Outcome Analysis of the Use of Cerament® in Patients with Chronic Osteomyelitis and Corticomedullary Defects
Abstract
:1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Birt, M.; Anderson, D.W.; Toby, E.B.; Wang, J. Osteomyelitis: Recent advances in pathophysiology and therapeutic strategies. J. Orthop. 2017, 14, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Mader, J.T.; Shirtliff, M.; Calhoun, J.H. Staging and staging application in osteomyelitis. Clin. Infect. Dis. 1997, 25, 1303–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabater-Martos, M.; Sigmund, I.K.; Loizou, C.; McNally, M. Surgical Treatment and Outcomes of Calcaneal Osteomyelitis in Adults: A Systematic Review. J. Bone Jt. Infect. 2019, 4, 146–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNally, M.A. Decision-making in infected nonunion: Is the surgery more important than the condition? Bone Joint. J. 2016, 98, 435–436. [Google Scholar] [CrossRef] [PubMed]
- Oliver, R.A.; Lovric, V.; Christou, C.; Walsh, W.R. Comparative osteoconductivity of bone void fillers with antibiotics in a critical size bone defect model. J. Mater. Sci. Mater. Med. 2020, 31, 80. [Google Scholar] [CrossRef]
- Kanakaris, N.; Gudipati, S.; Tosounidis, T.; Harwood, P.; Britten, S.; Giannoudis, P.V. The treatment of intramedullary osteomyelitis of the femur and tibia using the Reamer-Irrigator-Aspirator system and antibiotic cement rods. Bone Jt. J. 2014, 96, 783–788. [Google Scholar] [CrossRef]
- Aljawadi, A.; Naylor, T.; Islam, A.; Madhi, I.; Niazi, N.; Elmajee, M.; Pillai, A. Radiological Analysis of Gentamicin Eluting Synthetic Bone Graft Substitute Used in the Management of Patients with Traumatic Bone Voids. Cureus 2022, 14, e20969. [Google Scholar] [CrossRef]
- Freischmidt, H.; Armbruster, J.; Bonner, E.; Guehring, T.; Nurjadi, D.; Bechberger, M.; Sonntag, R.; Schmidmaier, G.; Grützner, P.A.; Helbig, L. Systemic Administration of PTH Supports Vascularization in Segmental Bone Defects Filled with Ceramic-Based Bone Graft Substitute. Cells 2021, 10, 2058. [Google Scholar] [CrossRef]
- Niazi, N.S.; Drampalos, E.; Morrissey, N.; Jahangir, N.; Wee, A.; Pillai, A. Adjuvant antibiotic loaded bio composite in the management of diabetic foot osteomyelitis—A multicentre study. Foot 2019, 39, 22–27. [Google Scholar] [CrossRef]
- McNally, M.A.; Ferguson, J.; Lau, A.C.K.; Diefenbeck, M.; Scarborough, M.; Ramsden, A.J.; Atkins, B.L. Single-stage treatment of chronic osteomyelitis with a new absorbable, gentamicin-loaded, calcium sulphate/hydroxyapatite biocomposite: A prospective series of 100 cases. Bone Joint J. 2016, 98, 1289–1296. [Google Scholar] [CrossRef] [Green Version]
