Ultrasonography Comparison of Pelvic Floor and Abdominal Wall Muscles in Women with and without Dyspareunia: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethical Considerations
2.3. Participants
2.4. Sample Size Calculation
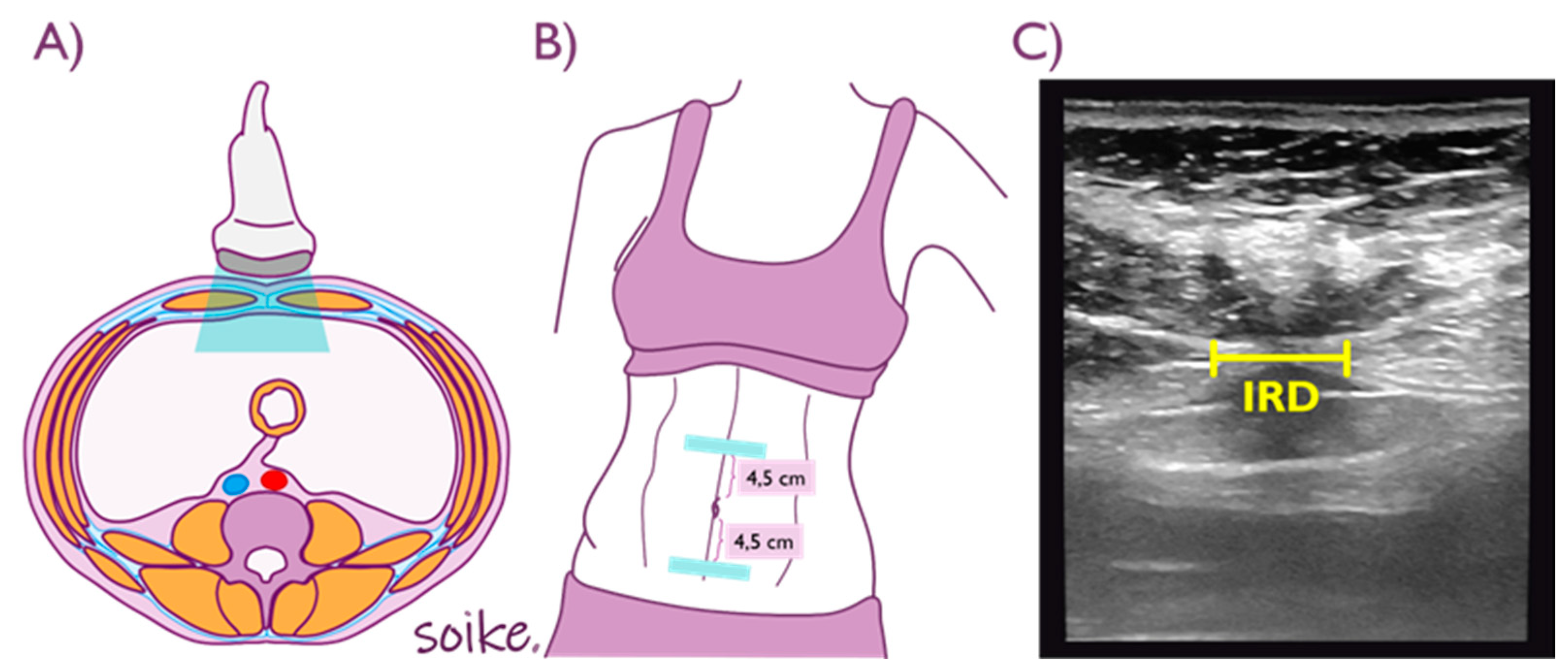
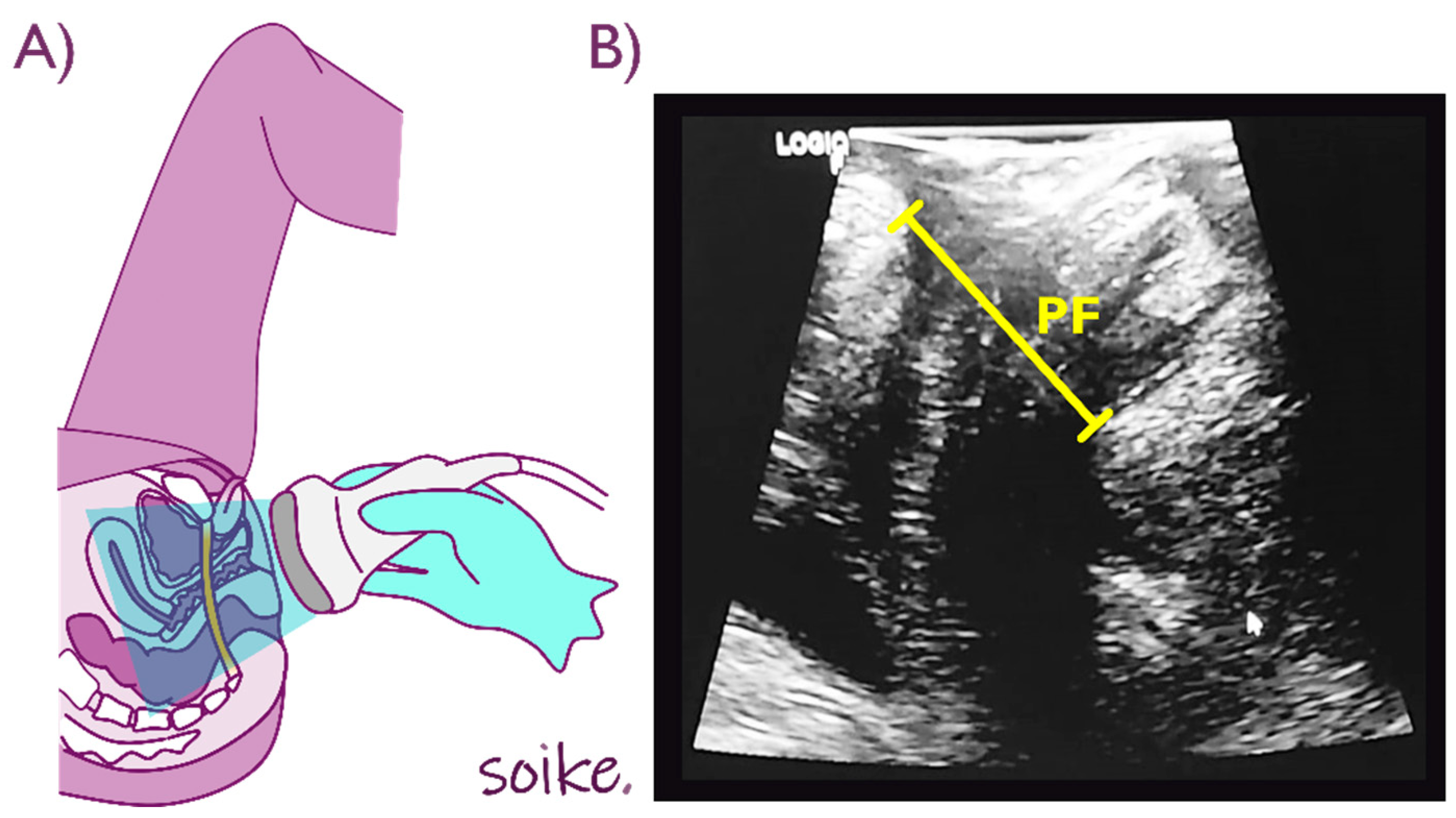
2.5. Outcome Measures
2.6. Statistical Analysis
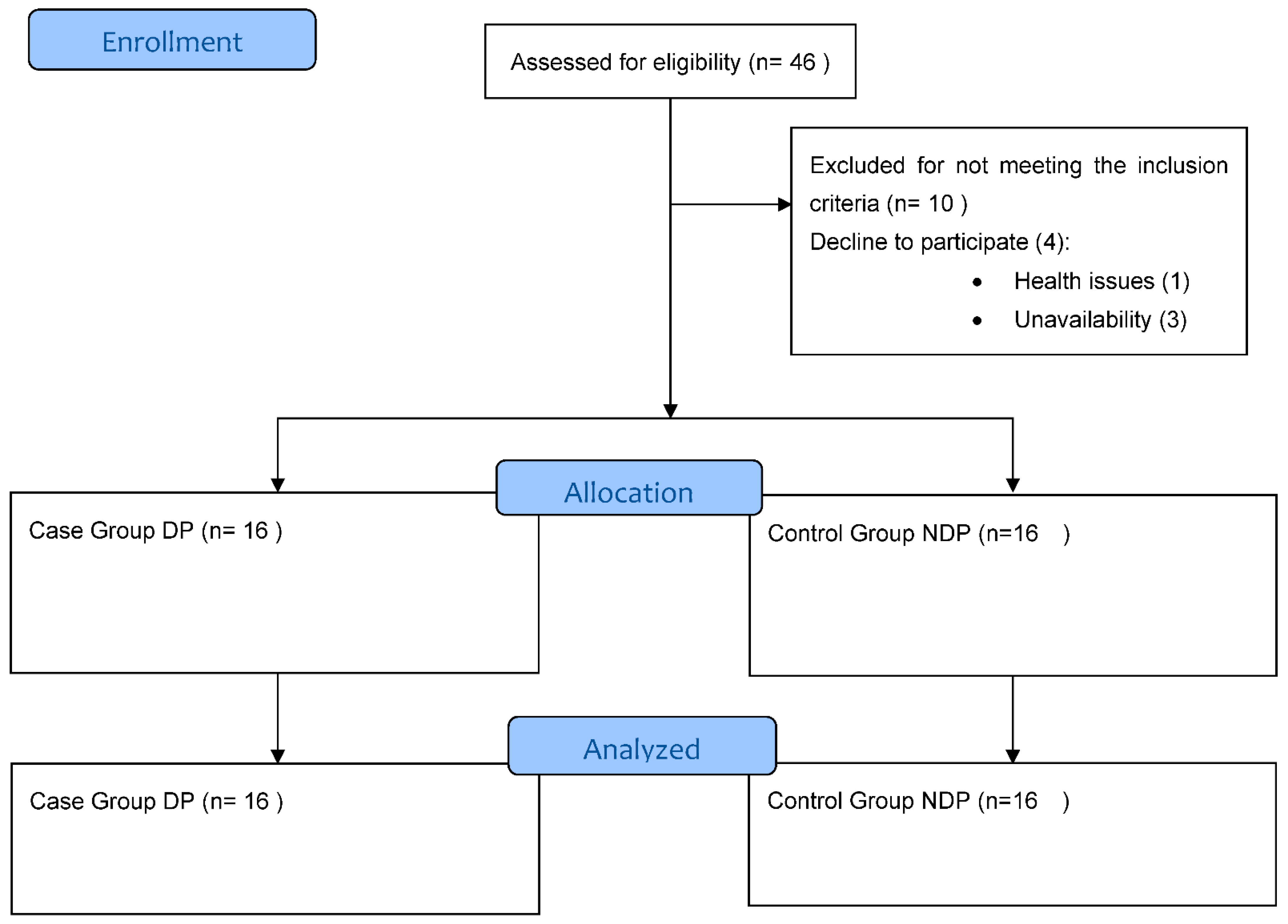
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wallace, S.L.; Miller, L.D.; Mishra, K. Pelvic floor physical therapy in the treatment of pelvic floor dysfunction in women. Curr. Opin. Obstet. Gynecol. 2019, 31, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Berghmans, B. Physiotherapy for pelvic pain and female sexual dysfunction: An untapped resource. Int. Urogynecol. J. 2018, 29, 631–638. [Google Scholar] [CrossRef]
- Alimi, Y.; Iwanaga, J.; Oskouian, R.J.; Loukas, M.; Tubbs, R.S. The clinical anatomy of dyspareunia: A review. Clin. Anat. 2018, 31, 1013–1017. [Google Scholar] [CrossRef]
- Laumann, E.O.; Paik, A.; Rosen, R.C. Sexual Dysfunction in the United StatesPrevalence and Predictors. JAMA 1999, 281, 537–544. [Google Scholar] [CrossRef]
- Ghaderi, F.; Bastani, P.; Hajebrahimi, S.; Jafarabadi, M.A.; Berghmans, B. Pelvic floor rehabilitation in the treatment of women with dyspareunia: A randomized controlled clinical trial. Int. Urogynecol. J. 2019, 30, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Morin, M.; Binik, Y.M.; Bourbonnais, D.; Khalifé, S.; Ouellet, S.; Bergeron, S. Heightened Pelvic Floor Muscle Tone and Altered Contractility in Women with Provoked Vestibulodynia. J. Sex. Med. 2017, 14, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, J.L. Ultrasound imaging of the lateral abdominal wall muscles in individuals with lumbopelvic pain and signs of concurrent hypocapnia. Man. Ther. 2008, 13, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, J.L.; Warner, M.B.; Stokes, M. Comparison of the sonographic features of the abdominal wall muscles and connective tissues in individuals with and without lumbopelvic