Predictive Value of Neutrophil–Lymphocyte Ratio as a Marker in Antiresorptive Agent-Related Osteonecrosis of the Jaw: A Retrospective Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Patients
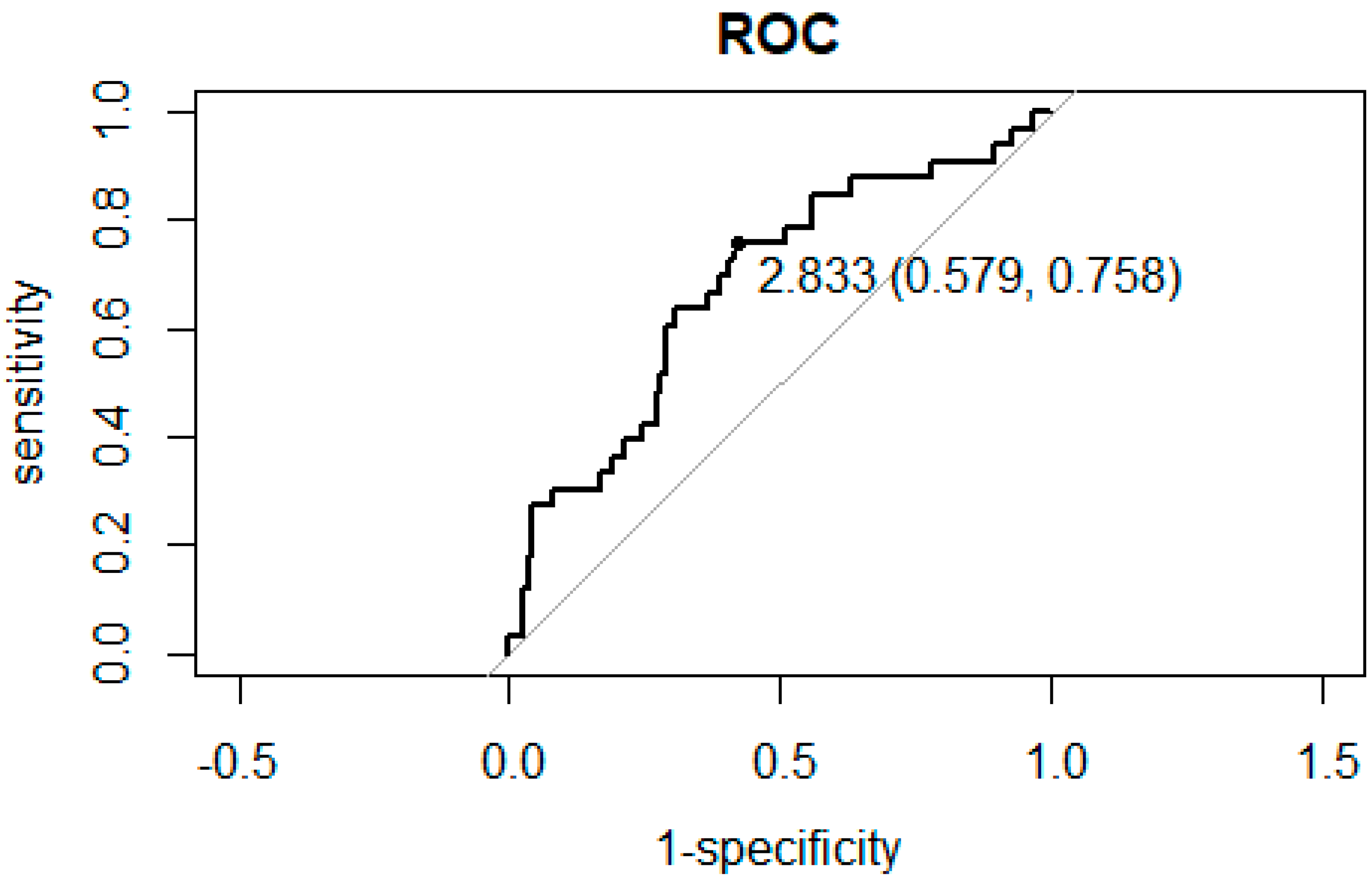
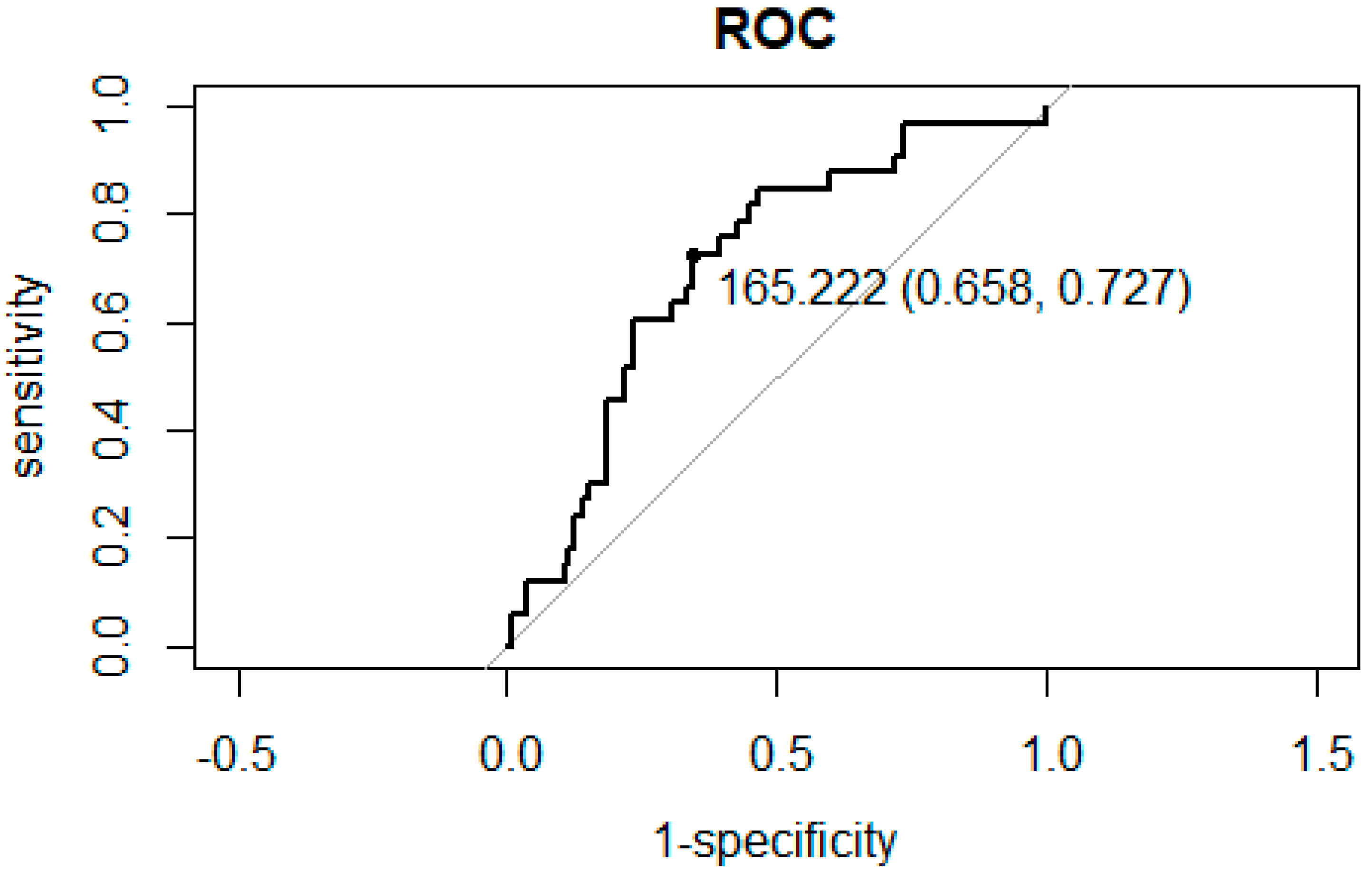
3.2. ROC Curve Analysis for the Association between NLR and PLR and Hospitalization
3.3. Relationship between NLR and Hospitalization for Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons American Association of Oral and Maxillofacial Surgeons Position Paper on Medication-Related Osteonecrosis of the Jaw—2014 Update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws—2022 Update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef] [PubMed]
- Keskinruzgar, A.; Bozdag, Z.; Aras, M.H.; Demir, T.; Yolcu, U.; Cetiner, S. Histopathological Effects of Teriparatide in Medication-Related Osteonecrosis of the Jaw: An Animal Study. J. Oral Maxillofac. Surg. 2016, 74, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Tanvetyanon, T.; Stiff, P.J. Management of the Adverse Effects Associated with Intravenous Bisphosphonates. Ann. Oncol. 2006, 17, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Toledano-Fonseca, M.; Cano, M.T.; Inga, E.; Gómez-España, A.; Guil-Luna, S.; García-Ortiz, M.V.; Mena-Osuna, R.; De la Haba-Rodriguez, J.R.; Rodríguez-Ariza, A.; Aranda, E. The Combination of Neutrophil-Lymphocyte Ratio and Platelet-Lymphocyte Ratio with Liquid Biopsy Biomarkers Improves Prognosis Prediction in Metastatic Pancreatic Cancer. Cancers 2021, 13, 1210. [Google Scholar] [CrossRef] [PubMed]
- Pirozzolo, G.; Gisbertz, S.S.; Castoro, C.; van Berge Henegouwen, M.I.; Scarpa, M. Neutrophil-to-Lymphocyte Ratio as Prognostic Marker in Esophageal Cancer: A Systematic Review and Meta-Analysis. J. Thorac. Dis. 2019, 11, 3136–3145. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.; Pyo, J.-S.; Chung, K.H.; Son, B.K. The Predicting Role of the Neutrophil-to-Lymphocyte Ratio for the Tumor Grade and Prognosis in Pancreatic Neuroendocrine Tumors. Diagnostics 2022, 12, 737. [Google Scholar] [CrossRef]
