Synchronous Periprosthetic Joint Infections: A Scoping Review of the Literature
Abstract
:1. Introduction
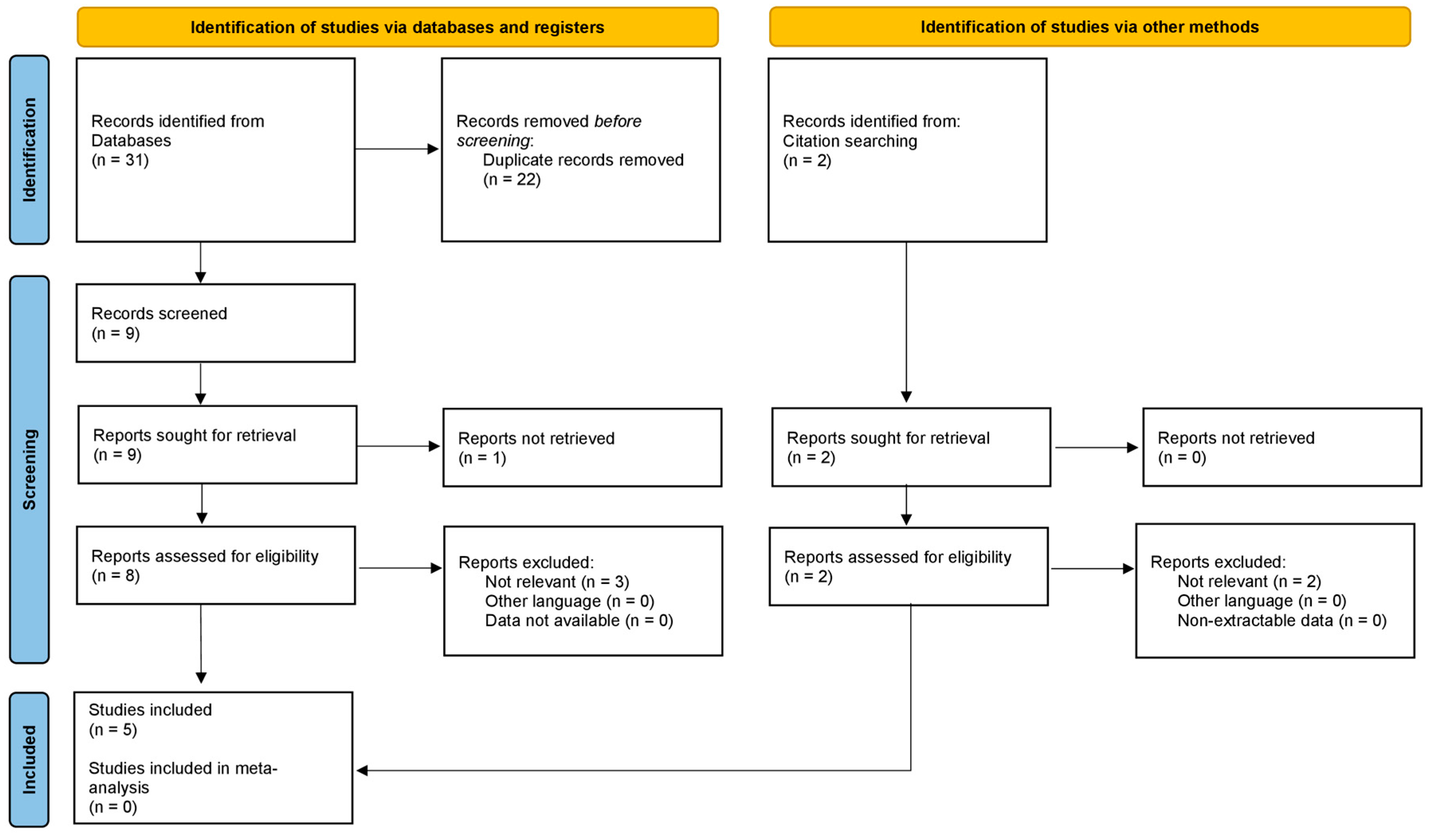
2. Materials and Methods
3. Results and Discussion
3.1. Epidemiology
3.2. Risk Factors
3.3. Diagnosis
3.4. Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Springer, B.D.; Cahue, S.; Etkin, C.D.; Lewallen, D.G.; McGrory, B.J. Infection burden in total hip and knee arthroplasties: An international registry-based perspective. Arthroplast. Today 2017, 3, 137–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, R.P.; Bourne, M.H.; Fitzgerald, R.H. Metachronous infections in patients who have had more than one total joint arthroplasty. J. Bone Jt. Surg. 1991, 73, 1469–1474. [Google Scholar] [CrossRef]
- Pina, M.; Gaukhman, A.D.; Hayden, B.; Smith, E.L. Three Concurrent Periprosthetic Joint Infections: A Case Report and Literature Review. Hip Pelvis 2019, 31, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Zeller, V.; Dedome, D.; Lhotellier, L.; Graff, W.; Desplaces, N.; Marmor, S. Concomitant multiple joint arthroplasty infections: Report on 16 cases. J. Arthroplast. 2016, 31, 2564–2568. [Google Scholar] [CrossRef]
- Thiesen, D.M.; Mumin-Gündüz, S.; Gehrke, T.; Klaber, I.; Salber, J.; Suero, E.; Mustafa, C. Synchronous periprosthetic joint infections: The need for all artificial joints to Be aspirated routinely. J. Bone Jt. Surg. 2020, 102, 283–291. [Google Scholar] [CrossRef]
- Gausden, E.B.; Pagnano, M.W.; Perry, K.I.; Suh, G.A.; Berry, D.J.; Abdel, M.P. Synchronous Periprosthetic Joint Infections: High Mortality, Reinfection, and Reoperation. J. Arthroplast. 2021, 36, 3556–3561. [Google Scholar] [CrossRef]
- Komnos, G.A.; Manrique, J.; Goswami, K.; Tan, T.L.; Restrepo, C.; Sherman, M.B.; Parvizi, J. Periprosthetic joint infection in patients who have multiple prostheses in place: What should Be done with the silent prosthetic joints. J. Bone Jt. Surg. 2020, 102, 1160–1168. [Google Scholar] [CrossRef]
- Abblitt, W.P.; Chan, E.W.; Shinar, A.A. Risk of Periprosthetic Joint Infection in Patients with Multiple Arthroplasties. J. Arthroplast. 2018, 33, 840–843. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Jafari, S.M.; Casper, D.S.; Restrepo, C.; Zmistowski, B.; Parvizi, J.; Sharkey, P.F. Periprosthetic joint infection: Are patients with multiple prosthetic joints at risk? J. Arthroplast. 2012, 27, 877–880. [Google Scholar] [CrossRef]
