Diagnostic Performance of Neutrophil to Lymphocyte Ratio, Monocyte to Lymphocyte Ratio, Platelet to Lymphocyte Ratio, and Platelet to Mean Platelet Volume Ratio in Periprosthetic Hip and Knee Infections: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
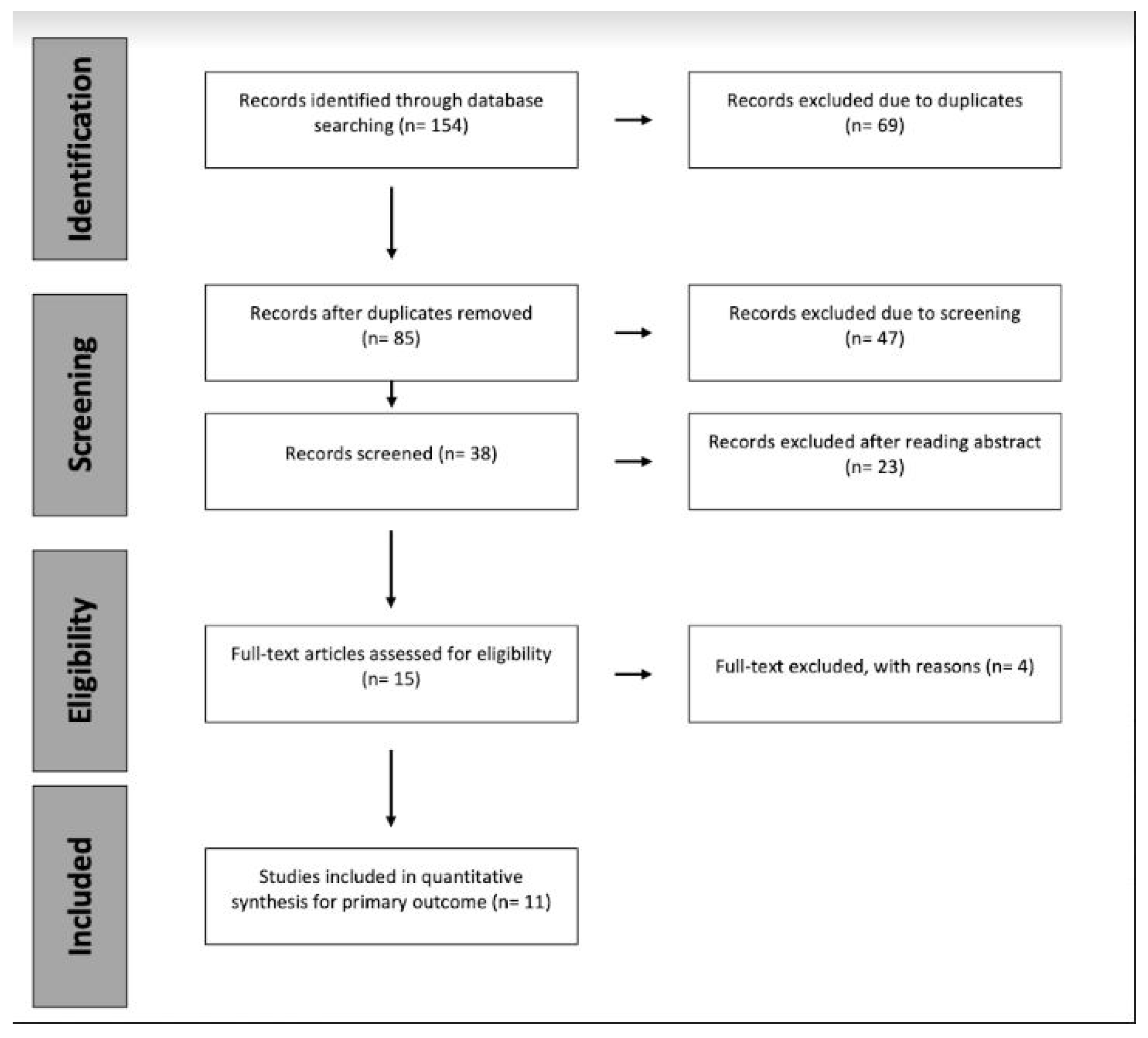
2. Materials and Methods
2.1. Search Strategy and Criteria
2.2. Study Assessment and Data Extraction
2.3. Statistical Analysis
3. Results
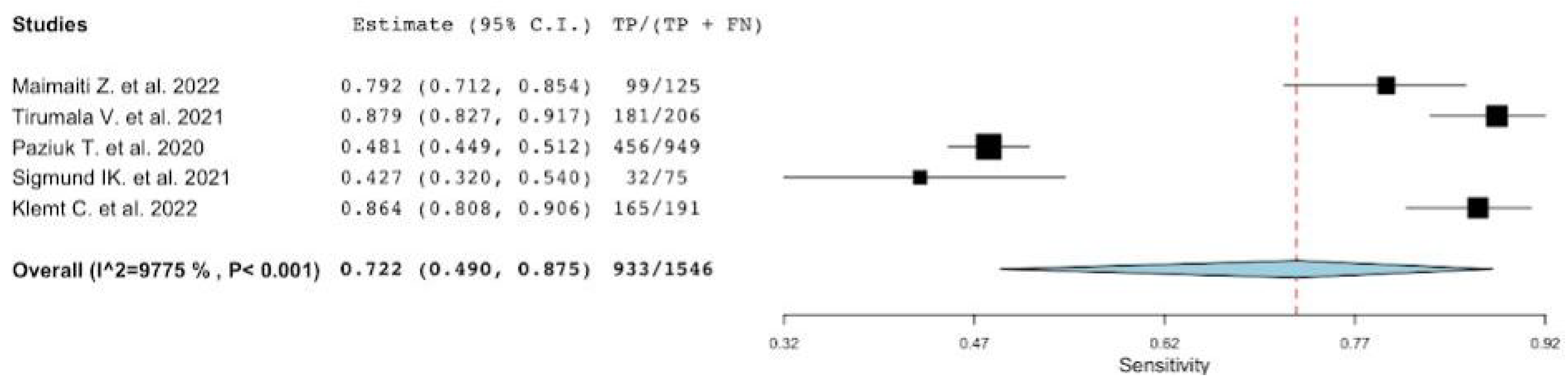
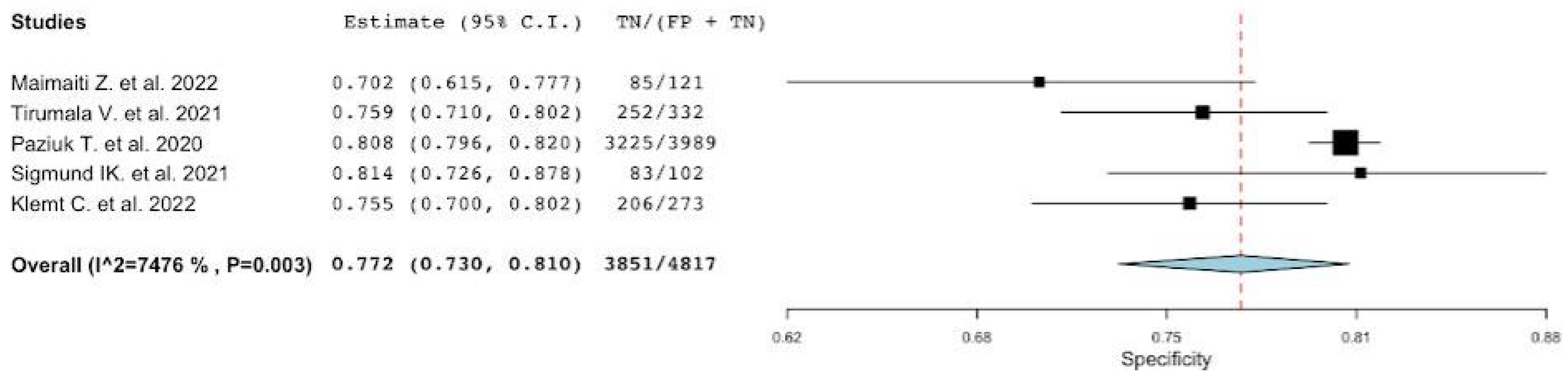
3.1. Diagnostic Accuracy of MLR
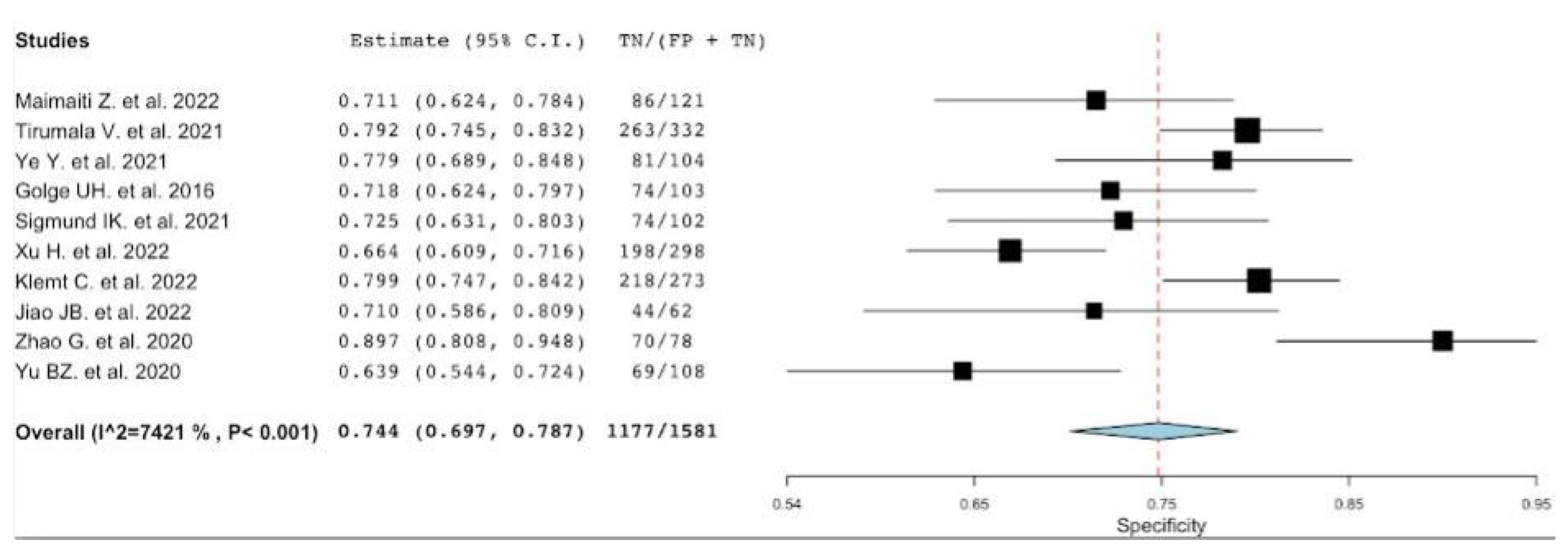
3.2. Diagnostic Accuracy of NLR
3.3. Diagnostic Accuracy of PVR
3.4. Diagnostic Accuracy of PLR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MLR | Monocyte to Lymphocyte ratio |
| NLR | Neutrophil to Lymphocyte ratio |
| PVR | Platelet to Mean Platelet Volume ratio |
| PLR | Platelet to Lymphocyte ratio |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| AUC | Area Under the Curve |
| PJI | Periprosthetic joint infection |
| DOR | Diagnostic odds ratio |
| QUADAS | Quality assessment tool for diagnostic accuracy studies |
| ICM | International Consensus on Musculoskeletal Infection |
| MSIS | MusculoSkeletal Infection Society |
| EBJS | The European Bone and Joint Infection Society |
References
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-joint infections. N. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J. Arthroplast. 2018, 5, 1309–1314.e2. [Google Scholar] [CrossRef] [PubMed]
- Alijanipour, P.; Bakhshi, H.; Parvizi, J. Diagnosis of periprosthetic joint infection: The threshold for serological markers. Clin. Orthop. Relat. Res. 2013, 471, 3186. [Google Scholar] [CrossRef]
- Berbari, E.; Mabry, T.; Tsaras, G.; Spangehl, M.; Erwin, P.J.; Murad, M.H.; Steckelberg, J.; Osmon, D. Inflammatory blood laboratory levels as markers of prosthetic joint infection: A systematic review and meta-analysis. J. Bone Jt. Surg. 2010, 92, 2102–2109. [Google Scholar] [CrossRef] [PubMed]
- Dugdale, E.M.; Uvodich, M.E.; Osmon, D.R.; Pagnano, M.W.; Berry, D.J.; Abdel, M.P. Laboratory Value Effectiveness in Predicting Early Postoperative Periprosthetic Joint Infection After Total Hip Arthroplasty. J. Arthroplast. 2022, 37, 574–580. [Google Scholar] [CrossRef]
- Pannu, T.S.; Villa, J.M.; Riesgo, A.M.; Patel, P.D.; Barsoum, W.K.; Higuera, C.A. Serum D-Dimer in the Diagnosis of Periprosthetic Knee Infection: Where Are We Today? J. Knee Surg. 2020, 33, 106–110. [Google Scholar] [CrossRef]
- Shahi, A.; Kheir, M.M.; Tarabichi, M.; Hosseinzadeh, H.R.S.; Tan, T.L.; Parvizi, J. Serum D Dimer Test Is Promising for the Diagnosis of Periprosthetic Joint Infection and Timing of Reimplantation. J. Bone Jt. Surg. 2017, 99, 1419–1427. [Google Scholar] [CrossRef]
- Balato, G.; De Franco, C.; Balboni, F.; De Matteo, V.; Ascione, T.; Baldini, A.; Lippi, G. The role of D-dimer in periprosthetic joint infection: A systematic review and meta-analysis. Diagnosis 2021, 9, 3–10. [Google Scholar] [CrossRef]