pain. J. Orthop. Sports Phys. Ther. 2013, 43, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, M.; Del Forno, S.; Spezzano, A.; Raimondo, D.; Arena, A.; Zanello, M.; Leonardi, D.; Paradisi, R.; Seracchioli, R. Painful Love: Superficial Dyspareunia and Three Dimensional Transperineal Ultrasound Evaluation of Pelvic Floor Muscle in Women with Endometriosis. J. Sex Marital Ther. 2020, 46, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Morin, M.; Bergeron, S.; Khalifé, S.; Mayrand, M.-H.; Binik, Y.M. Morphometry of the pelvic floor muscles in women with and without provoked vestibulodynia using 4D ultrasound. J. Sex. Med. 2014, 11, 776–785. [Google Scholar] [CrossRef]
- Delancey, J.O.; Hurd, W.W. Size of the urogenital hiatus in the levator ani muscles in normal women and women with pelvic organ prolapse. Obstet. Gynecol. 1998, 91, 364–368. [Google Scholar] [CrossRef]
- Dietz, H.P.; Shek, C.; Clarke, B. Biometry of the pubovisceral muscle and levator hiatus by three-dimensional pelvic floor ultrasound. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2005, 25, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Dietz, H.P.; Shek, C.; De Leon, J.; Steensma, A.B. Ballooning of the levator hiatus. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2008, 31, 676–680. [Google Scholar] [CrossRef]
- Roos, A.-M.; Speksnijder, L.; Steensma, A.B. Postpartum sexual function; the importance of the levator ani muscle. Int. Urogynecol. J. 2020, 31, 2261–2267. [Google Scholar] [CrossRef]
- Hsu, S.L.; Oda, H.; Shirahata, S.; Watanabe, M.; Sasaki, M. Effects of neuromuscular training on core stability. J. Phys. Ther. Sci. 2018, 30, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Abuín-Porras, V.; Maldonado-Tello, P.; de la Cueva-Reguera, M.; Rodríguez-Sanz, D.; Calvo-Lobo, C.; López-López, D.; Navarro-Flores, E.; Romero-Morales, C. Comparison of Lateral Abdominal Musculature Activation during Expiration with an Expiratory Flow Control Device Versus the Abdominal Drawing-in Maneuver in Healthy Women: A Cross-Sectional Observational Pilot Study. Medicina 2020, 56, 84. [Google Scholar] [CrossRef]