- Luessenhop, C.P.; Higgins, L.D.; Brause, B.D.; Ranawat, C.S. Multiple prosthetic infections after total joint arthroplasty. Risk factor analysis. J. Arthroplast. 1996, 11, 862–868. [Google Scholar] [CrossRef]
- Haverstock, J.P.; Somerville, L.E.; Naudie, D.D.; Howard, J.L. Multiple Periprosthetic Joint Infections: Evidence for Decreasing Prevalence. J. Arthroplast. 2016, 31, 2862–2866. [Google Scholar] [CrossRef]
- Unter Ecker, N.; Suero, E.M.; Gehrke, T.; Haasper, C.; Zahar, A.; Lausmann, C.; Hawi, N.; Citak, M. Serum C-reactive protein relationship in high- versus low-virulence pathogens in the diagnosis of periprosthetic joint infection. J. Med. Microbiol. 2019, 68, 910–917. [Google Scholar] [CrossRef]
- Gundtoft, P.H.; Pedersen, A.B.; Varnum, C.; Overgaard, S. Increased mortality after prosthetic joint infection in primary THA. Clin. Orthop. Relat. Res. 2017, 475, 2623–2631. [Google Scholar] [CrossRef] [Green Version]
- Natsuhara, K.M.; Shelton, T.J.; Meehan, J.P.; Lum, Z.C. Mortality during total hip periprosthetic joint infection. J. Arthroplast. 2019, 34, S337–S342. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, F.; Chen, W.; Liu, S.; Zhang, Q.; Zhang, Y. Risk factors for periprosthetic joint infection after total joint arthroplasty: A systematic review and meta-analysis. J. Hosp. Infect. 2015, 89, 82–89. [Google Scholar] [CrossRef]
- Kong, L.; Cao, J.; Zhang, Y.; Ding, W.; Shen, Y. Risk factors for periprosthetic joint infection following primary total hip or knee arthroplasty: A meta-analysis. Int. Wound J. 2017, 14, 529–536. [Google Scholar] [CrossRef]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J. Arthroplast. 2018, 33, 1309–1314.e2. [Google Scholar] [CrossRef]
- McNally, M.; Sousa, R.; Wouthuyzen-Bakker, M.; Chen, A.; Soriano, A.; Vogely, C.; Clauss, M.; Higuera, C.; Trebše, R. The EBJIS definition of periprosthetic joint infection. Bone Jt. J 2021, 103-B, 18–25. [Google Scholar] [CrossRef]
- Luthringer, T.A.; Fillingham, Y.A.; Okroj, K.; Ward, E.J.; Della Valle, C. Periprosthetic Joint Infection After Hip and Knee Arthroplasty: A Review for Emergency Care Providers. Ann. Emerg. Med. 2016, 68, 324–334. [Google Scholar] [CrossRef] [Green Version]
- Ting, N.T.; Della Valle, C.J. Diagnosis of Periprosthetic Joint Infection-An Algorithm-Based Approach. J. Arthroplast. 2017, 32, 2047–2050. [Google Scholar] [CrossRef]
- Parvizi, J.; Erkocak, O.F.; Della Valle, C.J. Culture-negative periprosthetic joint infection. J. Bone Jt. Surg. 2014, 96, 430–436. [Google Scholar] [CrossRef]