- Tischler, E.H.; Cavanaugh, P.K.; Parvizi, J. Leukocyte Esterase Strip Test: Matched for Musculoskeletal Infection Society Criteria. J. Bone Jt. Surg. 2014, 96, 1917–1920. [Google Scholar] [CrossRef]
- Wyatt, M.C.; Beswick, A.D.; Kunutsor, S.K.; Wilson, M.J.; Whitehouse, M.R.; Blom, A.W. The Alpha-Defensin Immunoassay and Leukocyte Esterase Colorimetric Strip Test for the Diagnosis of Periprosthetic Infection: A Systematic Review and Meta-Analysis. J. Bone Jt. Surg. 2016, 98, 992. [Google Scholar] [CrossRef]
- Wang, C.; Li, R.; Wang, Q.; Duan, J.; Wang, C. Leukocyte Esterase as a Biomarker in the Diagnosis of Periprosthetic Joint Infection. Med. Sci. Monit. 2017, 23, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, R.; Wang, Q.; Wang, C. Synovial Fluid Leukocyte Esterase in the Diagnosis of Peri-Prosthetic Joint Infection: A Systematic Review and Meta-Analysis. Surg. Infect. 2018, 19, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Deirmengian, C.; Kardos, K.; Kilmartin, P.; Cameron, A.; Schiller, K.; Parvizi, J. Combined measurement of synovial fluid α-Defensin and C-reactive protein levels: Highly accurate for diagnosing periprosthetic joint infection. J. Bone Jt. Surg. 2014, 96, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Felstead, A.; Kundasamy, P.; Penfold, G.; Whiting, K.; Buck, J.; Sturridge, S.; Meda, M. The combined measurement of synovial markers in the diagnosis of periprosthetic joint infection. Ann. R. Coll. Surg. Engl. 2022, 104, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Sigmund, I.K.; Holinka, J.; Gamper, J.; Staats, K.; Böhler, C.; Kubista, B.; Windhager, R. Qualitative α-defensin test (Synovasure) for the diagnosis of periprosthetic infection in revision total joint arthroplasty. Bone Jt. J. 2017, 99-B, 66–72. [Google Scholar] [CrossRef]
- Bonanzinga, T.; Ferrari, M.C.; Tanzi, P.; Vandenbulcke, F.; Zahar, A.; Marcacci, M. The role of alpha defensin in prosthetic joint infection (PJI) diagnosis: A literature review. EFORT Open Rev. 2019, 4, 10–13. [Google Scholar] [CrossRef]
- Balato, G.; Franceschini, V.; Ascione, T.; Lamberti, A.; D’Amato, M.; Ensini, A.; Baldini, A. High performance of α-defensin lateral flow assay (Synovasure) in the diagnosis of chronic knee prosthetic infections. Knee Surg. Sport. Traumatol. Arthrosc. 2017, 26, 1717–1722. [Google Scholar] [CrossRef]
- Balato, G.; de Matteo, V.; Ascione, T.; Di Donato, S.L.; De Franco, C.; Smeraglia, F.; Baldini, A.; Mariconda, M. Laboratory-based versus qualitative assessment of α-defensin in periprosthetic hip and knee infections: A systematic review and meta-analysis. Arch. Orthop. Trauma. Surg. 2019, 140, 293–301. [Google Scholar] [CrossRef]
- Ascione, T.; Balato, G.; Mariconda, M.; Rotondo, R.; Baldini, A.; Pagliano, P. Continuous Antibiotic Therapy Can Reduce Recurrence of Prosthetic Joint Infection in Patients Undergoing 2-Stage Exchange. J. Arthroplast. 2018, 34, 704–709. [Google Scholar] [CrossRef]