- Sapsford, R.R.; Hodges, P.W.; Richardson, C.A.; Cooper, D.H.; Markwell, S.J.; Jull, G.A. Co-activation of the abdominal and pelvic floor muscles during voluntary exercises. Neurourol. Urodyn. 2001, 20, 31–42. [Google Scholar] [CrossRef]
- Junginger, B.; Baessler, K.; Sapsford, R.; Hodges, P.W. Effect of abdominal and pelvic floor tasks on muscle activity, abdominal pressure and bladder neck. Int. Urogynecol. J. 2010, 21, 69–77. [Google Scholar] [CrossRef]
- Benjamin, D.R.; Frawley, H.C.; Shields, N.; van de Water, A.T.M.; Taylor, N.F. Relationship between diastasis of the rectus abdominis muscle (DRAM) and musculoskeletal dysfunctions, pain and quality of life: A systematic review. Physiotherapy 2019, 105, 24–34. [Google Scholar] [CrossRef]
- Payne, L.A.; Seidman, L.C.; Sim, M.S.; Rapkin, A.J.; Naliboff, B.D.; Zeltzer, L.K. Experimental evaluation of central pain processes in young women with primary dysmenorrhea. Pain 2019, 160, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Romero-Morales, C.; de la Cueva-Reguera, M.; Miñambres-Vallejo, B.; Ruiz-Ruiz, B.; Calvo-Lobo, C.; Casado-Hernández, I.; López-López, D.; Abuín-Porras, V. Ultrasound Assessment of the Abdominal Wall Muscles in Women with and without Primary Dysmenorrhea: A Cross-Sectional Study. Diagnostics 2020, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Rochera, M.B. Physiotherapy in Treating Sexual Pain Disorders in Women: A Systematic Review. Adv. Sex. Med. 2016, 6, 68337. [Google Scholar] [CrossRef][Green Version]
- Li-Yun-Fong, R.J.; Larouche, M.; Hyakutake, M.; Koenig, N.; Lovatt, C.; Geoffrion, R.; Brotto, L.A.; Lee, T.; Cundiff, G.W. Is Pelvic Floor Dysfunction an Independent Threat to Sexual Function? A Cross-Sectional Study in Women with Pelvic Floor Dysfunction. J. Sex. Med. 2017, 14, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Cyr, M.-P.; Dumoulin, C.; Bessette, P.; Pina, A.; Gotlieb, W.H.; Lapointe-Milot, K.; Morin, M. Characterizing Pelvic Floor Muscle Function and Morphometry in Survivors of Gynecological Cancer Who Have Dyspareunia: A Comparative Cross-Sectional Study. Phys. Ther. 2021, 101, pzab042. [Google Scholar] [CrossRef]