- Hotchen, A.J.; Dudareva, M.; Corrigan, R.A.; Ferguson, J.Y.; McNally, M.A. Can we predict outcome after treatment of long bone osteomyelitis? Bone Joint J. 2020, 102, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Radovanovic, D.; Seifert, B.; Urban, P.; Eberli, F.R.; Rickli, H.; Bertel, O.; Puhan, M.; Erne, P. Validity of Charlson Comorbidity Index in patients hospitalised with acute coronary syndrome. Insights from the nationwide AMIS Plus registry 2002–2012. Heart 2014, 100, 288–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNally, M.; Nagarajah, K. Osteomyelitis. Orthop. Trauma 2010, 24, 416–429. [Google Scholar] [CrossRef]
- Angst, F.; Schwyzer, H.K.; Aeschlimann, A.; Simmen, B.R.; Goldhahn, J. Measures of adult shoulder function: Disabilities of the Arm, Shoulder, and Hand Questionnaire (DASH) and its short version (QuickDASH), Shoulder Pain and Disability Index (SPADI), American Shoulder and Elbow Surgeons (ASES) Society standardized shoulder assessment form, Constant (Murley) Score (CS), Simple Shoulder Test (SST), Oxford Shoulder Score (OSS), Shoulder Disability Questionnaire (SDQ), and Western Ontario Shoulder Instability Index (WOSI). Arthritis Care Res. 2011, 63 (Suppl. S11), S174–S188. [Google Scholar]
- Odum, S.M.; Fehring, T.K.; Dennis, D.; Kim, R.; Goodman, S.; Huddleston, J.; Kazarian, G.; Lonner, J.H.; Suarez, J.C.; Patel, P.; et al. Can Original Knee Society Scores Be Used to Estimate New 2011 Knee Society Scores? Clin. Orthop. Relat. Res. 2017, 475, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Sierevelt, I.N.; Zwiers, R.; Schats, W.; Haverkamp, D.; Terwee, C.B.; Nolte, P.A.; Kerkhoffs, G.M.M.J. Measurement properties of the most commonly used Foot- and Ankle-Specific Questionnaires: The FFI, FAOS and FAAM. A systematic review. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 2059–2073. [Google Scholar] [CrossRef]
- Laucis, N.C.; Hays, R.D.; Bhattacharyya, T. Scoring the SF-36 in Orthopaedics: A Brief Guide. J. Bone Jt. Surg. Am. 2015, 97, 1628–1634. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, J.; Alexander, M.; Bruce, S.; O’Connell, M.; Beecroft, S.; McNally, M. A retrospective cohort study comparing clinical outcomes and healthcare resource utilisation in patients undergoing surgery for osteomyelitis in England: A case for reorganising orthopaedic infection services. J. Bone Jt. Infect. 2021, 6, 151–163. [Google Scholar] [CrossRef]
- Del Pozo, E.G.; Collazos, J.; Carton, J.A.; Camporro, D.; Asensi, V. Factors predictive of relapse in adult bacterial osteomyelitis of long bones. 11 Medical and Health Sciences 1103 Clinical Sciences. BMC Infect. Dis. 2018, 18, 635. [Google Scholar]
- Pincher, B.; Fenton, C.; Jeyapalan, R.; Barlow, G.; Sharma, H.K. A systematic review of the single-stage treatment of chronic osteomyelitis. J. Orthop. Surg. Res. 2019, 14, 393. [Google Scholar] [CrossRef] [Green Version]