- Schiefer, S.; Wirsik, N.M.; Kalkum, E.; Seide, S.E.; Nienhüser, H.; Müller, B.; Billeter, A.; Büchler, M.W.; Schmidt, T.; Probst, P. Systematic Review of Prognostic Role of Blood Cell Ratios in Patients with Gastric Cancer Undergoing Surgery. Diagnostics 2022, 12, 593. [Google Scholar] [CrossRef]
- Kong, W.; He, Y.; Bao, H.; Zhang, W.; Wang, X. Diagnostic Value of Neutrophil-Lymphocyte Ratio for Predicting the Severity of Acute Pancreatitis: A Meta-Analysis. Dis. Markers 2020, 2020, 9731854. [Google Scholar] [CrossRef]
- Paquissi, F.C. The Role of Inflammation in Cardiovascular Diseases: The Predictive Value of Neutrophil-Lymphocyte Ratio as a Marker in Peripheral Arterial Disease. Ther. Clin. Risk Manag. 2016, 12, 851–860. [Google Scholar] [CrossRef] [Green Version]
- Gasparyan, A.Y.; Ayvazyan, L.; Mukanova, U.; Yessirkepov, M.; Kitas, G.D. The Platelet-to-Lymphocyte Ratio as an Inflammatory Marker in Rheumatic Diseases. Ann. Lab. Med. 2019, 39, 345–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, Q.; Chen, K.; Rha, S.-W.; Lim, H.-E.; Li, G.; Liu, T. Usefulness of Neutrophil/Lymphocyte Ratio as a Predictor of Atrial Fibrillation: A Meta-Analysis. Arch. Med. Res. 2015, 46, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, X.; Zhang, W.; Ying, H.; Xu, Y.; Zhang, J.; Min, Q.; Chen, J. Neutrophil-Lymphocyte Ratio and Platelet-Lymphocyte Ratio Are 2 New Inflammatory Markers Associated with Pulmonary Involvement and Disease Activity in Patients with Dermatomyositis. Clin. Chim. Acta 2017, 465, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G.; Smith, C.W.; Entman, M.L. The Inflammatory Response in Myocardial Infarction. Cardiovasc. Res. 2002, 53, 31–47. [Google Scholar] [CrossRef]
- Badescu, M.C.; Rezus, E.; Ciocoiu, M.; Badulescu, O.V.; Butnariu, L.I.; Popescu, D.; Bratoiu, I.; Rezus, C. Osteonecrosis of the Jaws in Patients with Hereditary Thrombophilia/Hypofibrinolysis-From Pathophysiology to Therapeutic Implications. Int. J. Mol. Sci. 2022, 23, 640. [Google Scholar] [CrossRef] [PubMed]
- Soma, T.; Iwasaki, R.; Sato, Y.; Kobayashi, T.; Nakamura, S.; Kaneko, Y.; Ito, E.; Okada, H.; Watanabe, H.; Miyamoto, K.; et al. Tooth Extraction in Mice Administered Zoledronate Increases Inflammatory Cytokine Levels and Promotes Osteonecrosis of the Jaw. J. Bone Miner. Metab. 2021, 39, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Gao, L.; Ren, W.; Li, S.; Zheng, J.; Li, S.; Jiang, C.; Yang, S.; Zhi, K. The Role of the Immune Response in the Development of Medication-Related Osteonecrosis of the Jaw. Front. Immunol. 2021, 12, 606043. [Google Scholar] [CrossRef] [PubMed]
