Dental Implant Failure Risk in Post Oncological Patients, a Retrospective Study and Sapienza Head and Neck Unit Decisional Protocol- 7 Years of Follow-Up
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemotherapy/Other Medical Therapy
2.1.1. Cytotoxic Drugs for Chemotherapy
2.1.2. Antiresorptive (AR) Drugs
Bisphosphonates
Denosumab
2.1.3. Other ONJ-Related Drugs (Not Prescribed for Head and Neck Cancer)
- Drugs with a prevalent anti-angiogenic action (bevacizumab e aflibercept).
- Tyrosine kinase inhibitors (sunitinib, sorafenib, cabozantinib, regorafenib, axitinib)m-TOR inhibitors (temsirolimus, everolimus).
2.2. Radiotherapy
2.3. Bone Tissue Condition
2.3.1. Systemic Factors
- Patient’s age:
- General health:
- Smoking:
2.3.2. Local Factors
- Bone quality, quantity, and anatomical location:
2.4. Soft Tissue Condition
2.4.1. Tongue Mobility
2.4.2. Keratinized Gengiva
Red Code
- Chemotherapy: intravenous antiresorptive drugs assumption for bone metastases.
- Radiotherapy: future prosthesis zone directly irradiated with a total dose ≥66 Gy (2 Gy/fraction).
- Bone tissue condition: insufficient bone volume, the impossibility of bone augmentation for implant insertion, presence of jaw plates.
Yellow Code
- Adjuvant Chemotherapy/antiresorptive (AR) or antiangiogenic (AA) therapy
- The medical therapy specifies (drug type, dosage, the way of administration, and period of intake).
- The specifics of any additional pharmacological therapy (cortisone, anticoagulative, anti-aggregating drugs).
- The presence of any pathology that may interfere with the healing processes of the bone.
- 2.
- Radiotherapy
- 3.
- Bone tissue condition
- A bone height of at least 8 mm.
- A bone thickness that allows keeping at least 1 mm of bone around the implant itself.
- A healthy and good quality tissue.
- 4.
- Soft Tissue Condition.
Green Code
- No chemotherapy.
- No radiotherapy or a dose <50 Gy received by the future implant zone. Attention should be paid to obtain an appropriate result even in case of dose <50 Gy.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Lang, Y.; Dong, D. Cetuximab Plus Chemotherapy Versus Chemotherapy Alone in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: A Cost-Effectiveness Analysis. Cancer Manag. Res. 2020, 12, 11383–11390. [Google Scholar] [CrossRef] [PubMed]