- Huffman, L.B.; Hartenbach, E.M.; Carter, J.; Rash, J.K.; Kushner, D.M. Maintaining sexual health throughout gynecologic cancer survivorship: A comprehensive review and clinical guide. Gynecol. Oncol. 2016, 140, 359–368. [Google Scholar] [CrossRef]
- Del Forno, S.; Arena, A.; Pellizzone, V.; Lenzi, J.; Raimondo, D.; Cocchi, L.; Paradisi, R.; Youssef, A.; Casadio, P.; Seracchioli, R. Assessment of levator hiatal area using 3D/4D transperineal ultrasound in women with deep infiltrating endometriosis and superficial dyspareunia treated with pelvic floor muscle physiotherapy: Randomized controlled trial. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2021, 57, 726–732. [Google Scholar] [CrossRef]
- Falkert, A.; Willmann, A.; Endress, E.; Meint, P.; Seelbach-Göbel, B. Three-dimensional ultrasound of pelvic floor: Is there a correlation with delivery mode and persisting pelvic floor disorders 18-24 months after first delivery? Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2013, 41, 204–209. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Stetts, D.M.; Freund, J.E.; Allison, S.C.; Carpenter, G. A rehabilitative ultrasound imaging investigation of lateral abdominal muscle thickness in healthy aging adults. J. Geriatr. Phys. Ther. 2009, 32, 60–66. [Google Scholar] [CrossRef]
- van Veelen, G.A.; Schweitzer, K.J.; van der Vaart, C.H. Ultrasound imaging of the pelvic floor: Changes in anatomy during and after first pregnancy. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2014, 44, 476–480. [Google Scholar] [CrossRef]
- Hides, J.A.; Miokovic, T.; Belavý, D.L.; Stanton, W.R.; Richardson, C.A. Ultrasound Imaging Assessment of Abdominal Muscle Function During Drawing-in of the Abdominal Wall: An Intrarater Reliability Study. J. Orthop. Sport. Phys. Ther. 2007, 37, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Finucane, L.M.; Downie, A.; Mercer, C.; Greenhalgh, S.M.; Boissonnault, W.G.; Pool-Goudzwaard, A.L.; Beneciuk, J.M.; Leech, R.L.; Selfe, J. International Framework for Red Flags for Potential Serious Spinal Pathologies. J. Orthop. Sports Phys. Ther. 2020, 50, 350–372. [Google Scholar] [CrossRef] [PubMed]
