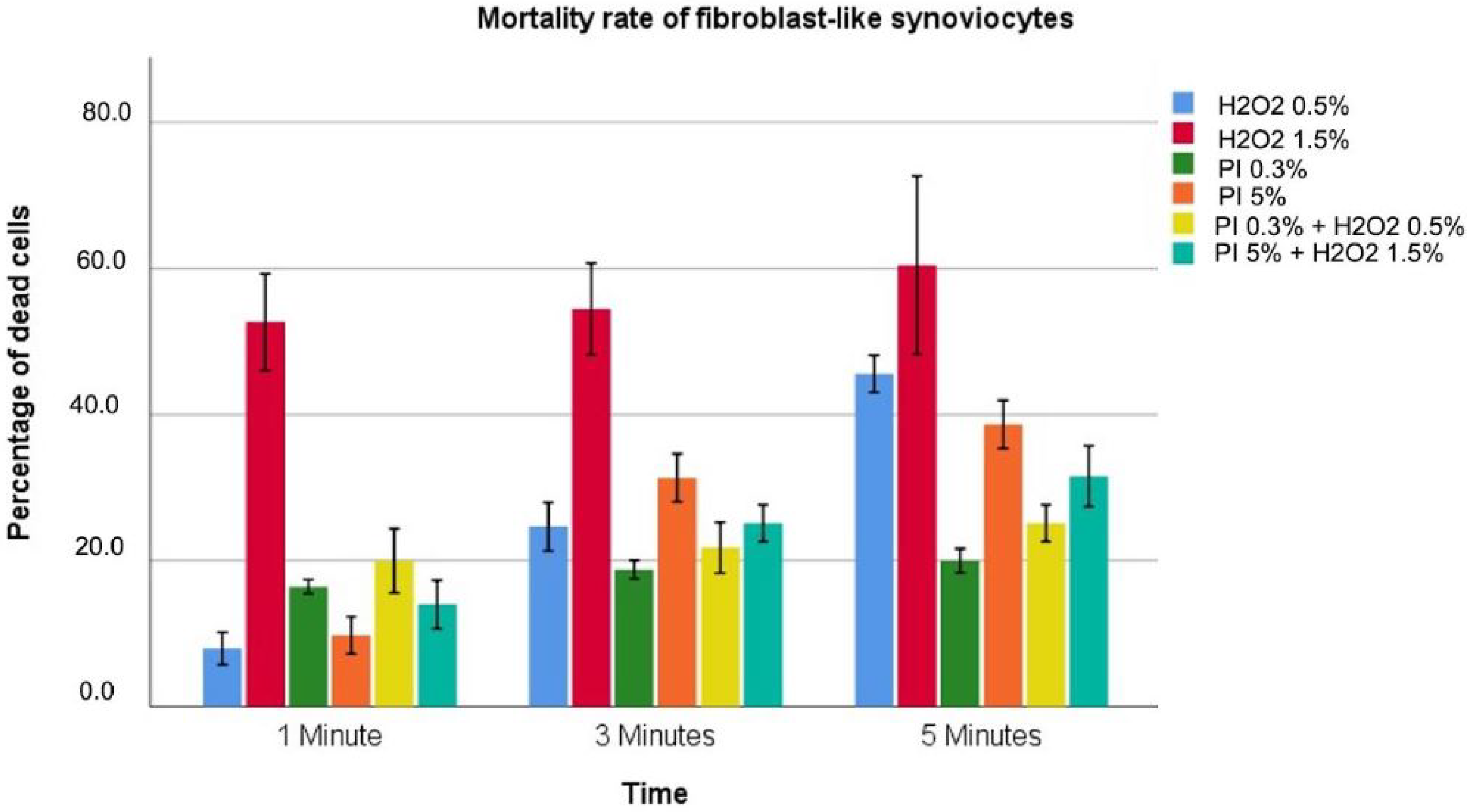
3.3. Fibroblast-like Synoviocytes
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PJIs | Periprosthetic joint infections |
| PI | Povidone iodine |
| HO | Hydrogen peroxide |
| HFLS | Human Fibroblast-Like Synoviocytes |
| NHAC-kn | Normal Human knee Articular Chondrocytes |
References
- Tande, A.J.; Patel, R. Prosthetic joint infection. Clin. Microbiol. Rev. 2014, 4, 302–345. [Google Scholar] [CrossRef] [Green Version]
- Goswami, K.; Cho, J.; Foltz, C.; Manrique, J.; Tan, T.L.; Fillingham, Y.; Higuera, C.; Della Valle, C.; Parvizi, J. Polymyxin and Bacitracin in the Irrigation Solution Provide No Benefit for Bacterial Killing in Vitro. Int. J. Bone Jt. Surg. Am. 2019, 101, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Zubko, E.I.; Zubko, M.K. Co-operative inhibitory effects of hydrogen peroxide and iodine against bacterial and yeast species. BMC Res. Notes 2013, 6, 272. [Google Scholar] [CrossRef] [Green Version]
- Bigliardi, P.L.; Alsagoff, S.A.L.; El-Kafrawi, H.Y.; Pyon, J.K.; Wa, C.T.C.; Villa, M.A. Povidone iodine in wound healing: A review of current concepts and practices. Int. J. Surg. 2017, 44, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Sambri, A.; Zucchini, R.; Giannini, C.; Donati, D.M.; De Paolis, M. Silver-coated megaprosthesis in prevention and treatment of peri-prosthetic infections: A systematic review and meta-analysis about efficacy and toxicity in primary and revision surgery. Eur. J. Orthop. Surg. Traumatol. 2021, 31, 201–220. [Google Scholar] [CrossRef]
- Berríos-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W.; Leas, B.; Stone, E.C.; Kelz, R.R.; Reinke, C.E.; Morgan, S.; Solomkin, J.S.; Mazuski, J.E.; et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection. JAMA Surg. 2017, 152, 784–791. [Google Scholar] [CrossRef]
- Barreto, R.; Barrois, B.; Lambert, J.; Malhotra-Kumar, S.; Santos-Fernandes, V.; Monstrey, S. Addressing the challenges in antisepsis: Focus on povidone–iodine. Int. J. Antimicrob. Agents 2020, 56, 106064. [Google Scholar] [CrossRef]
- Allegranzi, B.; Zayed, B.; Bischoff, P.; Kubilay, N.Z.; de Jonge, S.; de Vries, F.; Gomes, S.M.; Gans, S.; Wallert, E.D.; Wu, X.; et al. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: An evidence-based global perspective. Lancet Infect. Dis. 2016, 12, e288–e303. [Google Scholar] [CrossRef]
- Premkumar, A.; Nishtala, S.N.; Nguyen, J.T.; Bostrom, M.P.G.; Carli, A.V. The AAHKS Best Podium Presentation Research Award: Comparing the Efficacy of Irrigation Solutions on Staphylococcal Biofilm Formed on Arthroplasty Surfaces. Int. J. Arthroplast. 2021, 36, S26–S32. [Google Scholar] [CrossRef]