- Brauner, E.; Valentini, V.; Jamshir, S.; Guarino, G.; Battisti, A.; Fadda, M.T.; Pompa, G. Retrospective review of 78 rehabilitated head and neck postoncological patients: A new classification method. Minerva Stomatol. 2016, 65, 17–32. [Google Scholar] [PubMed]
- De la Plata, M.M.; Gías, L.N.; Díez, P.M.; Muñoz-Guerra, M.F.; González-García, R.; Lee, G.-Y.C.; Castrejón-Castrejón, S.; Rodríguez-Campo, F.J. Osseointegrated implant rehabilitation of irradiated oral cancer patients. J. Oral Maxillofac. Surg. 2012, 70, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Pompa, G.; Saccucci, M.; Di Carlo, G.; Brauner, E.; Valentini, V.; Di Carlo, S.; Gentile, T.; Guarino, G.; Polimeni, A. Survival of dental implants in patients with oral cancer treated by surgery and radiotherapy: A retrospective study. BMC Oral Health 2015, 15, 5. [Google Scholar] [CrossRef]
- Cox, J.F.; Zarb, G.A. The longitudinal clinical efficacy of osseointegrated implants—A 3-year report. Int. J. Oral Maxillofac. Implant. 1987, 2, 91–100. [Google Scholar]
- Dreizen, S.; Bodey, G.P.; Rodriguez, V. Oral Complications of Cancer Chemotherapy. Postgrad. Med. 1975, 58, 75–82. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw–2014 update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef]
- Favot, C.L.; Forster, C.; Glogauer, M. The effect of bisphosphonate therapy on neutrophil function: A potential biomarker. Int. J. Oral Maxillofac. Surg. 2013, 42, 619–626. [Google Scholar] [CrossRef]
- Campisi, G.; Mauceri, R.; Bertoldo, F.; Fusco, V.; Bedogni, A.; Campisi, G.; Mauceri, R.; Bertoldo, F.; Fusco Vittorio, B.A. Simplifying the Dental/Periodontal Management of Patients with Metabolic Bone Fragility Receiving Treatment with Denosumab; Qeios: London, UK, 2020. [Google Scholar]
- Brauner, E.; Mezi, S.; Ciolfi, A.; Ciolfi, C.; Pucci, R.; Cassoni, A.; Battisti, A.; Piesco, G.; De Felice, F.; Pranno, N.; et al. A New Medical Record Proposal to the Prognostic Risk Assessment for MRONJ in Oncologic Patients: “Sapienza Head and Neck Unit” Proposal. Int. J. Environ. Res. Public Health 2021, 18, 1851. [Google Scholar] [CrossRef]
- Bamias, A.; Kastritis, E.; Bamia, C.; Moulopoulos, L.A.; Melakopoulos, I.; Bozas, G.; Koutsoukou, V.; Gika, D.; Anagnostopoulos, A.; Papadimitriou, C.; et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: Incidence and risk factors. J. Clin. Oncol. 2005, 23, 8580–8587. [Google Scholar] [CrossRef]