- Maselli, F.; Palladino, M.; Barbari, V.; Storari, L.; Rossettini, G.; Testa, M. The diagnostic value of Red Flags in thoracolumbar pain: A systematic review. Disabil. Rehabil. 2022, 44, 1190–1206. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, C.M.; McAuley, J.A. Concurrent Validity of Calipers and Ultrasound Imaging to Measure Interrecti Distance. J. Orthop. Sport. Phys. Ther. 2013, 43, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Zhou, Q. Can We Evaluate Hiatal Ballooning by Measuring the Anteroposterior Diameter with 2-Dimensional Translabial Ultrasonography? J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2018, 37, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Thibault-Gagnon, S.; McLean, L.; Goldfinger, C.; Pukall, C.; Chamberlain, S. Differences in the Biometry of the Levator Hiatus at Rest, During Contraction, and During Valsalva Maneuver Between Women with and without Provoked Vestibulodynia Assessed by Transperineal Ultrasound Imaging. J. Sex. Med. 2016, 13, 243–252. [Google Scholar] [CrossRef]
- Vercellini, P.; Viganò, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef]
- Greene, A.D.; Lang, S.A.; Kendziorski, J.A.; Sroga-Rios, J.M.; Herzog, T.J.; Burns, K.A. Endometriosis: Where are we and where are we going? Reproduction 2016, 152, R63–R78. [Google Scholar] [CrossRef]
- Vercellini, P.; Buggio, L.; Frattaruolo, M.P.; Borghi, A.; Dridi, D.; Somigliana, E. Medical treatment of endometriosis-related pain. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 68–91. [Google Scholar] [CrossRef]
- Yong, P.J. Deep Dyspareunia in Endometriosis: A Proposed Framework Based on Pain Mechanisms and Genito-Pelvic Pain Penetration Disorder. Sex. Med. Rev. 2017, 5, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.-R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef] [PubMed]