- Ulivieri, S.; Toninelli, S.; Petrini, C.; Giorgio, A.; Oliveri, G. Prevention of post-operative infections in spine surgery by wound irrigation with a solution of povidone–iodine and hydrogen peroxide. Arch. Orthop. Trauma Surg. 2011, 131, 1203–1206. [Google Scholar] [CrossRef]
- George, D.A.; Konan, S.; Haddad, F.S. Single-Stage Hip and Knee Exchange for Periprosthetic Joint Infection. J. Arthroplast. 2015, 30, 2264–2270. [Google Scholar] [CrossRef] [PubMed]
- Balato, G.; De Matteo, V.; De Franco, C.; Lenzi, M.; Verrazzo, R.; de Giovanni, R.; Smeraglia, F.; Rizzo, M.; Ascione, T. Prevention and treatment of peri-prosthetic joint infection using surgical wound irrigation. J. Biol. Regul. Homeost. Agents 2020, 34 (Suppl. S5), 917–923. [Google Scholar]
- Schaumburger, J.; Beckmann, J.; Springorum, H.-R.; Handel, M.; Anders, S.; Kalteis, T.; Grifka, J.; Rath, B. Toxizität lokaler Antiseptika auf Chondrozyten in vitro. Z. Orthop. Unfall. 2010, 148, 39–54. [Google Scholar] [CrossRef]
- William, L.; Sally, M.; David, S.; Richard, H. Cellular and Bacterial Toxicities of Topical Antimicrobials. Plast. Reconstr. Surg. 1985, 75, 394–396. [Google Scholar] [CrossRef]
- Gocke, D.J.; Ponticas, S.; Pollack, W. In vitro studies of the killing of clinical isolates by povidone–iodine solutions. J. Hosp. Infect. 1986, 6, 59–66. [Google Scholar] [CrossRef]
- Zamora, J.L. Chemical and microbiologic characteristics and toxicity of povidone–iodine solutions. Am. J. Surg. 1896, 151, 406. [Google Scholar] [CrossRef]
- Muller, G.; Kramer, A. Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J. Antimicrob. Chemother. 2008, 61, 1281–1287. [Google Scholar] [CrossRef] [Green Version]
- von Keudell, A.; Canseco, J.A.; Gomoll, A.H. Deleterious Effects of Diluted Povidone–Iodine on Articular Cartilage. J. Arthroplast. 2013, 28, 918–921. [Google Scholar] [CrossRef]
- Campbell, S.T.; Goodnough, L.H.; Bennett, C.G.; Giori, N.J. Antiseptics Commonly Used in Total Joint Arthroplasty Interact and May Form Toxic Products. J. Arthroplast. 2017, 10, S0883540317309403. [Google Scholar] [CrossRef]
- Heidari, M.; Tahmasebi, M.; Etemad, S.; Salehkhou, S.; Heidari-Vala, H.; Akhondi, M.M. In vitro Human Chondrocyte Culture; A Modified Protocol. Middle-East J. Sci. Res. 2011, 9, 102–109. [Google Scholar]