- Hellstein, J.W.; Adler, R.A.; Edwards, B.; Jacobsen, P.L.; Kalmar, J.R.; Koka, S.; Migliorati, C.A.; Ristic, H. Managing the care of patients receiving antiresorptive therapy for prevention and treatment of osteoporosis: Executive summary of recommendations from the American Dental Association Council on Scientific Affairs. J. Am. Dent. Assoc. 2011, 142, 1243–1251. [Google Scholar] [CrossRef]
- Estilo, C.L.; Van Poznak, C.H.; Wiliams, T.; Bohle, G.C.; Lwin, P.T.; Zhou, Q.; Riedel, E.R.; Carlson, D.L.; Schoder, H.; Farooki, A.; et al. Osteonecrosis of the Maxilla and Mandible in Patients with Advanced Cancer Treated with Bisphosphonate Therapy. Oncologist 2008, 13, 911–920. [Google Scholar] [CrossRef][Green Version]
- Petrovic, I.; Rosen, E.B.; Matros, E.; Huryn, J.M.; Shah, J.P. Oral rehabilitation of the cancer patient: A formidable challenge. J. Surg. Oncol. 2018, 117, 1729–1735. [Google Scholar] [CrossRef]
- Syftestad, G.T.; Urist, M.R. Bone aging. Clin. Orthop. Relat. Res. 1982, 162, 288–297. [Google Scholar] [CrossRef]
- Köndell, P.A.; Landt, H.; Nordenram, A.; Carlsson, B.; Danielsson, K. The tissue-integrated prosthesis in the treatment of edentulous patients. A follow-up study. Swed. Dent. J. 1988, 12, 11–16. [Google Scholar]
- Jemt, T. Implant treatment in elderly patients. Int. J. Prosthodont. 1993, 6, 456–461. [Google Scholar]
- Ochi, S.; Morris, H.F.; Winkler, S. Patient Demographics and Implant Survival at Uncovering: Dental Implant Clinical Research Group Interim Report No. 6. Implant. Dent. 1994, 3, 247–251. [Google Scholar] [CrossRef]
- Matukas, V.J. Medical risks associated with dental implants. J. Dent. Educ. 1988, 52, 745–747. [Google Scholar] [CrossRef]
- Zoldos, J.; Kent, J.N. Healing of endosseous implants. In Endosseous Implants for Maxillofacial Reconstructions; Block, M.S., Kent, J.N., Eds.; W.B. Saunders Co.: Philadelphia, PA, USA, 1995; pp. 40–69. [Google Scholar]
- Esposito, M.; Hirsch, J.-M.; Lekholm, U.; Thomsen, P. Biological factors contributing to failures of osseointegrated oral implants. (II) Etiopathogenesis. Eur. J. Oral Sci. 1998, 106, 721–764. [Google Scholar] [CrossRef]
- Adell, R. The surgical principles of osseointegration. In Advanced Osseointegration Surgery: Applications in the Maxillofacial Region; Worthington, P., Bra·Nemark, P.-I., Eds.; Quintessence Publishing Co., Inc.: Chicago, IL, USA, 1992; pp. 94–107. [Google Scholar]