- López-Ruiz, M.; Losilla, J.M.; Monfort, J.; Portell, M.; Gutiérrez, T.; Poca, V.; Garcia-Fructuoso, F.; Llorente, J.; Garcia-Fontanals, A.; Deus, J. Central sensitization in knee osteoarthritis and fibromyalgia: Beyond depression and anxiety. PLoS ONE 2019, 14, e0225836. [Google Scholar] [CrossRef] [PubMed]
- van Griensven, H.; Schmid, A.; Trendafilova, T.; Low, M. Central Sensitization in Musculoskeletal Pain: Lost in Translation? J. Orthop. Sports Phys. Ther. 2020, 50, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Hides, J.; Wilson, S.; Stanton, W.; McMahon, S.; Keto, H.; McMahon, K.; Bryant, M.; Richardson, C. An MRI investigation into the function of the transversus abdominis muscle during “drawing-in” of the abdominal wall. Spine 2006, 31, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Urquhart, D.M.; Hodges, P.W.; Allen, T.J.; Story, I.H. Abdominal muscle recruitment during a range of voluntary exercises. Man. Ther. 2005, 10, 144–153. [Google Scholar] [CrossRef]
- Dias-Amaral, A.; Marques-Pinto, A. Female Genito-Pelvic Pain/Penetration Disorder: Review of the Related Factors and Overall Approach. Rev. Bras. Ginecol. Obstet. Rev. Fed. Bras. Soc. Ginecol. Obstet. 2018, 40, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Farnam, F.; Raisi, F.; Parsaeian, M. Prevalence of and Risk Factors for Genito-Pelvic Pain/Penetration Disorder: A Population-Based Study of Iranian Women. J. Sex. Med. 2019, 16, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
| Data | DP (n = 16) | NDP (n = 16) | p-Value Cases vs. Controls |
|---|---|---|---|
| Age, years | 25.38 ± 3.4 * | 26.75 ± 3.99 * | 0.303 ** |
| Weight, kg | 56.66 ± 10.36 * | 62.69 ± 7.24 * | 0.066 ** |
| Height, m | 1.61 ± 0.06 * | 1.66 ± 0.06 * | 0.04 ** |
| BMI, kg/m2 | 21.74 ± 2.86 * | 22.78 ± 1.70 * | 0.221 ** |
| Measurement | DP (n = 16) | NDP (n = 16) | p-Value |
|---|---|---|---|
| Distance (cm) | |||
| IRD SUP rest | 0.93 ± 0.48 *† | 1.3 ± 0.69 * | 0.149 ‡ |
| IRD SUP contraction | 0.87 ± 0.5 * | 1.14 ± 0.61 * | 0.173 ** |