- Miksad, R.A.; Lai, K.-C.; Dodson, T.B.; Woo, S.-B.; Treister, N.S.; Akinyemi, O.; Bihrle, M.; Maytal, G.; August, M.; Gazelle, G.S.; et al. Quality of Life Implications of Bisphosphonate-Associated Osteonecrosis of the Jaw. Oncologist 2011, 16, 121–132. [Google Scholar] [CrossRef] [Green Version]
- Caminha, R.-D.-G.; Alcantara, P.-L.; Carvalho, C.-G.; Reia, V.-C.-B.; Capelozza, A.-L.-A.; Santos, P.-S.S. The Impact of Medication-Related Osteonecrosis of the Jaws on the Quality of Life in Cancer Patients. J. Clin. Exp. Dent. 2020, 12, e725–e729. [Google Scholar] [CrossRef]
- Kuiper, J.W.P.; Forster, C.; Sun, C.; Peel, S.; Glogauer, M. Zoledronate and Pamidronate Depress Neutrophil Functions and Survival in Mice. Br. J. Pharmacol. 2012, 165, 532–539. [Google Scholar] [CrossRef] [Green Version]
- Favot, C.L.; Forster, C.; Glogauer, M. The Effect of Bisphosphonate Therapy on Neutrophil Function: A Potential Biomarker. Int. J. Oral Maxillofac. Surg. 2013, 42, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Zahorec, R. Ratio of Neutrophil to Lymphocyte Counts--Rapid and Simple Parameter of Systemic Inflammation and Stress in Critically Ill. Bratisl. Lek. Listy 2001, 102, 5–14. [Google Scholar] [PubMed]
- Baetta, R.; Corsini, A. Role of Polymorphonuclear Neutrophils in Atherosclerosis: Current State and Future Perspectives. Atherosclerosis 2010, 210, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Erturk, M.; Cakmak, H.A.; Surgit, O.; Celik, O.; Aksu, H.U.; Akgul, O.; Gurdogan, M.; Bulut, U.; Ozalp, B.; Akbay, E.; et al. Predictive Value of Elevated Neutrophil to Lymphocyte Ratio for Long-Term Cardiovascular Mortality in Peripheral Arterial Occlusive Disease. J. Cardiol. 2014, 64, 371–376. [Google Scholar] [CrossRef] [Green Version]
- Kaito, K.; Otsubo, H.; Usui, N.; Yoshida, M.; Tanno, J.; Kurihara, E.; Matsumoto, K.; Hirata, R.; Domitsu, K.; Kobayashi, M. Platelet Size Deviation Width, Platelet Large Cell Ratio, and Mean Platelet Volume Have Sufficient Sensitivity and Specificity in the Diagnosis of Immune Thrombocytopenia. Br. J. Haematol. 2005, 128, 698–702. [Google Scholar] [CrossRef]