- Tenore, G.; Nuvoli, A.; Mohsen, A.; Cassoni, A.; Battisti, A.; Terenzi, V.; Della Monaca, M.; Raponi, I.; Brauner, E.; De Felice, F.; et al. Tobacco, alcohol and family history of cancer as risk factors of Oral Squamous Cell Carcinoma: Case-control retrospective study. Appl. Sci. 2020, 10, 3896. [Google Scholar] [CrossRef]
- Lindhe, J.; Meyle, J.; Group D of European Workshop on Periodontology. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35 (Suppl. S8), 282–285. [Google Scholar] [CrossRef] [PubMed]
- Meffert, R.M.; Singleton, D.G. Reactors’ Summary Clinical Trials on Endosseous Implants, Part I. Ann. Periodontol. 1997, 2, 314. [Google Scholar] [CrossRef]
- Esposito, M.; Hirsch, J.-M.; Lekholm, U.; Thomsen, P. Biological factors contributing to failures of osseointegrated oral implants. (I) Success criteria and epidemiology. Eur. J. Oral Sci. 1998, 106, 527–551. [Google Scholar] [CrossRef] [PubMed]
- Friberg, B.; Jemt, T.; Lekholm, U. Early failures in 4641 consecutively placed Branemark dental Implants: A study from stage 1 surgery to the connection of completed prostheses. Int. J. Oral Maxillofac. Implant. 1991, 6, 142–146. [Google Scholar]
- Truhlar, R.S.; Farish, S.E.; Scheitler, L.E.; Morris, H.F.; Ochi, S. Bone quality and implant design-related outcomes through stage II surgical uncovering of spectra-system root form implants. J. Oral Maxillofac. Surg. 1997, 55 (Suppl. S5), 46–54. [Google Scholar] [CrossRef]
- Patrick, D.; Zosky, J.; Lubar, R.; Buchs, A. The longitudinal clinical efficacy of Core-Vent dental implants: A five-year report. J. Oral Implant. 1989, 15, 95–103. [Google Scholar]
- Drago, C.J. Rates of osseointegration of dental implants with regard to anatomical location. J. Prosthodont. 1992, 1, 29–31. [Google Scholar] [CrossRef]
- Wedgwood, D.; Jennings, K.J.; Critchlow, H.A.; Watkinson Quayle, A.A. Experience with ITI osseointegrated implants at five centres in the UK. Br. J. Oral Maxillofac. Surg. 1992, 30, 377–381. [Google Scholar] [CrossRef]
- Fugazzotto, P.A.; Gulbransen, H.J.; Wheeler, S.L.; Lindsay, J.A. The use of IMZ osseointegrated implants in partially and completely edentulous patients: Success and failure rates of 2023 implant cylinders up to 60+ months in function. Int. J. Oral Maxillofac. Implant. 1993, 8, 617–621. [Google Scholar]
- Babbush, C.A.; Shimuram. Five-year statistical and clinical observations with the IMZ two-stage osteointegrated implant system. Int. J. Oral Maxillofac. Implant. 1993, 8, 245–253. [Google Scholar] [CrossRef]