- Dudareva, M.; Kümin, M.; Vach, W.; Kaier, K.; Ferguson, J.; McNally, M.; Scarborough, M. Short or Long Antibiotic Regimes in Orthopaedics (SOLARIO): A randomised controlled open-label non-inferiority trial of duration of systemic antibiotics in adults with orthopaedic infection treated operatively with local antibiotic therapy. Trials 2019, 20, 693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tice, A.D.; Hoaglund, P.; Shoultz, D.A. Outcomes of osteomyelitis among patients treated with outpatient parenteral antimicrobial therapy. Am. J. Med. 2003, 114, 723–728. [Google Scholar] [CrossRef]
- Kendall, J.V.; McNally, M.; Taylor, C.; Ferguson, J.; Galitzine, S.; Critchley, P.; Giele, H.; Ramsden, A.J. The Effect of Age on Outcome in Excision of Chronic Osteomyelitis with Free Muscle Flap Reconstruction. J. Bone Jt. Infect. 2019, 4, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Parsons, B.; Strauss, E. Surgical management of chronic osteomyelitis. Am. J. Surg. 2004, 188, 57–66. [Google Scholar] [CrossRef]
- Evans, R.P.; Nelson, C.L. Gentamicin-impregnated polymethylmethacrylate beads compared with systemic antibiotic therapy in the treatment of chronic osteomyelitis. Clin. Orthop. Relat. Res. 1993, 295, 37–42. [Google Scholar] [CrossRef]
- Shi, X.; Wu, Y.; Ni, H.; Li, M.; Zhang, C.; Qi, B.; Wei, M.; Wang, T.; Xu, Y. Antibiotic-loaded calcium sulfate in clinical treatment of chronic osteomyelitis: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2022, 17, 104. [Google Scholar] [CrossRef]
- Branstetter, J.G.; Jackson, S.R.; Haggard, W.O.; Richelsoph, K.C.; Wenke, J.C. Locally-administered antibiotics in wounds in a limb. J. Bone Jt. Surg. Br. 2009, 91, 1106–1109. [Google Scholar] [CrossRef] [Green Version]
- Dvorzhinskiy, A.; Perino, G.; Chojnowski, R.; van der Meulen, M.C.H.; Bostrom, M.P.G.; Yang, X. Ceramic composite with gentamicin decreases persistent infection and increases bone formation in a rat model of debrided osteomyelitis. J. Bone Jt. Infect. 2021, 6, 283–293. [Google Scholar] [CrossRef]
- Horstmann, P.F.; Hettwer, W.H.; Kaltoft, N.S.; Petersen, M.M. Early Clinical and Radiological Experience with a Ceramic Bone Graft Substitute in the Treatment of Benign and Borderline Bone Lesions. Sci. Rep. 2018, 8, 15384. [Google Scholar] [CrossRef]
- Ferguson, J.; Athanasou, N.; Diefenbeck, M.; McNally, M. Radiographic and Histological Analysis of a Synthetic Bone Graft Substitute Eluting Gentamicin in the Treatment of Chronic Osteomyelitis. J. Bone Jt. Infect. 2019, 4, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Logoluso, N.; Drago, L.; Gallazzi, E.; George, D.; Morelli, I.; Romanò, C. Calcium-Based, Antibiotic-Loaded Bone Substitute as an Implant Coating: A Pilot Clinical Study. J. Bone Jt. Infect. 2016, 1, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Andreacchio, A.; Alberghina, F.; Paonessa, M.; Cravino, M.; De Rosa, V.; Canavese, F. Tobramycin-impregnated calcium sulfate pellets for the treatment of chronic osteomyelitis in children and adolescents. J. Pediatr. Orthop. Part B 2019, 28, 189–195. [Google Scholar] [CrossRef] [PubMed]
