Efficacy of Endobiliary Radiofrequency Ablation in Preserving Survival, Performance Status and Chemotherapy Eligibility of Patients with Unresectable Distal Cholangiocarcinoma: A Case-Control Study
Abstract
1. Introduction
2. Materials and Methods
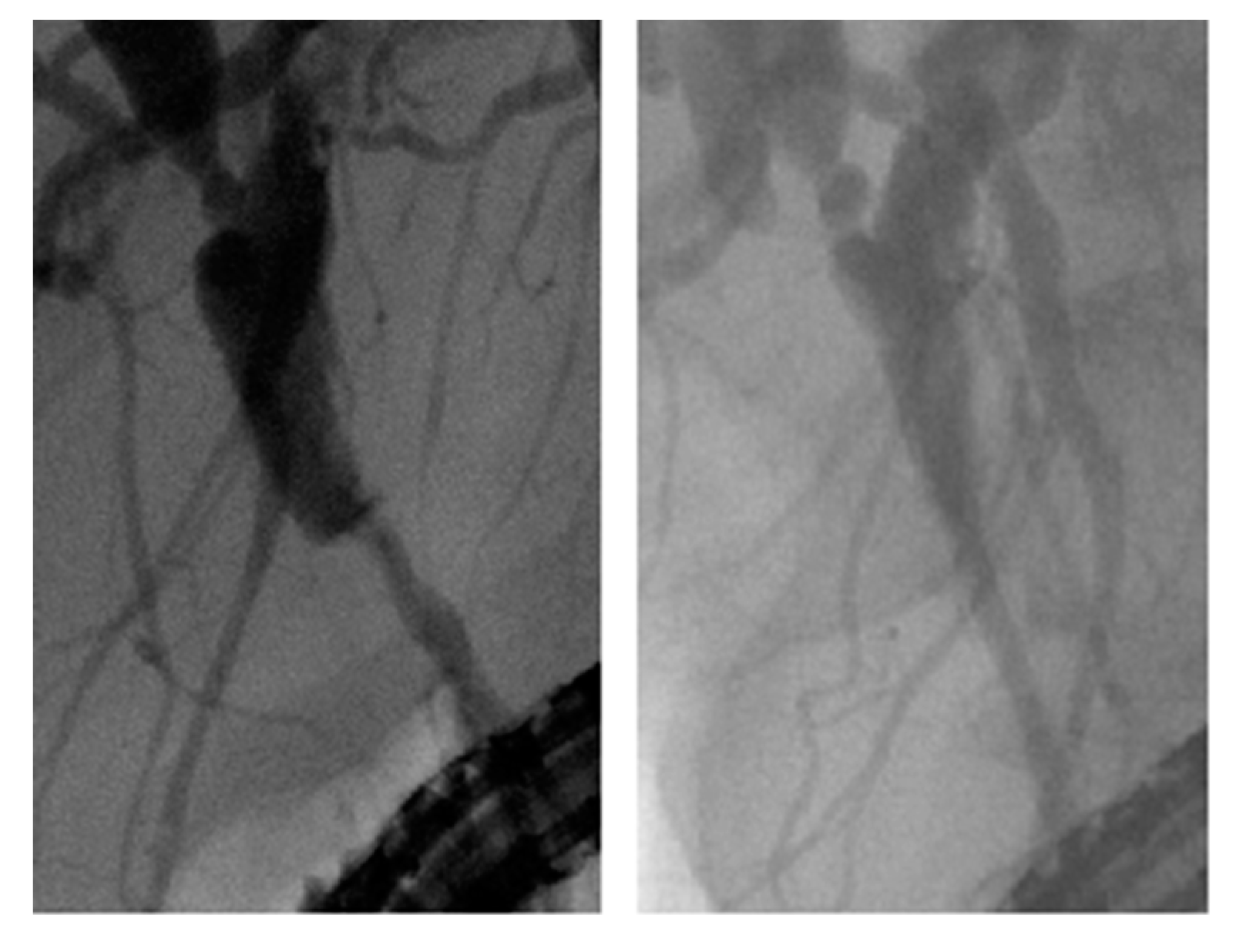
2.1. Patients, Procedures, and Study Protocol
2.2. Statistical Analysis and Methodology
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blechacz, B. Cholangiocarcinoma: Current Knowledge and New Developments. Gut Liver 2017, 11, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Chen, C.P.; Wu, E. Precision Medicine in Cholangiocarcinoma: Past, Present, and Future. Life 2022, 12, 829. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Hu, Z.; Song, J.; Wu, Y.; Zhang, B.; Zhang, L. The state of therapy modalities in clinic for biliary tract cancer. Front. Biosci.-Landmark 2022, 27, 185. [Google Scholar] [CrossRef] [PubMed]
- Zografos, G.N.; Farfaras, A.; Zagouri, F.; Chrysikos, D.; Karaliotas, K. Cholangiocarcinoma: Principles and current trends. Hepatobiliary Pancreat. Dis. Int. 2011, 10, 10–20. [Google Scholar] [CrossRef]
- Jang, S.; Stevens, T.; Parsi, M.A.; Bhatt, A.; Kichler, A.; Vargo, J.J. Superiority of Self-Expandable Metallic Stents over Plastic Stents in Treatment of Malignant Distal Biliary Strictures. Clin. Gastroenterol. Hepatol. 2022, 20, e182–e195. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, B.S.; Sandulescu, L.; Şurlin, V.; Spârchez, Z.; Săftoiu, A. Surgical hepatic resection vs. ultrasonographic guided radiofrequency ablation in colorectal liver metastases: What should we choose? Med. Ultrason. 2014, 16, 145–151. [Google Scholar] [CrossRef][Green Version]
- Trojan, J.; Hoffmeister, A.; Neu, B.; Kasper, S.; Dechêne, A.; Jürgensen, C.; Schirra, J.; Jakobs, R.; Palmer, D.; Selbo, P.K.; et al. Photochemical Internalization of Gemcitabine Is Safe and Effective in Locally Advanced Inoperable Cholangiocarcinoma. Oncologist 2022, 27, e430–e433. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, J.; Niu, L.; Liu, Y.; Ye, G.; Jiang, M.; Qi, Z. Clinical Safety and Efficacy of Locoregional Therapy Combined with Adoptive Transfer of Allogeneic γδ T Cells for Advanced Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J. Vasc. Interv. Radiol. 2022, 33, 19–27.e3. [Google Scholar] [CrossRef]
- Bokemeyer, A.; Matern, P.; Bettenworth, D.; Cordes, F.; Nowacki, T.M.; Heinzow, H.; Kabar, I.; Schmidt, H.; Ullerich, H.; Lenze, F. Endoscopic Radiofrequency Ablation Prolongs Survival of Patients with Unresectable Hilar Cholangiocellular Carcinoma—A Case-Control Study. Sci. Rep. 2019, 9, 13685. [Google Scholar] [CrossRef]