- Lill, W.; Thornton, B.; Reichsthaler, J.; Schneider, B. Statistical analyses on the success potential of osseointegrated implants: A retrospective single-dimension statistical analysis. J. Prosthet. Dent. 1993, 69, 176–185. [Google Scholar] [CrossRef]
- Buser, D.; Mericske-Stern, R.; Bernard, J.P.; Behneke, A.; Behneke, N.; Hirt, H.P.; Belser, U.C.; Lang, N.P. Long-term evaluation of non-submerged ITI implants. Part 1, 8-year life table analysis of prospective multi-center study with 2359 implants. Clin. Oral Implant. Res. 1997, 8, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Friberg, B.; Nilson, H.; Olsson, M.; Palmquist, C. Mk II: The self-tapping Branemark implant: 5-year results of a prospective 3-center study. Clin. Oral Implant. Res. 1997, 8, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Von Wowern, N. Variations in bone mass within the cortices of the mandible. Scand. J. Dent. Res. 1977, 85, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Von Wowern, N. Variations in structure within the trabecular bone of the mandible. Scand. J. Dent. Res. 1977, 85, 613–622. [Google Scholar] [CrossRef]
- Johns, R.B.; Jemt, T.; Heath, M.R.; Hutton, J.E.; Mckenna, S.; Mcnamara, D.C.; Van Steenberghe, D.; Taylor, R.; Watson, R.M.; Herrmann, I. A multicenter study of overdentures supported by Branemark implants. Int. J. Oral Maxillofac. Implant. 1992, 7, 513–522. [Google Scholar] [CrossRef]
- Orenstein, I.H.; Synan, W.J.; Truhlar, R.S.; Morris, H.F.; Ochi, S. Bone quality in patients receiving endosseous implants: DICRG interim report No. 1. Implant Dent. 1994, 3, 90–94. [Google Scholar] [CrossRef]
- Friberg, B.; Sennerby, L.; Roos, J.; Lekholm, U. Identification of bone quality in conjunction with insertion of titanium implants. A pilot study in jaw autopsy specimens. Clin. Oral Implant. Res. 1995, 6, 213–219. [Google Scholar] [CrossRef]
- Truhlar, R.S.; Orenstein, I.H.; Morris, H.F.; Ochi, S. Distribution of bone quality in patients receiving endosseous dental implants. J. Oral Maxillofac. Surg. 1997, 55 (Suppl. S5), 36–45. [Google Scholar] [CrossRef]
- Lekholm, U.; Zarb, G.A. Patient selection and preparation. In Tissue-Integrated Prostheses; Branemark, P.-I., Zarb, G.A., Albrektsson, T., Eds.; Quintessence Publishing Co., Inc.: Chicago, IL, USA, 1985; pp. 199–209. [Google Scholar]
- Brauner, E.; Di Carlo, S.; Ciolfi, A.; Pompa, G.; Jamshir, S.; De Angelis, F.; Della Monaca, M.; Valentini, V. Use of Porous Implants for the Prosthetic Rehabilitation of Fibula Free Flap Reconstructed Patients. J. Craniofac. Surg. 2019, 30, 1163–1169. [Google Scholar] [CrossRef]