- Di Meglio, F.; Sacco, A.M.; Belviso, I.; Romano, V.; Sirico, F.; Loiacono, C.; Palermi, S.; Pempinello, C.; Montagnani, S.; Nurzynska, D.; et al. Influence of Supplements and Drugs used for the Treatment of Musculoskeletal Disorders on Adult Human Tendon-Derived Stem Cells. Muscles Ligaments I Tendons J. (MLTJ) 2020, 10, 376–384. [Google Scholar] [CrossRef]
- Akuji, M.A.; Chambers, D.J. Hydrogen peroxide: More harm than good? Br. J. Anaesth. 2017, 6, 958–959. [Google Scholar] [CrossRef] [Green Version]
- Van den Broek, P.J.; Buys, L.F.; Van Furth, R. Interaction of povidone–iodine compounds, phagocytic cells, and microorganisms. Antimicrob. Agents Chemother. 1982, 22, 593–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oosting, R.S.; van Bree, L.; van Iwaarden, J.F.; van Golde, L.M.; Verhoef, J. Impairment of phagocytic functions of alveolar macrophages by hydrogen peroxide. Am. J.-Physiol.-Lung Cell. Mol. Physiol. 1990, 259, L87–L94. [Google Scholar] [CrossRef] [PubMed]
- Busquets-Cortés, C.; Capó, X.; Argelich, E.; Ferrer, M.D.; Mateos, D.; Bouzas, C.; Abbate, M.; Tur, J.A.; Sureda, A.; Pons, A. Effects of Millimolar Steady-State Hydrogen Peroxide Exposure on Inflammatory and Redox Gene Expression in Immune Cells from Humans with Metabolic Syndrome. Nutrients 2018, 10, 1920. [Google Scholar] [CrossRef] [Green Version]
- Eming, A.; Smola-Hess, S.; Kurschat, P.; Hirche, D.; Krieg, T.; Smola, H. A novel property of povidon-iodine: Inhibition of excessive protease levels in chronic non-healing wounds. J. Investig. Dermatol. 2006, 126, 2731–2733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loo, A.E.; Halliwell, B. Effects of hydrogen peroxide in a keratinocyte-fibroblast co-culture model of wound healing. Biochem. Biophys. Res. Commun. 2012, 423, 253–258. [Google Scholar] [CrossRef] [PubMed]
| Solutions Tested | Materials 1 |
|---|---|
| Hydrogen Peroxide 0.5% | 417 mL NaCl 0.9%/83 mL HO 3% |
| Hydrogen Peroxide 1.5% | 250 mL NaCl 0.9%/250 mL HO 3% |
| Povidone–iodine 0.3% | 485 mL NaCl 0.9%/15 mL PI 10% |
| Povidone–iodine 5% | 250 mL NaCl 0.9%/250 mL PI 10% |
| Povidone–iodine 0.3%/Hydrogen Peroxide 0.5% | 15 mL PI 10%/83 mL HO 3%/402 mL NaCl 0.9% |
| Povidone–iodine 5%/Hydrogen Peroxide 1.5% | 250 mL PI 10%/250 mL HO 3% |
| Tested Solutions | 1 min | 3 min | 5 min | p Value |
|---|---|---|---|---|
| Hydrogen Peroxide 0.5% | 10.3 ± 3 | 31.6 ± 8.1 | 44.8 ± 10.5 | <0.001 |
| Hydrogen Peroxide 1.5% | 54.7 ± 1.7 | 63.7 ± 0.35 | 74.8 ± 1.6 | <0.001 |
| Povidone–iodine 0.3% | 18.8 ± 0.53 | 21.9 ± 0.63 | 20.7 ± 2.42 | 0.061 |
| Povidone–iodine 5% | 9.33 ± 1.2 | 31.5 ± 5.54 | 44.2 ± 4.53 | <0.001 |