- Ascione, T.; Balato, G.; Mariconda, M.; Smeraglia, F.; Baldini, A.; De Franco, C.; Pandolfo, G.; Siciliano, R.; Pagliano, P. Synovial Cell Count Before Reimplantation Can Predict the Outcome of Patients with Periprosthetic Knee Infections Undergoing Two-stage Exchange. Clin. Orthop. Relat. Res. 2021, 479, 2061–2068. [Google Scholar] [CrossRef]
- Abdelaziz, H.; Aljawabra, A.; Rossmann, M.; Tien, C.S.; Citak, M.; Klatte, T.O.; Gehrke, T. What Is the Impact of Automated Synovial Cell Counting on Different Aseptic Causes and Periprosthetic Conditions Associated with Revision THA? Clin. Orthop. Relat. Res. 2022, 480, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Tirumala, V.; Klemt, C.; Xiong, L.; Chen, W.; Kieboom, J.V.D.; Kwon, Y.-M. Diagnostic Utility of Platelet Count/Lymphocyte Count Ratio and Platelet Count/Mean Platelet Volume Ratio in Periprosthetic Joint Infection Following Total Knee Arthroplasty. J. Arthroplast. 2020, 36, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Paziuk, T.; Rondon, A.J.; Goswami, K.; Tan, T.L.; Parvizi, J. A Novel Adjunct Indicator of Periprosthetic Joint Infection: Platelet Count and Mean Platelet Volume. J. Arthroplast. 2020, 35, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xie, J.-W.; Liu, L.; Wang, D.; Huang, Z.-Y.; Zhou, Z.-K. Combination of CRP with NLR is a sensitive tool for screening fixation-related infection in patients undergoing conversion total hip arthroplasty after failed internal fixation for femoral neck fracture. Bone Jt. J. 2021, 103-B, 1534–1540. [Google Scholar] [CrossRef]
- Gao, K.; Zhu, W.; Liu, W.; Ma, D.; Li, H.; Yu, W.; Wang, L.; Cao, Y.; Jiang, Y. Diagnostic value of the blood monocyte-lymphocyte ratio in knee osteoarthritis. J. Int. Med. Res. 2019, 47, 4413–4421. [Google Scholar] [CrossRef]
- Djordjevic, D.; Rondovic, G.; Surbatovic, M.; Stanojevic, I.; Udovicic, I.; Andjelic, T.; Zeba, S.; Milosavljevic, S.; Stankovic, N.; Abazovic, D.; et al. Neutrophil-to-Lymphocyte Ratio, Monocyte-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio, and Mean Platelet Volume-to-Platelet Count Ratio as Biomarkers in Critically Ill and Injured Patients: Which Ratio to Choose to Predict Outcome and Nature of Bacteremia? Mediat. Inflamm. 2018, 2018, 3758068. [Google Scholar]
- Klinger, M.H.; Jelkmann, W. Review: Role of Blood Platelets in Infection and Inflammation. J. Interf. Cytokine Res. 2002, 22, 913–922. [Google Scholar] [CrossRef]
- Robbins, G.; Barnard, D.L. Mean platelet volume changes in infection. J. Clin. Pathol. 1983, 36, 1320. [Google Scholar] [CrossRef]
- Paziuk, T.; Korniluk, A.; Koper-Lenkiewicz, O.M.; Kamińska, J.; Kemona, H.; Dymicka-Piekarska, V. Mean Platelet Volume (MPV): New Perspectives for an Old Marker in the Course and Prognosis of Inflammatory Conditions. Mediat. Inflamm. 2019, 2019, 9213074. [Google Scholar] [CrossRef]
- Van der Lelie, J.; Von dem Borne, A.K. Increased mean platelet volume in septicaemia. J. Clin. Pathol. 1983, 36, 693. [Google Scholar] [CrossRef]
- Warny, M.; Helby, J.; Nordestgaard, B.G.; Birgens, H.; Bojesen, S.E. Lymphopenia and risk of infection and infection-related death in 98,344 individuals from a prospective Danish population-based study. PLoS Med. 2018, 15, e1002685. [Google Scholar] [CrossRef] [PubMed]