- Zavattero, E.; Ramieri, G.; Agrò, G.; Fasolis, M.; Garzino-Demo, P.; Borbon, C. Implant Dental Rehabilitation of Fibula-Free Flap Reconstructed Jaws. J. Craniofacial Surg. 2021, 32, e134–e136. [Google Scholar] [CrossRef] [PubMed]
- Hayter, J.; Cawood, J. Cawood. Oral rehabilitation with endosteal implants and free flaps. Int. J. Oral Maxillofac. Surg. 1996, 25, 3–12. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods, and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Spiro, R.H.; Strong, E.W. Surgical treatment of cancer of the tongue. Surg. Clin. N. Am. 1974, 54, 759–765. [Google Scholar] [CrossRef]
- Chang, E.I.; Yu, P.; Skoracki, R.J.; Liu, J.; Hanasono, M.M. Comprehensive analysis of functional outcomes and survival after microvascular reconstruction of glossectomy defects. Ann. Surg. Oncol. 2015, 22, 3061–3069. [Google Scholar] [CrossRef]
- Bressmann, T.; Sader, R.; Whitehill, T.L.; Samman, N. Consonant intelligibility and tongue motility in patients with partial glossectomy. J. Oral Maxillofac. Surg. 2004, 62, 298–303. [Google Scholar] [CrossRef]
- Huang, Z.S.; Chen, W.L.; Huang, Z.Q.; Yang, Z.H. Dysphagia in Tongue Cancer Patients before and after Surgery. J. Oral Maxillofac. Surg. 2016, 74, 2067–2072. [Google Scholar] [CrossRef]
- Li, X.; Sun, Q.; Guo, S. Functional assessments in patients undergoing radial forearm flap following hemiglossectomy. J. Craniofacial Surg. 2016, 27, e172–e175. [Google Scholar] [CrossRef]
- Hsiao, H.T.; Leu, Y.S.; Liu, C.J.; Tung, K.Y.; Lin, C.C. Radial Forearm versus Anterolateral Thigh Flap Reconstruction after Hemiglossectomy: Functional Assessment of Swallowing and Speech. J. Reconstr. Microsurg. 2008, 24, 85–88. [Google Scholar] [CrossRef]
- Lang, N.P.; Löe, H. The relationship between the width of keratinized gingiva and gingival health. J. Periodontol. 1972, 43, 623–627. [Google Scholar] [CrossRef]
- Esper, L.A.; Ferreira, S.B., Jr.; Kaizer Rde, O.; de Almeida, A.L. The role of keratinized mucosa in peri-implant health. Cleft Palate-Craniofac. J. 2012, 49, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Panchal, H.; Shamsunder, M.G.; Petrovic, I.; Rosen, E.B.; Allen, R.J.; Hernandez, M.; Ganly, I.; Boyle, J.O.; Matros, E.; Nelson, J.A. Dental Implant Survival in Vascularized Bone Flaps: A Systematic Review and Meta-Analysis. Plast. Reconstr. Surg. 2020, 146, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Pompa, G.; Brauner, E.; Jamshir, S.; De Angelis, F.; Giardino, R.; Di Carlo, S. Quality of life in patients rehabilitated with palatal obturator without reconstruction versus fixed implant-prosthesis after reconstruction of maxillectomy defects. J. Int. Dent. Med. Res. 2017, 10, 1–8. [Google Scholar]