- McKee, M.D.; Wild, L.M.; Schemitsch, E.H.; Waddell, J.P. The Use of an Antibiotic-Impregnated, Osteoconductive, Bioabsorbable Bone Substitute in the Treatment of Infected Long Bone Defects: Early Results of a Prospective Trial. J. Orthop. Trauma 2002, 16, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Gitelis, S.; Brebach, G.T. The treatment of chronic osteomyelitis with a biodegradable antibiotic-impregnated implant. J. Orthop. Surg. 2002, 10, 53–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.J.; Xue, L.; Wu, C.B.; Zhou, Q. Use of Vancomycin-Impregnated Calcium Sulfate in the Treatment of Osteomyelitis of the Jaw. J. Oral Maxillofac. Surg. 2017, 75, 119–128. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, G.; Zhang, Y.; Luo, W.; Liu, S.; Liu, Y.; Zhou, Y.; Zhang, Y. The effect of calcium sulfate/calcium phosphate composite for the treatment of chronic osteomyelitis compared with calcium sulfate. Ann. Palliat. Med. 2020, 9, 1821833. [Google Scholar] [CrossRef]
- Ferrando, A.; Part, J.; Baeza, J. Treatment of Cavitary Bone Defects in Chronic Osteomyelitis: Bioactive glass S53P4 vs. Calcium Sulphate Antibiotic Beads. J. Bone Jt. Infect. 2017, 2, 194–201. [Google Scholar] [CrossRef]
- Humm, G.; Noor, S.; Bridgeman, P.; David, M.; Bose, D. Adjuvant treatment of chronic osteomyelitis of the tibia following exogenous trauma using OSTEOSET Ò-T: A review of 21 patients in a regional trauma centre. Strateg. Trauma Limb Reconstr. 2014, 9, 157–161. [Google Scholar] [CrossRef] [Green Version]
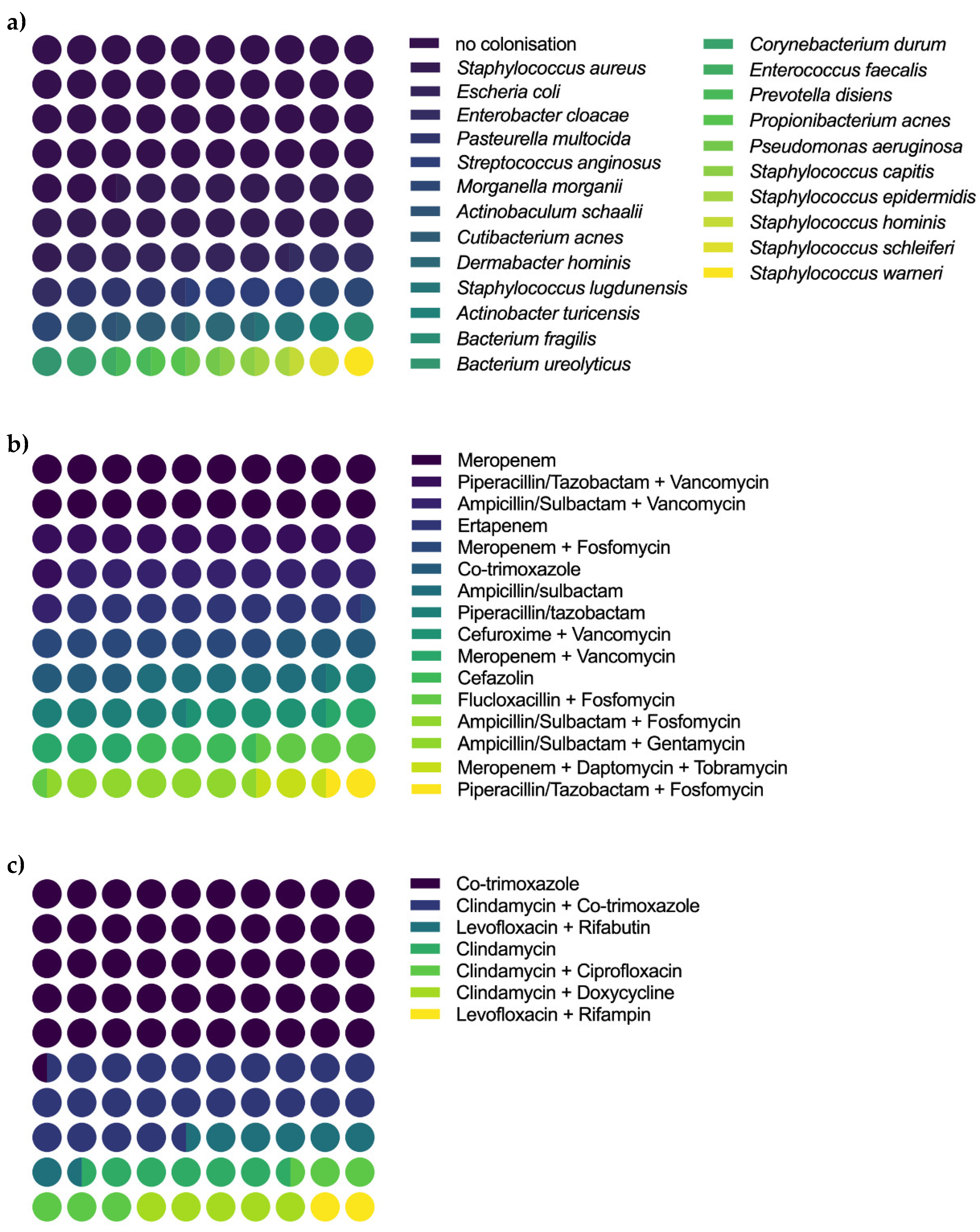

| Total Sample (N = 20) | Revised (N = 10) | Not Revised (N = 10) | Statistic * | |
|---|---|---|---|---|
| Gender (female [%]/male [%]) | 8 (40.0%)/12 (60.0%) | 4 (40.0%)/6 (60.0%) | 4 (40.0%)/6 (60.0%) | p > 0.9 |
| age (years) | 46.4 ± 16.3 (95%CI 38.8–54.0) | 45.6 ± 17.0 (95%CI 33.4–57.8) | 47.2 ± 16.5 (95%CI 35.4–59.0) | p = 0.7 |
| BMI | 24.7 ± 5.0 (95%CI 22.3–27.0) | 25.1 ± 6.4 (95%CI 20.5–29.7) | 24.2 ± 3.3 (95%CI 21.8–26.6) | p = 0.8 |
| CCI | 1.3 ± 1.8 (95%CI 0.5–2.1) | 0.9 ± 1.0 (95%CI 0.2–1.6) | 1.7 ± 2.3 (95%CI 0.1–3.3) | p = 0.7 |
| ASA | 1.8 ± 0.6 (95%CI 1.5–2.1) | 1.8 ± 0.6 (95%CI 1.4–2.3) | 1.8 ± 0.6 (95%CI 1.4–2.3) | p > 0.9 |
| Previous revision surgeries | 8.8 ± 15.7 (95%CI 0.4–17.1) | 8.7 ± 12.3 (95%CI −4.2–21.5) | 8.8 ± 18.1 (95%CI −4.1–21.7) | p = 0.8 |
| Disease duration (months) | 149.7 ± 168.2 (95%CI 70.9–228.4) | 162.9 ± 180.6 (95%CI 33.7–292.1) | 136.4 ± 163.5 (95%CI 19.4–253.4) | p = 0.9 |