- Ahmed, O.; Lee, J.H. Modern gastrointestinal endoscopic techniques for biliary tract cancers. Chin. Clin. Oncol. 2020, 9, 3. [Google Scholar] [CrossRef]
- Dumonceau, J.M.; Kapral, C.; Aabakken, L.; Papanikolaou, I.S.; Tringali, A.; Vanbiervliet, G.; Beyna, T.; Dinis-Ribeiro, M.; Hritz, I.; Mariani, A.; et al. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2020, 52, 127–149. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhara, V.; Khashab, M.A.; Muthusamy, V.R.; Acosta, R.D.; Agrawal, D.; Bruining, D.H.; Eloubeidi, M.A.; Fanelli, R.D.; Faulx, A.L.; Gurudu, S.R.; et al. Adverse events associated with ERCP. Gastrointest. Endosc. 2017, 85, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Kim, E.J.; Chung, D.H.; Kim, Y.J.; Kim, Y.S.; Park, Y.H.; Kim, K.K.; Cho, J.H. Endobiliary radiofrequency ablation for distal extrahepatic cholangiocarcinoma: A clinicopathological study. PLoS ONE 2018, 13, e0206694. [Google Scholar] [CrossRef] [PubMed]
- Dolak, W.; Schreiber, F.; Schwaighofer, H.; Gschwantler, M.; Plieschnegger, W.; Ziachehabi, A.; Mayer, A.; Kramer, L.; Kopecky, A.; Schrutka-Kölbl, C.; et al. Endoscopic radiofrequency ablation for malignant biliary obstruction: A nationwide retrospective study of 84 consecutive applications. Surg. Endosc. 2014, 28, 854–860. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Zhou, H.; Zhou, Y.; Wang, Y.; Jin, H.; Lou, Q.; Zhang, X. Efficacy and safety of endoscopic radiofrequency ablation for unresectable extrahepatic cholangiocarcinoma: A randomized trial. Endoscopy 2018, 50, 751–760. [Google Scholar] [CrossRef]
- Inoue, T.; Naitoh, I.; Kitano, R.; Ibusuki, M.; Kobayashi, Y.; Sumida, Y.; Nakade, Y.; Ito, K.; Yoneda, M. Endobiliary Radiofrequency Ablation Combined with Gemcitabine and Cisplatin in Patients with Unresectable Extrahepatic Cholangiocarcinoma. Curr. Oncol. 2022, 29, 2240–2251. [Google Scholar] [CrossRef]
- Camus, M.; Napoléon, B.; Vienne, A.; Le Rhun, M.; Leblanc, S.; Barret, M.; Chaussade, S.; Robin, F.; Kaddour, N.; Prat, F. Efficacy and safety of endobiliary radiofrequency ablation for the eradication of residual neoplasia after endoscopic papillectomy: A multicenter prospective study. Gastrointest. Endosc. 2018, 88, 511–518. [Google Scholar] [CrossRef]
- Hu, B.; Gao, D.J.; Zhang, X.; Zhang, Y.C. 121 Endobiliary Radiofrequency Ablation Improve Overall Survival of Cholangiocarcinoma: A Multi-Center Randomized Control Study. Gastrointest. Endosc. 2016, 83, AB126. [Google Scholar] [CrossRef]