- Brauner, E.; Quarato, A.; De Angelis, F.; Pompa, G.; Jamshir, S.; Valentini, V.; Di Carlo, S. Prosthetic rehabilitation involving the use of implants following a fibula free flap reconstruction in the treatment of Osteosarcoma of the maxilla: A case report. Clin. Ter. 2017, 168, e392–e396. [Google Scholar] [CrossRef]
- Brauner, E.; Valentini, V.; De Angelis, F.; Jamshir, S.; Visca, A.; Romeo, U.; Tenore, G.; Pompa, G.; Di Carlo, S. Gingival hyperplasia around dental implants in jaws reconstructed with free vascularized flaps: A case report series. J. Int. Dent. Med. Res. 2018, 11, 1–7. [Google Scholar]
- Linsen, S.S.; Martini, M.; Stark, H. Long-term results of endosteal implants following radical oral cancer surgery with and without adjuvant radiation therapy. Clin. Implant Dent. Relat. Res. 2012, 14, 250–258. [Google Scholar] [CrossRef]
- Papi, P.; Brauner, E.; Di Carlo, S.; Musio, D.; Tombolini, M.; De Angelis, F.; Valentini, V.; Tombolini, V.; Polimeni, A.; Pompa, G. Crestal bone loss around dental implants placed in head and neck cancer patients treated with different radiotherapy techniques: A prospective cohort study. Int. J. Oral Maxillofac. Surg. 2019, 48, 691–696. [Google Scholar] [CrossRef]
- Brauner, E.; Musio, D.; Mezi, S.; Ciolfi, A.; Maghella, F.; Cassoni, A.; De Angelis, F.; Guarino, G.; Romeo, U.; Tenore, G.; et al. Implant placement in oral squamous cells carcinoma patients treated with chemoradiotherapy: “Sapienza Head and Neck Unit” clinical recommendations. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9923–9930. [Google Scholar]

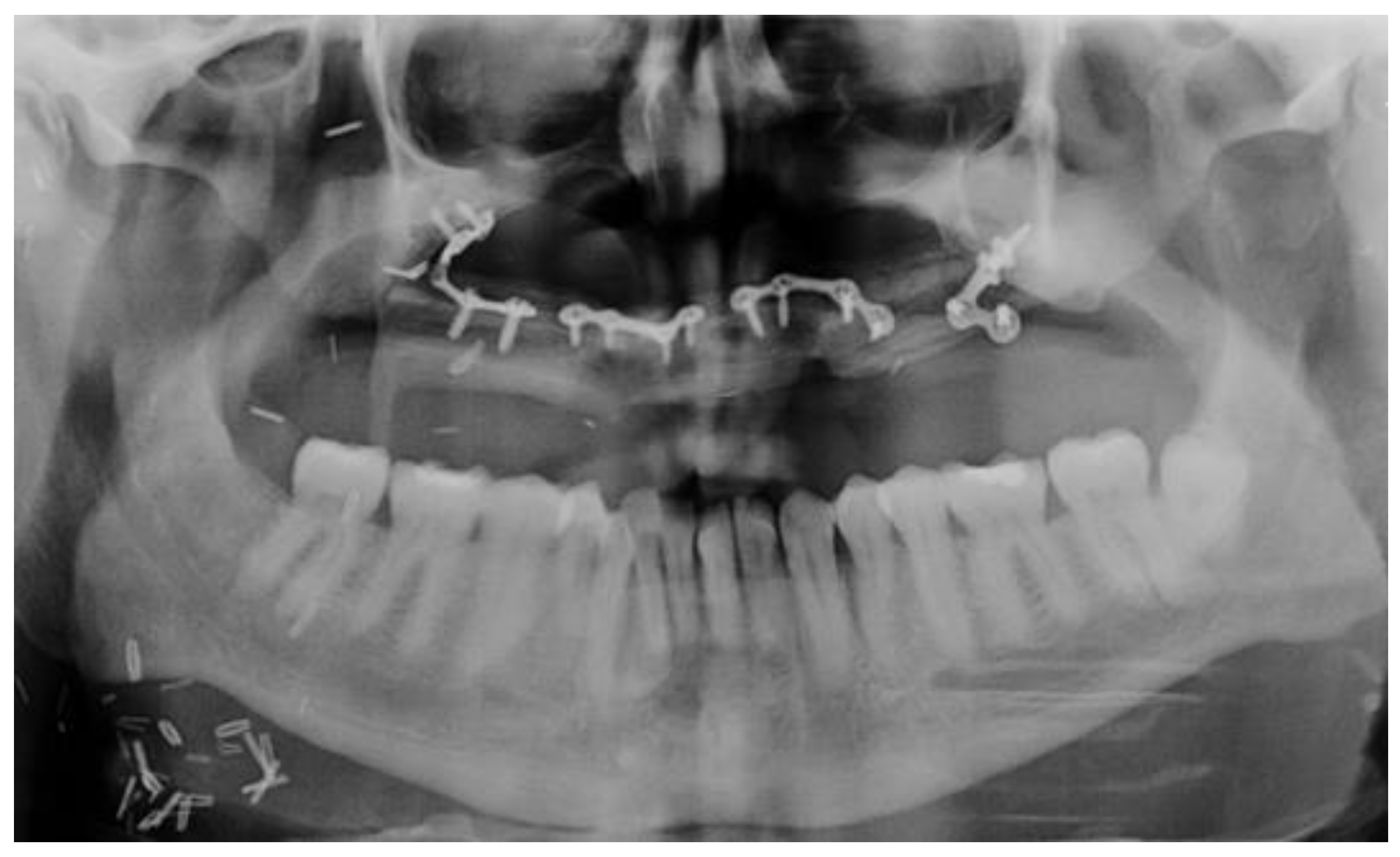

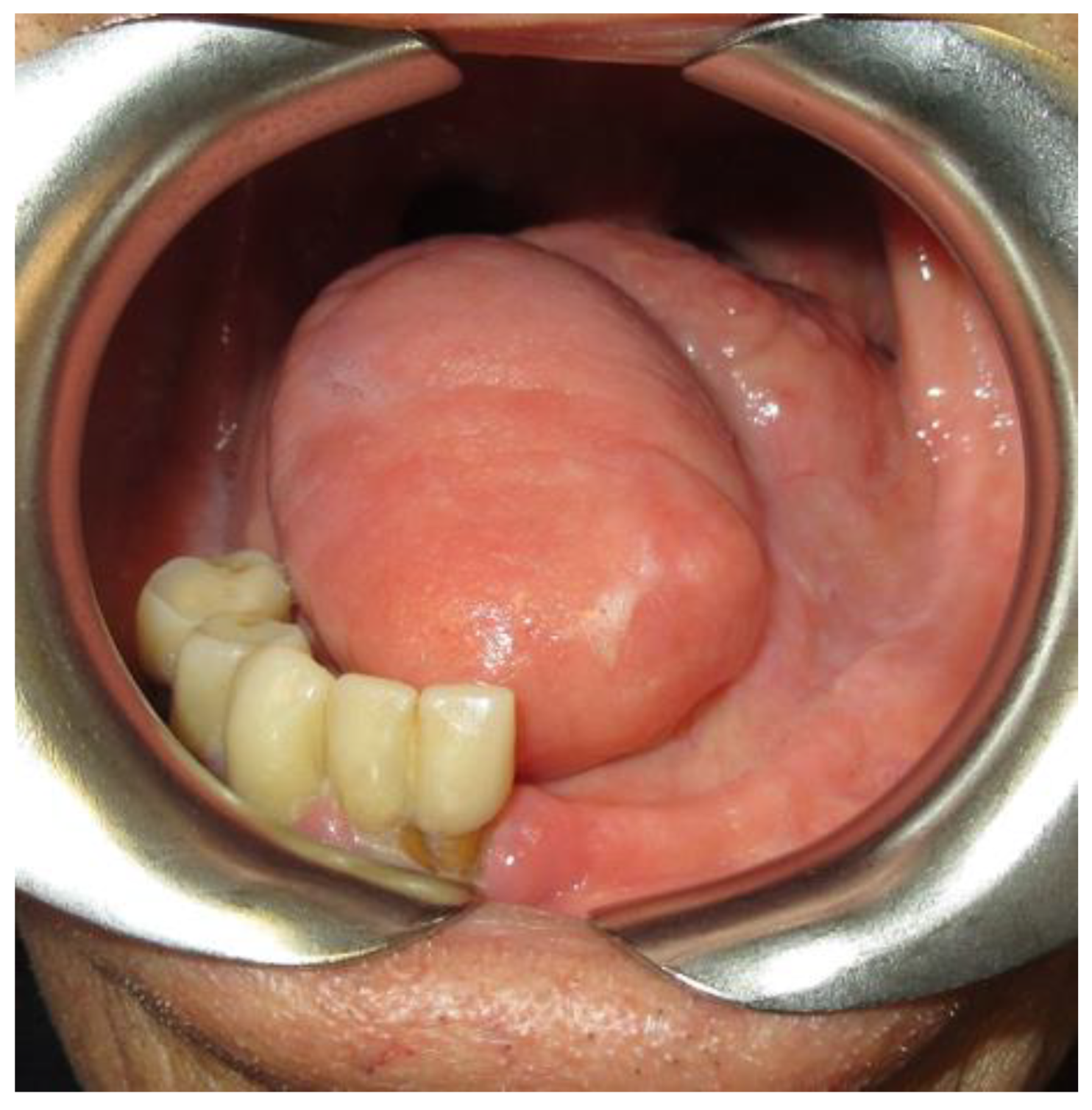

| Implant Failure Risk Factors | Risk Entity | |
|---|---|---|
| CHEMOTHERAPY or OTHER MEDICAL THERAPY | FOR BONE METASTASES | |
| ADJUVANT CHEMOTHERAPY | ||
| OSTEOMETABOLIC DRUG THERAPY | ||
| NO CHEMOTHERAPY OR OTHER DRUG THERAPY | ||
| RADIOTHERAPY (dose at the implant zone) | DOSE ≥ 66 Gy | |
| 50 Gy ≥ DOSE < 66 Gy | ||
| DOSE < 50 Gy | ||
| NO RADIOTHERAPY | ||
| BONE TISSUE | NO BONE PRESENT/JAW PLATES PRESENT | |