| Povidone–iodine 0.3%/Hydrogen Peroxide 0.5% | 22.4 ± 1 | 27.9 ± 1.3 | 29.6 ± 1.35 | <0.001 |
| Povidone–iodine 5%/Hydrogen Peroxide 1.5% | 16.2 ± 1 | 31.9 ± 2.4 | 45.9 ± 3.1 | <0.001 |
| p value | <0.001 | <0.001 | <0.001 |
| Tested Solutions | 1 min | 3 min | 5 min | p Value |
|---|---|---|---|---|
| Hydrogen Peroxide 0.5% | 7.2 ± 3.4 | 23.6 ± 2.3 | 42.2 ± 1 | <0.001 |
| Hydrogen Peroxide 1.5% | 46.4 ± 1.6 | 54.8 ± 3.6 | 63.3 ± 2 | <0.001 |
| Povidone–iodine 0.3% | 15.8 ± 1.7 | 16.9 ± 2.3 | 18.9 ± 1.7 | 0.214 |
| Povidone–iodine 5% | 7.6 ± 1 | 20.4 ± 2 | 29.3 ± 1.8 | <0.001 |
| Povidone–iodine 0.3%/Hydrogen Peroxide 0.5% | 20.9 ± 1 | 23.3 ± 2 | 25.6 ± 1.7 | 0.034 |
| Povidone–iodine 5%/Hydrogen Peroxide 1.5% | 13.6 ± 1.4 | 26.4 ± 2 | 36.2 ± 3.1 | <0.001 |
| p value | <0.001 | <0.001 | <0.001 |
| Tested Solutions | 1 min | 3 min | 5 min | p Value |
|---|---|---|---|---|
| Hydrogen Peroxide 0.5% | 8.0 ± 0.8 | 24.7 ± 1.3 | 45.6 ± 1 | <0.001 |
| Hydrogen Peroxide 1.5% | 52.7 ± 2.6 | 54.4 ± 2.5 | 60.4 ± 4.9 | <0.001 |
| Povidone–iodine 0.3% | 16.4 ± 0.3 | 18.9 ± 0.5 | 20.0 ± 0.7 | <0.001 |
| Povidone–iodine 5% | 9.8 ± 1 | 31.3 ± 1.3 | 38.7 ± 1.3 | <0.001 |
| Povidone–iodine 0.3%/Hydrogen Peroxide 0.5% | 20.0 ± 1.8 | 21.8 ± 1.4 | 25.1 ± 1 | 0.012 |
| Povidone–iodine 5%/Hydrogen Peroxide 1.5% | 14.0 ± 1.3 | 25.1 ± 1 | 31.6 ± 1.7 | <0.001 |
| p value | <0.001 | <0.001 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, V.; Di Gennaro, D.; Sacco, A.M.; Festa, E.; Roscetto, E.; Basso, M.A.; Ascione, T.; Balato, G. Cell Toxicity Study of Antiseptic Solutions Containing Povidone–Iodine and Hydrogen Peroxide. Diagnostics 2022, 12, 2021. https://doi.org/10.3390/diagnostics12082021
Romano V, Di Gennaro D, Sacco AM, Festa E, Roscetto E, Basso MA, Ascione T, Balato G. Cell Toxicity Study of Antiseptic Solutions Containing Povidone–Iodine and Hydrogen Peroxide. Diagnostics. 2022; 12(8):2021. https://doi.org/10.3390/diagnostics12082021
Chicago/Turabian StyleRomano, Veronica, Donato Di Gennaro, Anna Maria Sacco, Enrico Festa, Emanuela Roscetto, Morena Anna Basso, Tiziana Ascione, and Giovanni Balato. 2022. "Cell Toxicity Study of Antiseptic Solutions Containing Povidone–Iodine and Hydrogen Peroxide" Diagnostics 12, no. 8: 2021. https://doi.org/10.3390/diagnostics12082021
APA StyleRomano, V., Di Gennaro, D., Sacco, A. M., Festa, E., Roscetto, E., Basso, M. A., Ascione, T., & Balato, G. (2022). Cell Toxicity Study of Antiseptic Solutions Containing Povidone–Iodine and Hydrogen Peroxide. Diagnostics, 12(8), 2021. https://doi.org/10.3390/diagnostics12082021