- Ozcan Cetin, E.H.; Cetin, M.S.; Aras, D.; Topaloglu, S.; Temizhan, A.; Kisacik, H.L.; Aydogdu, S. Platelet to Lymphocyte Ratio as a Prognostic Marker of In-Hospital and Long-Term Major Adverse Cardiovascular Events in ST-Segment Elevation Myocardial Infarction. Angiology 2016, 67, 336–345. [Google Scholar] [CrossRef]
- Ferroni, P.; Basili, S.; Falco, A.; Davì, G. Platelet Activation in Type 2 Diabetes Mellitus. J. Thromb. Haemost. 2004, 2, 1282–1291. [Google Scholar] [CrossRef]
- Stark, K.; Massberg, S. Interplay between Inflammation and Thrombosis in Cardiovascular Pathology. Nat. Rev. Cardiol. 2021, 18, 666–682. [Google Scholar] [CrossRef]
- Verschoor, A.; Langer, H.F. Crosstalk between Platelets and the Complement System in Immune Protection and Disease. Thromb. Haemost. 2013, 110, 910–919. [Google Scholar] [CrossRef]
- Glueck, C.J.; Freiberg, R.; Tracy, T.; Stroop, D.; Wang, P. Thrombophilia and Hypofibrinolysis: Pathophysiologies of Osteonecrosis. Clin. Orthop. Relat. Res. 1997, 334, 43–56. [Google Scholar] [CrossRef]
| Patient Characteristics | n (%) | |||
|---|---|---|---|---|
| Total | 147 | |||
| Sex | Male | 63 (42.9) | ||
| Female | 84 (57.1) | |||
| Main disease | Malignant tumor | 85 (57.8) | ||
| Osteoporosis | 41 (27.9) | |||
| Autoimmune disease | 21 (14.3) | |||
| Allergic history | 37(25.1) | |||
| BMA | Denosumab | 62 (42.2) | ||
| Bisphosphonate related | 85 (57.8) | |||
| Hospitalization for acute inflammation of ARONJ | 33 (22.4) | |||
| NAO | 49 (33.3) | |||
| ARONJ stage (%) | 0 | 10 (6.3) | ||
| 1 | 45 (30.6) | |||
| 2 | 86 (58.5) | |||
| 3 | 6 (4.1) | |||
| Patient Characteristics | Mean (SD) | |||
| Age (years) | 73.2 (10.7) | |||
| Period of BMA use | 43.9 (48.1) | months | ||
| Period of stable condition | 19.5 (17.9) | months | ||
| Laboratory parameters at ARONJ diagnosis | White blood cells | 6613.1 (2439.6) | ×103/μL | |
| Neutrophils | 4456.3 (2362.4) | /μL | ||
| Lymphocytes | 1461.7 (601.9) | /μL | ||
| Platelets | 253.2 (97.9) | ×103/μL | ||
| NLR | 4.01 (4.37) | |||
| PLR | 214.5 (158.9) | |||