- Stojkovic Lalosevic, M.; Pavlovic Markovic, A.; Stankovic, S.; Stojkovic, M.; Dimitrijevic, I.; Radoman Vujacic, I.; Lalic, D.; Milovanovic, T.; Dumic, I.; Krivokapic, Z. Combined Diagnostic Efficacy of Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), and Mean Platelet Volume (MPV) as Biomarkers of Systemic Inflammation in the Diagnosis of Colorectal Cancer. Dis. Markers 2019, 2019, 6036979. [Google Scholar] [CrossRef]
- Qin, B.; Ma, N.; Tang, Q.; Wei, T.; Yang, M.; Fu, H.; Hu, Z.; Liang, Y.; Yang, Z.; Zhong, R. Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) were useful markers in assessment of inflammatory response and disease activity in SLE patients. Mod. Rheumatol. 2016, 26, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; He, L.; Zhang, G.; Yu, J.; Chen, Y.; Yin, H.; Goyal, H.; Zhang, G.-M.; Xiao, Y.; Gu, C.; et al. Normal Reference Intervals of Neutrophil-To-Lymphocyte Ratio, Platelet-To-Lymphocyte Ratio, Lymphocyte-To-Monocyte Ratio, and Systemic Immune Inflammation Index in Healthy Adults: A Large Multi-Center Study from Western China. Clin. Lab. 2019, 65, 3. [Google Scholar] [CrossRef] [PubMed]
- Asik, Z. The Role of the NLR and PLR in Urinary Tract Infection. Clin. Lab. 2021, 67, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, J.; Wang, J.; Xie, T.; Zhang, Q.; Feng, S.; Deng, H.; Zhong, B. Platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte ratio (NLR) are associated with chronic hepatitis B virus (HBV) infection. Int. Immunopharmacol. 2017, 51, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.D.; Parajuli, A.; Gale, H.J.; Bulteel, N.S.; Schuetz, P.; de Jager, C.P.; Loonen, A.J.; Merekoulias, G.I.; Baillie, J.K. The utility of peripheral blood leucocyte ratios as biomarkers in infectious diseases: A systematic review and meta-analysis. J. Infect. 2019, 78, 339–348. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Reitsma, J.B.; Glas, A.S.; Rutjes, A.W.; Scholten, R.J.; Bossuyt, P.M.; Zwinderman, A.H. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J. Clin. Epidemiol. 2005, 58, 982–990. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef] [PubMed]
- Maimaiti, Z.; Xu, C.; Fu, J.; Chai, W.; Zhou, Y.; Chen, J. The Potential Value of Monocyte to Lymphocyte Ratio, Platelet to Mean Platelet Volume Ratio in the Diagnosis of Periprosthetic Joint Infections. Orthop. Surg. 2021, 14, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Chen, W.; Gu, M.; Liu, Q.; Xian, G.; Pan, B.; Zheng, L.; Chen, X.; Zhang, Z.; Sheng, P. Limited value of serum neutrophil-to-lymphocyte ratio in the diagnosis of chronic periprosthetic joint infection. J. Orthop. Traumatol. 2021, 22, 37. [Google Scholar] [CrossRef] [PubMed]
- Golge, U.; Kaymaz, B.; Pazarci, Ö.; Kilinc, S.; Öztemür, Z.; Bulut, O. Neutrophil to Lymphocyte Ratio May Be a Diagnostic Marker for Prosthetic Joint Infection. J. Clin. Anal. Med. 2016, 7, 218–221. [Google Scholar]