- Sambri, A.; Spinnato, P.; Tedeschi, S.; Zamparini, E.; Fiore, M.; Zucchini, R.; Giannini, C.; Caldari, E.; Crombé, A.; Viale, P.; et al. Bone and Joint Infections: The Role of Imaging in Tailoring Diagnosis to Improve Patients’ Care. J. Pers. Med. 2021, 11, 1317. [Google Scholar] [CrossRef]
- Wouthuyzen-Bakker, M.; Sebillotte, M.; Arvieux, C.; Fernandez-Sampedro, M.; Senneville, E.; Barbero, J.M.; Lora-Tamayo, J.; Aboltins, C.; Trebse, R.; Salles, M.J.; et al. How to Handle Concomitant Asymptomatic Prosthetic Joints During an Episode of Hematogenous Periprosthetic Joint Infection, a Multicenter Analysis. Clin. Infect. Dis. 2021, 73, e3820–e3824. [Google Scholar] [CrossRef]
- Karczewski, D.; Winkler, T.; Renz, N.; Trampuz, A.; Lieb, E.; Perka, C.; Müller, M. A standardized interdisciplinary algorithm for the treatment of prosthetic joint infections. Bone Jt. J. 2019, 101-B, 132–139, Erratum in Bone Jt. J. 2019, 101-B, 1032. [Google Scholar] [CrossRef]
- Sambri, A.; Fiore, M.; Tedeschi, S.; De Paolis, M. The Need for Multidisciplinarity in Modern Medicine: An Insight into Orthopaedic Infections. Microorganisms 2022, 10, 756. [Google Scholar] [CrossRef]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R. Infectious Diseases Society of America. Diagnosis and management of prosthetic joint infection: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, e1–e25. [Google Scholar] [CrossRef] [Green Version]
- Zeller, V.; Lhotellier, L.; Marmor, S.; Leclerc, P.; Krain, A.; Graff, W.; Ducroquet, F.; Biau, D.; Leonard, P.; Desplaces, N.; et al. One-stage exchange arthroplasty for chronic periprosthetic hip infection: Results of a large prospective cohort study. J. Bone Jt. Surg. 2014, 96, e1. [Google Scholar] [CrossRef] [PubMed]
- Charette, R.S.; Melnic, C.M. Two-Stage Revision Arthroplasty for the Treatment of Prosthetic Joint Infection. Curr. Rev. Musculoskelet. Med. 2018, 11, 332–340. [Google Scholar] [CrossRef] [PubMed]
| Study | Zeller et al. [4] | Gausden et al. [5] | Thiensen et al. [6] | Komnos et al. [7] | Abblitt et al. [8] |
|---|---|---|---|---|---|
| Q1: was the hypothesis/aim/objective of the study clearly stated? | yes | yes | yes | yes | yes |
| Q2: was the study conducted prospectively? | no | no | no | no | no |
| Q3: were the cases collected in more than one centre? | no | no | no | no | no |
| Q4: were patients recruited consecutively? | yes | yes | yes | yes | yes |