| Hemoglobin | 11.5 (1.76) | g/dL | ||
| Total protein | 6.92 (0.644) | g/dL | ||
| Albumin | 3.93 (0.452) | g/dL | ||
| CRP | 1.71 (5.144) | mg/dL | ||
| Low NLR (<4) | High NLR (≥4) | p | |||
|---|---|---|---|---|---|
| n | 100 | 47 | |||
| Sex (%) | Male | 40 (40.0) | 23 (48.9) | 0.3722 | |
| Main disease (%) | Malignant tumor | 61 (61.0) | 24 (51.1) | ||
| Osteoporosis | 29 (29.0) | 12 (25.5) | |||
| Autoimmune disease | 10 (10.0) | 11 (23.4) | |||
| Allergic history (%) | 27 (27.0) | 10 (21.3) | 0.5434 | ||
| BMA (%) | Denosumab | 58 (58.0) | 27 (57.4) | 1 | |
| Hospitalization for acute inflammation of ARONJ (%) | 17 (17.0) | 16 (34.0) | 0.0328 * | ||
| NAO (%) | 18 (18.0) | 15 (31.9) | 0.08863 | ||
| Age (mean (SD)) | 72.93 (11.24) | 73.85 (9.38) | 0.8958 | ||
| Period of BMA use (mean (SD)) | 43.35 (46.39) | 45.14 (52.28) | months | 0.8966 | |
| Period of stable condition (mean (SD)) | 20.42 (19.31) | 25.11 (21.10) | months | 0.1363 | |
| ARONJ stage (mean (SD)) | 1.58 (0.68) | 1.64 (0.67) | 0.595 | ||
| Laboratory parameters at ARONJ diagnosis (mean (SD)) | White blood cells | 5798.60 (1655.33) | 8345.96 (2912.36) | ×103/μL | <0.001 * |
| Neutrophils | 3542.58 (1231.92) | 6713.11 (2731.28) | /μL | <0.001 * | |
| Lymphocytes | 1658.41 (576.95) | 1043.04 (412.71) | /μL | <0.001 * | |
| Platelets | 232.40 (79.12) | 297.45 (118.37) | ×103/μL | <0.001 * | |
| NLR | 2.29 (0.82) | 7.68 (6.24) | <0.001 * | ||
| PLR | 156.47 (79.94) | 337.98 (208.57) | <0.001 * | ||
| Hemoglobin | 11.72 (1.81) | 11.16 (1.61) | g/dL | 0.0441 * | |
| Total protein | 7.01 (0.62) | 6.74 (0.66) | g/dL | 0.0154 * | |
| Albumin | 4.03 (0.44) | 3.72 (0.40) | g/dL | <0.001 * | |
| CRP | 1.21 (5.94) | 2.68 (2.90) | mg/dL | <0.001 * | |
| NLR < 2.833 | NLR ≥ 2.833 | p | |||
|---|---|---|---|---|---|
| n | 74 | 73 | |||
| Sex (%) | Male | 31 (41.9) | 32 (43.8) | 0.8683 | |
| Main disease (%) | Malignant tumor | 43 (58.1) | 42 (57.5) | 0.054 | |
| Osteoporosis | 25 (33.8) | 16 (21.9) | |||
| Autoimmune disease | 6 (8.1) | 15 (20.5) | |||
| Allergic history (%) | 20 (27.0) | 17 (23.3) | 0.7045 | ||
| BMA (%) | Denosumab | 41 (55.4) | 44 (60.3) | 0.6174 | |
| Hospitalization for acute inflammation of ARONJ (%) | 8 (10.8) | 25 (34.2) | <0.001 * | ||