| IRD INF rest | 0.15 ± 0.15 * | 0.21 ± 0.14 * | 0.212 ** |
| IRD SUP contraction | 0.18 ± 0.16 | 0.22 ± 0.15 * | 0.428 ** |
| Thickness (cm) | |||
| RA rest | 0.93 ± 0.16 * | 0.93 ± 0.14 * | 0.958 ** |
| RA contraction | 1.17 ± 0.18 * | 1.21 ± 0.25 * | 0.624 ** |
| TrAb rest | 0.26 ± 0.06 * | 0.28 ± 0.06 * | 0.477 ** |
| TrAb contraction | 0.46 ± 0.11 * | 0.46 ± 0.14 * | 0.989 ** |
| IO rest | 0.49 ± 0.1 * | 0.55 ± 0.12 * | 0.131 ** |
| IO contraction | 0.59 ± 0.17 * | 0.65 ± 0.15 * | 0.27 ** |
| EO rest | 0.35 ± 0.07 * | 0.39 ± 0.11 * | 0.243 ** |
| EO contraction | 0.48 ± 0.15 * | 0.52 ± 0.17 * | 0.482 ** |
| APH (cm) | |||
| Rest | 3.75 ± 0.57 * | 3.66 ± 0.5 * | 0.635 ** |
| Contraction | 3.29 ± 0.46 * | 3.24 ± 0.35 * | 0.723 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castellanos-López, E.; Castillo-Merino, C.; Abuín-Porras, V.; López-López, D.; Romero-Morales, C. Ultrasonography Comparison of Pelvic Floor and Abdominal Wall Muscles in Women with and without Dyspareunia: A Cross-Sectional Study. Diagnostics 2022, 12, 1827. https://doi.org/10.3390/diagnostics12081827
Castellanos-López E, Castillo-Merino C, Abuín-Porras V, López-López D, Romero-Morales C. Ultrasonography Comparison of Pelvic Floor and Abdominal Wall Muscles in Women with and without Dyspareunia: A Cross-Sectional Study. Diagnostics. 2022; 12(8):1827. https://doi.org/10.3390/diagnostics12081827
Chicago/Turabian StyleCastellanos-López, Elena, Camila Castillo-Merino, Vanesa Abuín-Porras, Daniel López-López, and Carlos Romero-Morales. 2022. "Ultrasonography Comparison of Pelvic Floor and Abdominal Wall Muscles in Women with and without Dyspareunia: A Cross-Sectional Study" Diagnostics 12, no. 8: 1827. https://doi.org/10.3390/diagnostics12081827
APA StyleCastellanos-López, E., Castillo-Merino, C., Abuín-Porras, V., López-López, D., & Romero-Morales, C. (2022). Ultrasonography Comparison of Pelvic Floor and Abdominal Wall Muscles in Women with and without Dyspareunia: A Cross-Sectional Study. Diagnostics, 12(8), 1827. https://doi.org/10.3390/diagnostics12081827