- Sigmund, I.K.; Holinka, J.; Staats, K.; Sevelda, F.; Lass, R.; Kubista, B.; Giurea, A.; Windhager, R. Inferior performance of established and novel serum inflammatory markers in diagnosing periprosthetic joint infections. Int. Orthop. 2020, 45, 837–846. [Google Scholar] [CrossRef]
- Xu, H.; Xie, J.; Zhang, S.; Wang, D.; Huang, Z.; Zhou, Z. Potential Blood Biomarkers for Diagnosing Periprosthetic Joint Infection: A Single-Center, Retrospective Study. Antibiotics 2022, 11, 505. [Google Scholar] [CrossRef]
- Klemt, C.; Tirumala, V.; Smith, E.J.; Xiong, L.; Kwon, Y.-M. Complete blood platelet and lymphocyte ratios increase diagnostic accuracy of periprosthetic joint infection following total hip arthroplasty. Arch. Orthop. Trauma. Surg. 2022, 1–9. [Google Scholar] [CrossRef]
- Jiao, J.-B.; Huang, J.-C.; Chen, X.; Jin, Y. Albumin to Globulin ratio, Neutrophil to Lymphocyte ratio, and Globulin levels do not outperform ESR or CRP when diagnosing periprosthetic joint infection. BMC Musculoskelet. Disord. 2022, 23, 404. [Google Scholar] [CrossRef]
- Zhao, G.; Chen, J.; Wang, J.; Wang, S.; Xia, J.; Wei, Y.; Wu, J.; Huang, G.; Chen, F.; Shi, J.; et al. Predictive values of the postoperative neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and lymphocyte-to-monocyte ratio for the diagnosis of early periprosthetic joint infections: A preliminary study. J. Orthop. Surg. Res. 2020, 15, 571. [Google Scholar] [CrossRef]
- Yu, B.Z.; Fu, J.; Chai, W.; Hao, L.B.; Chen, J.Y. Neutrophil to lymphocyte ratio as a predictor for diagnosis of early Periprosthetic joint infection. BMC Musculoskelet. Disord. 2020, 21, 706. [Google Scholar] [CrossRef]
- Bogut, A.; Niedźwiadek, J.; Kozioł-Montewka, M.; Strzelec-Nowak, D.; Blacha, J.; Mazurkiewicz, T.; Marczyński, W.; Plewik, D. Characterization of Staphylococcus epidermidis and Staphyloccocus warneri small-colony variants associated with prosthetic-joint infections. J. Med. Microbiol. 2014, 63 Pt 2, 176–185. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Naess, A.; Nilssen, S.S.; Mo, R.; Eide, G.E.; Sjursen, H. Role of neutrophil to lymphocyte and monocyte to lymphocyte ratios in the diagnosis of bacterial infection in patients with fever. Infection 2016, 45, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Yombi, J.C.; Schwab, P.E.; Thienpont, E. Neutrophil-to-lymphocyte ratio (NLR) distribution shows a better kinetic pattern than C-reactive protein distribution for the follow-up of early inflammation after total knee arthroplasty. Knee Surgery Sports Traumatol. Arthrosc. 2015, 24, 3287–3292. [Google Scholar] [CrossRef] [PubMed]
- Menges, T.; Engel, J.; Welters, I.; Wagner, R.M.; Little, S.; Ruwoldt, R.; Wollbrueck, M.; Hempelmann, G. Changes in blood lymphocyte populations after multiple trauma: Association with posttraumatic complications. Crit. Care Med. 1999, 27, 733–740. [Google Scholar] [CrossRef]
- Heffernan, D.S.; Monaghan, S.F.; Thakkar, R.K.; Machan, J.T.; Cioffi, W.G.; Ayala, A. Failure to normalize lymphopenia following trauma is associated with increased mortality, independent of the leukocytosis pattern. Crit. Care 2012, 16, R12. [Google Scholar] [CrossRef]