| NAO (%) | 13 (17.6) | 20 (27.4) | 0.1708 | ||
| Age (mean (SD)) | 73.38 (11.28) | 73.07 (10.06) | 0.6911 | ||
| Period of BMA use (mean (SD)) | 43.21 (50.09) | 44.63 (46.19) | months | 0.5536 | |
| Period of stable condition (mean (SD)) | 21.95 (20.29) | 21.89 (19.73) | months | 0.8921 | |
| The stage of ARONJ (mean (SD)) | 1.51(0.71) | 1.68(0.64) | 0.1612 | ||
| Laboratory parameters at ARONJ diagnosis (mean (SD)) | White blood cells | 5747.70 (1598.59) | 7490.27 (2815.55) | ×103/μL | <0.001 * |
| Neutrophils | 3320.57 (1129.17) | 5808.93 (2619.85) | /μL | <0.001 * | |
| Lymphocytes | 1790.15 (555.20) | 1128.67 (445.91) | /μL | <0.001 * | |
| Platelets | 229.38 (67.07) | 277.34 (117.06) | ×103/μL | 0.0128 * | |
| NLR | 1.92 (0.59) | 6.13 (5.41) | <0.001 * | ||
| PLR | 138.64 (55.58) | 291.41 (190.18) | <0.001 * | ||
| Hemoglobin | 11.87 (1.80) | 11.21 (1.67) | g/dL | 0.0128 * | |
| Total protein | 7.03 (0.63) | 6.82 (0.64) | g/dL | 0.0326 * | |
| Albumin | 4.04 (0.45) | 3.82 (0.43) | g/dL | 0.00148 * | |
| CRP | 1.48 (6.88) | 1.94 (2.61) | mg/dL | <0.001 * | |
| PLR < 165.2 | PLR ≥ 165.2 | p | |||
|---|---|---|---|---|---|
| n | 84 | 63 | |||
| Sex (%) | Male | 40 (47.6) | 23 (36.5) | 0.238 | |
| Main disease (%) | Malignant tumor | 48 (57.1) | 37 (58.7) | 0.487 | |
| Osteoporosis | 26 (31.0) | 15 (23.8) | |||
| Autoimmune disease | 10 (11.9) | 11 (17.5) | |||
| Allergic history (%) | 21 (25.0) | 16 (25.4) | 1 | ||
| BMA (%) | Denosumab | 49 (58.3) | 36 (57.1) | 1 | |
| Hospitalization for acute inflammation of ARONJ (%) | 9 (10.7) | 24 (38.1) | <0.001 * | ||
| NAO (%) | 16 (19.0) | 17 (27.0) | 0.319 | ||
| Age (mean (SD)) | 72.57 (11.08) | 74.10 (10.09) | 0.372 | ||
| Period of BMA use (mean (SD)) | 42.61 (47.74) | 45.68 (48.90) | months | 0.721 | |
| Period of stable condition (mean (SD)) | 22.65 (19.17) | 20.94 (21.05) | months | 0.352 | |
| ARONJ stage (mean (SD)) | 1.56 (0.66) | 1.65 (0.70) | 0.433 | ||
| Laboratory parameters at ARONJ diagnosis (mean (SD)) | White blood cells | 6504.64 (2075.96) | 6757.62 (2864.99) | ×103/μL | 0.813 |
| Neutrophils | 4089.79 (1852.49) | 5178.29 (2803.54) | /μL | 0.007 * | |
| Lymphocytes | 1783.02 (533.21) | 1033.17 (382.21) | /μL | <0.001 * | |