| Follow up (months) | 20.2 ± 17.2 (95%CI 12.1–28.3) | 21.0 ± 18.1 (95%CI 8.0–34.0) | 19.4 ± 17.3 (95%CI 7.1–31.8) | p = 0.9 |
| Location | Total Sample | OM stage According to Cierny and Mader | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Sample | Revised | Not Revised | |||||||||||
| III A | III BL | III BS | III BLS | III A | III BL | III BS | III BLS | III A | III BL | III BS | III BLS | ||
| Humerus | 2 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Ulna | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Femur | 3 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 |
| Tibia | 11 | 5 | 2 | 4 | 0 | 1 | 1 | 3 | 0 | 4 | 1 | 1 | 0 |
| Calcaneum | 3 | 1 | 0 | 2 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| Total | 20 | 8 | 3 | 7 | 2 | 3 | 1 | 6 | 0 | 5 | 2 | 1 | 2 |
| Total Sample (N = 20) | Revised (N = 10) | Not Revised (N = 10) | Statistic * | |
|---|---|---|---|---|
| Surgery duration (minutes) | 114.7 ± 54.0 (95%CI 89.5–139.9) | 107.6 ± 49.6 (95%CI 72.1–143.1) | 121.8 ± 59.8 (95%CI 79.1–164.5) | p = 0.7 |
| LOS (days) | 21.0 ± 12.7 (95%CI 15.0–26.9) | 21.4 ± 9.8 (95%CI 14.4–28.4) | 20.5 ± 15.7 (95%CI 9.3–31.7) | p = 0.5 |
| Intravenous antibiotics (days) | 18.9 ± 14.3 (95%CI 12.2–25.6) | 16.2 ± 9.3 (95%CI 9.6–22.9) | 21.6 ± 18.1 (95%CI 8.7–34.5) | p = 0.6 |
| Oral antibiotics (days) | 29.7 ± 38.4 (95%CI 11.7–47.7) | 24.6 ± 50.6 (95%CI −11.6–60.8) | 34.8 ± 22.4 (95%CI 18.8–50.5) | p = 0.1 |
| Following revisions | 2.0 ± 1.3 (95%CI 1.1–2.9) | 2.0 ± 1.3 (95%CI 1.1–2.9) | n. a. | n. a. |
| Duration until revision (days) | 60.1 ± 65.7 (95%CI 9.6–110.6) | 60.1 ± 65.7 (95%CI 9.6–110.6) | n. a. | n. a. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niemann, M.; Graef, F.; Ahmad, S.S.; Braun, K.F.; Stöckle, U.; Trampuz, A.; Meller, S. Outcome Analysis of the Use of Cerament® in Patients with Chronic Osteomyelitis and Corticomedullary Defects. Diagnostics 2022, 12, 1207. https://doi.org/10.3390/diagnostics12051207
Niemann M, Graef F, Ahmad SS, Braun KF, Stöckle U, Trampuz A, Meller S. Outcome Analysis of the Use of Cerament® in Patients with Chronic Osteomyelitis and Corticomedullary Defects. Diagnostics. 2022; 12(5):1207. https://doi.org/10.3390/diagnostics12051207
Chicago/Turabian StyleNiemann, Marcel, Frank Graef, Sufian S. Ahmad, Karl F. Braun, Ulrich Stöckle, Andrej Trampuz, and Sebastian Meller. 2022. "Outcome Analysis of the Use of Cerament® in Patients with Chronic Osteomyelitis and Corticomedullary Defects" Diagnostics 12, no. 5: 1207. https://doi.org/10.3390/diagnostics12051207
APA StyleNiemann, M., Graef, F., Ahmad, S. S., Braun, K. F., Stöckle, U., Trampuz, A., & Meller, S. (2022). Outcome Analysis of the Use of Cerament® in Patients with Chronic Osteomyelitis and Corticomedullary Defects. Diagnostics, 12(5), 1207. https://doi.org/10.3390/diagnostics12051207