- Müller, I.; Munder, M.; Kropf, P.; Hänsch, G.M. Polymorphonuclear neutrophils and T lymphocytes: Strange bedfellows or brothers in arms? Trends Immunol. 2009, 30, 522–530. [Google Scholar] [CrossRef]
- Ferrone, C.; Dranoff, G. Dual Roles for Immunity in Gastrointestinal Cancers. J. Clin. Oncol. 2010, 28, 4045–4051. [Google Scholar] [CrossRef]
- Chen, P.; Liu, Y.; Lin, X.; Tang, S.; Wang, T.; Zheng, K.; Lin, D.; Lin, C.; Yu, B.; Chen, B.; et al. Diagnostic Value of the Blood Neutrophil-to-Lymphocyte Ratio and Monocyte-to-Lymphocyte Ratio in Tibia Fracture-Related Infection. Dis. Markers 2022, 2022, 6119583. [Google Scholar] [CrossRef]
- Dib, P.R.B.; Quirino-Teixeira, A.C.; Merij, L.B.; Pinheiro, M.B.M.; Rozini, S.V.; Andrade, F.B.; Hottz, E.D. Innate immune receptors in platelets and platelet-leukocyte interactions. J. Leukoc. Biol. 2020, 108, 1157–1182. [Google Scholar] [CrossRef]

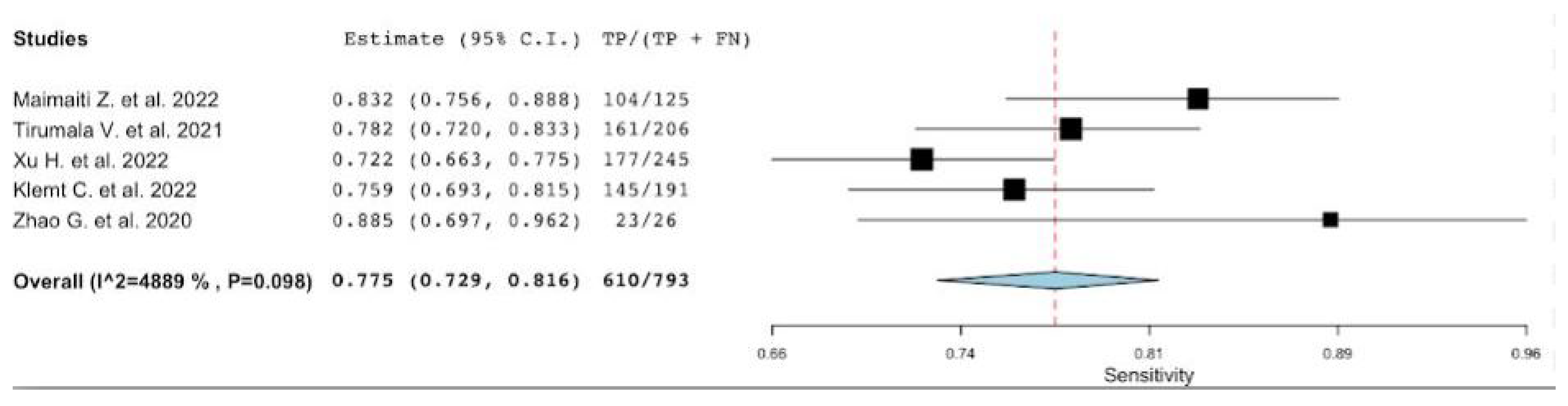
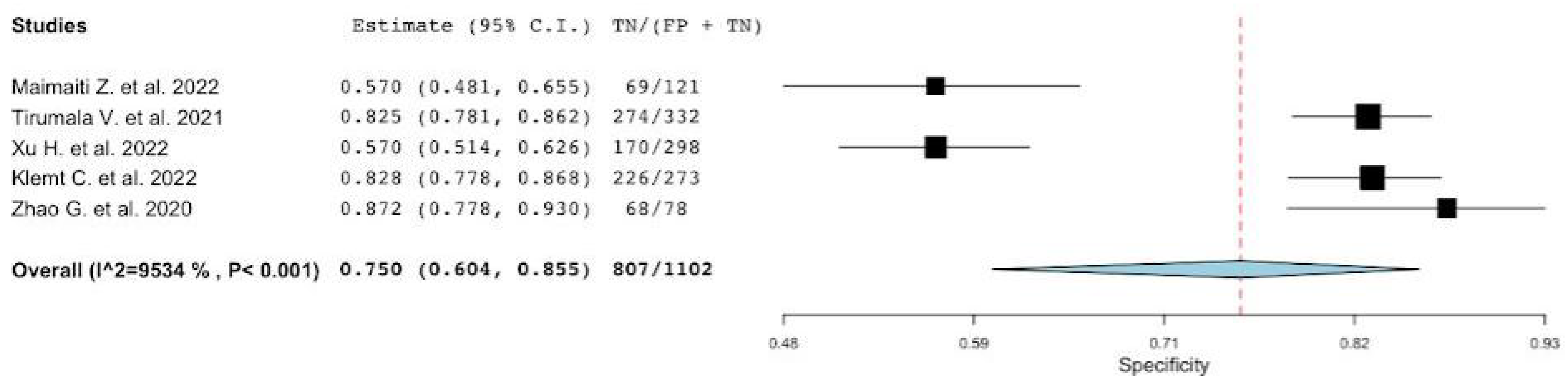
| Lead Author, Publication Date | Site of Arthroplasty in Infected Group Hip/Knee | No Patients a (PJIs n%) | Reference Standard | Parameters Evaluated MLR/NLR/MLR/PVR |
|---|---|---|---|---|
| Maimaiti Z. et al., 2022 [42] | 57/68 | 125/246 (50%) | MSIS | MLR,NLR,PVR,PLR |
| Tirumala V. et al., 2021 [22] | 0/206 | 206/538 (38%) | ICM 2018 | MLR,NLR,PVR,PLR |
| Paziuk T. et al., 2020 [23] | NA | 949/4938 (19%) | MSIS | PVR |
| Ye Y. et al 2021 [43] | 27/27 | 54/158 (34%) | ICM 2018 | NLR |
| Golge UH. et al., 2016 [44] | 30/0 | 30/133 (22%) | NA | NLR |
| Sigmund IK. et al., 2021 [45] | 36/39 | 75/177 (42%) | EBJS criteria | NLR,PVR |
| Xu H. et al., 2022 [46] | NA | 245/543 (45%) | ICM 2013 | MLR,NLR,PLR |
| Klemt C. et al., 2022 [47] | 464/0 | 191/464 (41%) | ICM 2018 | MLR,NLR,PVR,PLR |
| Jiao JB. et al., 2022 [48] | NA | 53/115 (46%) | MSIS | NLR |
| Zhao G. et al., 2020 [49] | NA | 26/104 (25%) | MSIS | NLR,PLR |
| Yu BZ. et al., 2020 [50] | 37/17 | 20/121 (16%) | MSIS | NLR |
| a QUADAS-2 Score | 1 * | 2 * | 3 * | Bias | Appl. | 4 † | 5 † | Bias | Appl. | 6 ‡ | 7 ‡ | Bias | Appl. | 8 ** | 9 ** | 10 ** | 11 ** | Bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maimaiti Z. et al., 2022 [42] | Yes | Yes | Yes | Low | Low | No | No | Low | Low | Yes | Yes | Low | Low | Yes | Yes | Yes | Yes | Low |
| Tirumala V. et al., 2021 [22] | Yes | Yes | Yes | Low | Low | No | No | Low | Low | Yes | Yes | Low | Low | Yes | Yes | Yes | Yes | Low |
| Paziuk T. et al., 2020 [23] | Yes | Yes | Yes | Low | Low | No | No | Low | Low | Yes | Yes | Low | Low | Yes | Yes | Yes | Yes | Low |
| Ye Y. et al. 2021 [43] | Yes | Yes | Yes | Low | Low | No | No | Low | Low | Yes | Yes | Low | Low | Yes | Yes | Yes | Yes | Low |
| Golge UH. et al., 2016 [44] | Yes | Yes | NC | NC | NC | No | No | Low | Low | Yes | Yes | Low | Low | Yes | Yes | Yes | Yes | Low |
| Sigmund IK. et al., 2021 [45] | Yes | Yes | Yes | Low | Low | No | No | Low | Low | Yes | Yes | Low | Low | Yes | Yes | Yes | Yes | Low |