| PARTIALLY PRESENT OR UNFAVORABLE SISTEMIC/LOCAL CONDITIONS | ||
| PRESENT | ||
| RECONSTRUCTED BONE | ||
| SOFT TISSUE | LOW TONGUE MOBILITY | |
| DEFICIENCY/ABSENCE OF KERATINIZED GENGIVA IN FUTURE IMPLANT INSERTION ZONE | ||
| SUFFICIENT KERATINIZED GENGIVA IN FUTURE IMPLANT INSERTION ZONE | ||
| NORMAL TONGUE MOBILITY | ||
| LEGEND | ||
| HIGH RISK OF IMPLANT FAILURE | ||
| MODERATE RISK OF IMPLANT FAILURE | ||
| LOW RISK OF IMPLANT FAILURE | ||
| Total Number of Patients Rehabilitated with Implants | Total Number of Insterted Implants | Total Number of Lost Implants | % of Lost Implants |
|---|---|---|---|
| 42 | 200 | 9 | 4.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brauner, E.; Valentini, V.; Romeo, U.; Cantore, M.; Laudoni, F.; Rajabtork Zadeh, O.; Formisano, V.; Cassoni, A.; Della Monaca, M.; Battisti, A.; et al. Dental Implant Failure Risk in Post Oncological Patients, a Retrospective Study and Sapienza Head and Neck Unit Decisional Protocol- 7 Years of Follow-Up. Diagnostics 2022, 12, 1863. https://doi.org/10.3390/diagnostics12081863
Brauner E, Valentini V, Romeo U, Cantore M, Laudoni F, Rajabtork Zadeh O, Formisano V, Cassoni A, Della Monaca M, Battisti A, et al. Dental Implant Failure Risk in Post Oncological Patients, a Retrospective Study and Sapienza Head and Neck Unit Decisional Protocol- 7 Years of Follow-Up. Diagnostics. 2022; 12(8):1863. https://doi.org/10.3390/diagnostics12081863
Chicago/Turabian StyleBrauner, Edoardo, Valentino Valentini, Umberto Romeo, Marco Cantore, Federico Laudoni, Oriana Rajabtork Zadeh, Valeria Formisano, Andrea Cassoni, Marco Della Monaca, Andrea Battisti, and et al. 2022. "Dental Implant Failure Risk in Post Oncological Patients, a Retrospective Study and Sapienza Head and Neck Unit Decisional Protocol- 7 Years of Follow-Up" Diagnostics 12, no. 8: 1863. https://doi.org/10.3390/diagnostics12081863
APA StyleBrauner, E., Valentini, V., Romeo, U., Cantore, M., Laudoni, F., Rajabtork Zadeh, O., Formisano, V., Cassoni, A., Della Monaca, M., Battisti, A., Mezi, S., Cirillo, A., De Felice, F., Botticelli, A., Tombolini, V., De Vincentiis, M., Colizza, A., Tenore, G., Polimeni, A., & Di Carlo, S. (2022). Dental Implant Failure Risk in Post Oncological Patients, a Retrospective Study and Sapienza Head and Neck Unit Decisional Protocol- 7 Years of Follow-Up. Diagnostics, 12(8), 1863. https://doi.org/10.3390/diagnostics12081863