| Platelets | 212.21 (55.17) | 307.84 (114.84) | ×103/μL | <0.001 * | |
| NLR | 2.45 (1.21) | 6.09 (5.94) | <0.001 * | ||
| PLR | 123.68 (28.05) | 335.61 (179.83) | <0.001 * | ||
| Hemoglobin | 12.02 (1.56) | 10.91 (1.83) | g/dL | <0.001 * | |
| Total protein | 7.02 (0.56) | 6.79 (0.73) | g/dL | 0.052 * | |
| Albumin | 4.08 (0.35) | 3.73 (0.50) | g/dL | <0.001 * | |
| CRP | 1.33 (6.49) | 2.18 (2.74) | mg/dL | <0.001 * | |
| NLR | ||
| Variables | HR (95%CI) | p |
| Age | 1.01 (0.973–1.06) | 0.508 |
| Albumin | 0.642 (0.251–1.72) | 0.359 |
| ARONJ stage | 1.59 (0.834–3.22) | 0.172 |
| NLR | 1.15 (1.01–1.31) | 0.036 * |
| PLR | ||
| Variables | HR (95%CI) | p |
| Age | 1.01 (0.973–1.06) | 0.519 |
| Albumin | 0.665 (0.246–1.87) | 0.422 |
| ARONJ stage | 1.42 (0.750–2.81) | 0.296 |
| PLR | 1.00 (0.999–1.01) | 0.151 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurohara, K.; Shimizu, K.; Murata, T.; Koizumi, G.; Takigawa, A.; Nagata, K.; Okumura, K.; Arai, N. Predictive Value of Neutrophil–Lymphocyte Ratio as a Marker in Antiresorptive Agent-Related Osteonecrosis of the Jaw: A Retrospective Analysis. Diagnostics 2022, 12, 1836. https://doi.org/10.3390/diagnostics12081836
Kurohara K, Shimizu K, Murata T, Koizumi G, Takigawa A, Nagata K, Okumura K, Arai N. Predictive Value of Neutrophil–Lymphocyte Ratio as a Marker in Antiresorptive Agent-Related Osteonecrosis of the Jaw: A Retrospective Analysis. Diagnostics. 2022; 12(8):1836. https://doi.org/10.3390/diagnostics12081836
Chicago/Turabian StyleKurohara, Kazuto, Kasumi Shimizu, Taku Murata, Gaku Koizumi, Akira Takigawa, Kokoro Nagata, Kenya Okumura, and Naoya Arai. 2022. "Predictive Value of Neutrophil–Lymphocyte Ratio as a Marker in Antiresorptive Agent-Related Osteonecrosis of the Jaw: A Retrospective Analysis" Diagnostics 12, no. 8: 1836. https://doi.org/10.3390/diagnostics12081836
APA StyleKurohara, K., Shimizu, K., Murata, T., Koizumi, G., Takigawa, A., Nagata, K., Okumura, K., & Arai, N. (2022). Predictive Value of Neutrophil–Lymphocyte Ratio as a Marker in Antiresorptive Agent-Related Osteonecrosis of the Jaw: A Retrospective Analysis. Diagnostics, 12(8), 1836. https://doi.org/10.3390/diagnostics12081836