| Xu H. et al., 2022 [46] | Yes | Yes | Yes | Low | Low | No | No | Low | Low | Yes | Yes | Low | Low | Yes | Yes | Yes | No | Low |
| Klemt C. et al., 2022 [47] | Yes | Yes | Yes | Low | Low | No | No | Low | Low | Yes | Yes | Low | Low | Yes | Yes | Yes | Yes | Low |
| Jiao JB. et al., 2022 [48] | Yes | Yes | Yes | Low | Low | No | No | Low | Low | Yes | Yes | Low | Low | Yes | Yes | Yes | No | Low |
| Zhao G. et al., 2020 [49] | Yes | Yes | Yes | Low | Low | No | No | Low | Low | Yes | Yes | Low | Low | Yes | Yes | Yes | Yes | Low |
| Yu BZ. et al., 2020 [50] | Yes | Yes | Yes | Low | Low | No | No | Low | Low | Yes | Yes | Low | Low | Yes | Yes | Yes | Yes | Low |
| Sensitivity | Specificity | Positive LR | Negative LR | DOR | |
|---|---|---|---|---|---|
| Maimaiti Z. et al., 2022 [42] | 0.6 [0.51–0.68] | 0.81 [0.73–0.87] | 3.1 [2.22–4.68] | 0.49 [0.33–0.73] | 6.39 [3.59–11.39] |
| Tirumala V. et al., 2021 [22] | 0.81 [0.76–0.86] | 0.78 [0.74–0.82] | 3.7 [3.03–4.66] | 0.23 [0.19–0.29] | 13.19 [8.70–19.9] |
| Xu H. et al., 2022 [46] | 0.54 [0.47–0.6] | 0.79 [0.74–0.83] | 2.6 [2.02–3.32] | 0.58 [0.45–0.75] | 4.45 [3.05–6.48] |
| Klemt C. et al., 2022 [47] | 0.75 [0.69–0.81] | 0.80 [0.75–0.85] | 3.9 [3.01–5.01] | 0.31 [0.24–0.39] | 12.72 [8.15–19.85] |
| Pooled | NA | NA | NA | NA | NA |
| Sensitivity | Specificity | Positive LR | Negative LR | DOR | |
|---|---|---|---|---|---|
| Maimaiti Z. et al., 2022 [42] | 0.67 [0.58–0.75] | 0.71 [0.62–0.78] | 2.3 [1.71–3.15] | 0.46 [0.34–0.63] | 5.03 [2.93–8.66] |
| Tirumala V. et al., 2021 [22] | 0.76 [ 0.70–0.82] | 0.79 [0.74–0.83] | 3.7 [2.95–4.61] | 0.29 [0.23–0.37] | 12.55 [8.26–19.05] |
| Ye Y. et al. 2021 [43] | 0.57 [0.44–0.70] | 0.77 [0.69–0.85] | 2.6 [1.69–3.98] | 0.55 [0.36–0.84] | 4.75 [2.33–9.66] |
| Golge UH. et al., 2016 [44] | 0.90 [0.73–0.97] | 0.72 [0.62–0.60] | 3.2 [2.30–4.45] | 0.13 [0.1–0.19] | 22.97 [6.46–81.59] |
| Sigmund IK. et al., 2021 [45] | 0.62 [0.51–0.73] | 0.72 [0.63–0.80] | 2.3 [1.59–3.27] | 0.51 [0.36–0.74] | 4.44 [2.34–8.40] |
| Xu H. et al., 2022 [46] | 0.62 [0.56–0.69] | 0.66 [0.61–0.72] | 1.9 [1.55–2.26] | 0.56 [0.46–0.67] | 3.35 [2.35–4.77] |
| Klemt C. et al., 2022 [47] | 0.75 [0.69–0.81] | 0.79 [0.75–0.84] | 3.7 [2.93–4.83] | 0.30 [0.23–0.39] | 12.49 [8.01–19.49] |
| Jiao JB. et al., 2022 [48] | 0.73 [0.60–0.84] | 0.71 [0.59–0.81] | 2.5 [1.66–3.86] | 0.36 [0.24–0.57] | 6.81 [2.99–15.47] |
| Zhao G. et al., 2020 [49] | 0.84 [0.65–0.94] | 0.90 [0.81–0.95] | 8.2 [4.19–16.23] | 0.17 [0.09–0.34] | 48.12 [13.22–175.23] |
| Yu BZ. et al., 2020 [50] | 0.85 [0.62–0.95] | 0.68 [0.58–0.76] | 2.7 [1.88–3.67] | 0.22 [0.16–0.31] | 11.85 [3.24–43.28] |
| Pooled | 0.72 [0.66–0.77] | 0.74 [0.70–0,79] | 2.8 [2.30–3.54] | 0.33 [0.25–0.44] | 8.13 [5.13–12.89] |
| Sensitivity | Specificity | Positive LR | Negative LR | DOR | |
|---|---|---|---|---|---|
| Maimaiti Z. et al., 2022 [42] | 0.79 [0.71–0.85] | 0.68 [0.61–0.78] | 2.4 [1.99–3.55] | 0.3 [0.22–0.39] | 8.99 [5.02–16.09] |
| Tirumala V. et al., 2021 [22] | 0.88 [0.83–0.92] | 0.76 [0.71–0.80] | 3.6 [2.99–4.44] | 0.16 [0.13–0.19] | 22.81 [14–37.15] |