| Q5: were the characteristics of the patients included in the study described? | yes | yes | yes | yes | yes |
| Q6: were the eligibility criteria (i.e., inclusion and exclusion criteria) for entry into the study clearly stated? | yes | yes | yes | yes | yes |
| Q7: did patients enter the study at a similar point in the disease? | yes | yes | yes | yes | yes |
| Q8: was the intervention of interest clearly described? | yes | yes | yes | yes | yes |
| Q9: were additional interventions (co-interventions) clearly described? | no | no | no | no | no |
| Q10: were relevant outcome measures established a priori? | yes | yes | yes | yes | yes |
| Q11: were outcome assessors blinded to the intervention that patients received? | no | no | no | no | no |
| Q12: were the relevant outcomes measured using appropriate objective/subjective methods? | yes | yes | yes | yes | yes |
| Q13: were the relevant outcome measures made before and after the intervention? | no | no | no | no | no |
| Q14: were the statistical tests used to assess the relevant outcomes appropriate? | no | yes | yes | yes | yes |
| Q15: was follow-up long enough for important events and outcomes to occur? | yes | yes | yes | yes | yes |
| Q16: were losses to follow-up reported? | yes | no | no | no | no |
| Q17: did the study provided estimates of random variability in the data analysis of relevant outcomes? | no | yes | yes | yes | no |
| Q18: were the adverse events reported? | yes | yes | yes | no | no |
| Q19: were the conclusions of the study supported by results? | yes | yes | yes | yes | yes |
| Q20: were both competing interests and sources of support for the study reported? | no | no | yes | yes | yes |
| TOTAL (yes/no/unclear) | 12/8/0 | 13/7/0 | 14/6/0 | 13/7/0 | 12/8/0 |
| Study | Patients with Multiple Arthroplasties (n°) | Synchronous PJI (n°) | Percent |
|---|---|---|---|
| Zeller et al. [4] | 1185 | 16 | 1.4% |
| Gausden et al. [5] | 2671 | 34 | 1.3% |
| Thiensen et al. [6] | 644 | 26 | 4% |
| Komnos et al. [7] | 197 | 11 | 5% |
| Abblitt et al. [8] | 76 | 4 | 5% |
| Study | Joints Involved |
|---|---|
| Zeller et al. [4] | 8 bilateral THA, 3 bilateral TKA, 4 TKA and THA, 1 bilateral TKA + THA + toe arthroplasty |
| Gausden et al. [5] | 27 bilateral TKA, 3 bilateral THA, 1 TKA + TSA, 1 TKA + TEA, 1 bilateral THA + TKA |
| Thiensen et al. [6] | 20 THA, 15 TKA, 7 TSA |
| Komnos et al. [7] | 19 THA, 4 TKA |
| Abblitt et al. [8] | 3 bilateral TKA, 1 THA + TKA |