| Paziuk T. et al., 2020 [23] | 0.48 [0.45–0.51] | 0.81 [0.80–0.82] | 2.5 [2.29–2.75] | 0.64 [0.58–0.70] | 3.90 [3.36–4.53] |
| Sigmund IK. et al., 2021 [45] | 0.43 [0.32–0.54] | 0.81 [0.73–0.88] | 2.3 [1.41–3.71] | 0.71 [0.43–1.14] | 3.25 [1.65–6.39] |
| Klemt C. et al., 2022 [47] | 0.86 [0.81–0.91] | 0.75 [0.7–0.80] | 3.5 [2.84–4.37] | 0.18 [0.14–0.22] | 19.51 [11.87–32.07] |
| Pooled | 0.72 [0.49–0.87] | 0.77 [0.73–0.81] | 2.9 [2.41–3.58] | 0.33 [0.16–0.66] | 8.73 [3.71–20.54] |
| Sensitivity | Specificity | Positive LR | Negative LR | DOR | |
|---|---|---|---|---|---|
| Maimaiti Z. et al., 2022 [42] | 0.83 [0.76–0.89] | 0.57 [0.48–0.66] | 1.9 [1.55–2.41] | 0.29 [0.24–0.37] | 6.57 [3.64–11.87] |
| Tirumala V. et al., 2021 [22] | 0.78 [0.72–0.83] | 0.82 [0.78–0.86] | 4.5 [3.5–5.71] | 0.26 [0.21–0.34]] | 16.90 [10.94–26.12] |
| Xu H. et al., 2022 [46] | 0.72 [0.66–0.77] | 0.57 [0.51–0.63] | 1.7 [1.44–1.96] | 0.49 [0.42–0.57] | 3.46 [2.41–4.96] |
| Klemt C. et al., 2022 [47] | 0.76 [0.69–0.82] | 0.83 [0.78–0.87] | 4.4 [3.36–5.79] | 0.29 [0.22–0.38] | 15.16 [9.60–23.94] |
| Zhao G. et al., 2020 [49] | 0.88 [0.70–0.96] | 0.87 [0.78–0.93] | 6.9 [3.81–12.51] | 0.13 [0.07–0.24] | 52.13 [13.19–20.6] |
| Pooled | 0.77 [0.73–0.82] | 0.75 [0.60–0.85] | 3.3 [1.2–5.35] | 0.3 [0.20–0.40] | 11.16 [5–24.9] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Festa, E.; Ascione, T.; Bernasconi, A.; Di Gennaro, D.; Basso, M.A.; Guarino, A.; Balato, G. Diagnostic Performance of Neutrophil to Lymphocyte Ratio, Monocyte to Lymphocyte Ratio, Platelet to Lymphocyte Ratio, and Platelet to Mean Platelet Volume Ratio in Periprosthetic Hip and Knee Infections: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 2033. https://doi.org/10.3390/diagnostics12092033
Festa E, Ascione T, Bernasconi A, Di Gennaro D, Basso MA, Guarino A, Balato G. Diagnostic Performance of Neutrophil to Lymphocyte Ratio, Monocyte to Lymphocyte Ratio, Platelet to Lymphocyte Ratio, and Platelet to Mean Platelet Volume Ratio in Periprosthetic Hip and Knee Infections: A Systematic Review and Meta-Analysis. Diagnostics. 2022; 12(9):2033. https://doi.org/10.3390/diagnostics12092033
Chicago/Turabian StyleFesta, Enrico, Tiziana Ascione, Alessio Bernasconi, Donato Di Gennaro, Morena Anna Basso, Amedeo Guarino, and Giovanni Balato. 2022. "Diagnostic Performance of Neutrophil to Lymphocyte Ratio, Monocyte to Lymphocyte Ratio, Platelet to Lymphocyte Ratio, and Platelet to Mean Platelet Volume Ratio in Periprosthetic Hip and Knee Infections: A Systematic Review and Meta-Analysis" Diagnostics 12, no. 9: 2033. https://doi.org/10.3390/diagnostics12092033
APA StyleFesta, E., Ascione, T., Bernasconi, A., Di Gennaro, D., Basso, M. A., Guarino, A., & Balato, G. (2022). Diagnostic Performance of Neutrophil to Lymphocyte Ratio, Monocyte to Lymphocyte Ratio, Platelet to Lymphocyte Ratio, and Platelet to Mean Platelet Volume Ratio in Periprosthetic Hip and Knee Infections: A Systematic Review and Meta-Analysis. Diagnostics, 12(9), 2033. https://doi.org/10.3390/diagnostics12092033