| Study | S. aureus (n°) | S. epidermidis (n°) | Streptococcus spp. (n°) | E. coli (n°) | P. mirabilis (n°) | N. meningitidis (n°) | Enterococcus spp. (n°) | R. Ornithinolytica (n°) | M. Chelonae (n°) | Unknown (n°) |
|---|---|---|---|---|---|---|---|---|---|---|
| Zeller et al. [4] | 8 (50%) | 1 (6%) | 6 (38%) | 1 (6%) | / | / | / | / | / | / |
| Gausden et al. [5] | 12 (35%) | 1 (3%) | 4 (12%) | 1 (3%) | 1 (3%) | 1 (3%) | / | 1 (3%) | 1 (3%) | 12 (35%) |
| Thiensen et al. [6] | 5 (19.2%) | 9 (34.6%) | 2 (7.7%) | 2 (7.7%) | / | / | 3 (11.5%) | / | / | 4 (11.5%) |
| Komnos et al. [7] | 4 (36%) | 3 (27%) | 1 (9%) | 1 (9%) | / | / | / | / | / | 2 (18%) |
| Abblitt et al. [8] | 3 (75%) | / | 1 (25%) | / | / | / | / | / | / | / |
| Study | Risk Factors for Synchronous PJI |
|---|---|
| Zeller et al. [4] | Staphylococcal or streptococcal bacteremia |
| Gausden et al. [5] | Bacteremia |
| Thiensen et al. [6] | 3 or more prosthetic joints, rheumatoid arthritis, neoplasia, immune-modulating therapy, bacteremia, sepsis |
| Komnos et al. [7] | Bacteremia |
| Abblitt et al. [8] | Bacteremia |
| Jafari et al. [10] | Immunosuppression |
| Luessenhop et al. [11] | Rheumatoid arthritis |
| Haverstock et al. [12] | Bacteremia |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sambri, A.; Caldari, E.; Fiore, M.; Giannini, C.; Filippini, M.; Morante, L.; Rondinella, C.; Zamparini, E.; Tedeschi, S.; Viale, P.; et al. Synchronous Periprosthetic Joint Infections: A Scoping Review of the Literature. Diagnostics 2022, 12, 1841. https://doi.org/10.3390/diagnostics12081841
Sambri A, Caldari E, Fiore M, Giannini C, Filippini M, Morante L, Rondinella C, Zamparini E, Tedeschi S, Viale P, et al. Synchronous Periprosthetic Joint Infections: A Scoping Review of the Literature. Diagnostics. 2022; 12(8):1841. https://doi.org/10.3390/diagnostics12081841
Chicago/Turabian StyleSambri, Andrea, Emilia Caldari, Michele Fiore, Claudio Giannini, Matteo Filippini, Lorenzo Morante, Claudia Rondinella, Eleonora Zamparini, Sara Tedeschi, Pierluigi Viale, and et al. 2022. "Synchronous Periprosthetic Joint Infections: A Scoping Review of the Literature" Diagnostics 12, no. 8: 1841. https://doi.org/10.3390/diagnostics12081841
APA StyleSambri, A., Caldari, E., Fiore, M., Giannini, C., Filippini, M., Morante, L., Rondinella, C., Zamparini, E., Tedeschi, S., Viale, P., & De Paolis, M. (2022). Synchronous Periprosthetic Joint Infections: A Scoping Review of the Literature. Diagnostics, 12(8), 1841. https://doi.org/10.3390/diagnostics12081841