- Yu, T.; Zhang, W.; Li, C.; Wang, C.; Gong, G.; Wang, L.; Li, G.; Chen, Y.; Wang, X. Percutaneous intraductal radiofrequency ablation combined with biliary stent placement for treatment of malignant biliary obstruction. Abdom. Radiol. 2020, 45, 3690–3697. [Google Scholar] [CrossRef]
- Sofi, A.A.; Khan, M.A.; Das, A.; Sachdev, M.; Khuder, S.; Nawras, A.; Lee, W. Radiofrequency ablation combined with biliary stent placement versus stent placement alone for malignant biliary strictures: A systematic review and meta-analysis. Gastrointest. Endosc. 2018, 87, 944–951.e1. [Google Scholar] [CrossRef] [PubMed]
- Buerlein, R.; Strand, D.S.; Patrie, J.T.; Sauer, B.G.; Shami, V.M.; Scheiman, J.M.; Zaydfudim, V.M.; Bauer, T.W.; Adams, R.B.; Wang, A.Y. 544 ERCP-directed biliary ablation prolongs survival in patients with unresectable perihilar cholangiocarcinoma compared to stenting alone. Gastrointest. Endosc. 2019, 89, AB91–AB92. [Google Scholar] [CrossRef]
- Teoh, A.Y.; Cheung, S.Y.; Chong, C.; Lee, K.F.; Ng, E.K.; Lai, P.B.; Lau, J.Y. 613 endoscopic biliary radiofrequency ablation for malignant distal common bile duct strictures does not improve survival. A randomized controlled trial. Gastrointest. Endosc. 2018, 87, AB104–AB105. [Google Scholar] [CrossRef]
- Albers, D.; Schmidt, A.; Schiemer, M.; Caca, K.; Wannhoff, A.; Sauer, P.; Wiesweg, M.; Schumacher, B.; Dechene, A. Impact of endobiliary radiofrequency ablation on biliary drainage in patients with malignant biliary strictures treated with uncovered self-expandable metal stents: A randomized controlled multicenter-trial. Gastrointest. Endosc. 2022, in press. [Google Scholar] [CrossRef]
- Pereira, P.; Santos, A.L.; Morais, R.; Vilas-Boas, F.; Rodrigues-Pinto, E.; Santos-Antunes, J.; Macedo, G. Endoscopic radiofrequency ablation for palliative treatment of hilar cholangiocarcinoma. Video GIE 2021, 6, 195–198. [Google Scholar] [CrossRef]
- Wang, F.; Li, Q.; Zhang, X.; Jiang, G.; Ge, X.; Yu, H.; Nie, J.; Ji, G.; Miao, L. Endoscopic radiofrequency ablation for malignant biliary strictures. Exp. Ther. Med. 2016, 11, 2484–2488. [Google Scholar] [CrossRef][Green Version]
- Nair, P.; Rao, H.; Koshy, A.K.; Krishnapriya, S.; Venu, R.P. Safety and Efficacy of Endobiliary Radio-Frequency Ablation in Hilar Cholangiocarcinoma. J. Gastroenterol. Hepatol. Res. 2022, 11, 3658–3664. [Google Scholar]
- Steel, A.W.; Postgate, A.J.; Khorsandi, S.; Nicholls, J.; Jiao, L.; Vlavianos, P.; Habib, N.; Westaby, D. Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointest. Endosc. 2011, 73, 149–153. [Google Scholar] [CrossRef]
- Abou Ali, E.; Barret, M. Radiofrequency ablation for cholangiocarcinoma: Do we need to be more precise? Endosc. Int. Open 2019, 7, E1301–E1302. [Google Scholar] [CrossRef]



| Patients | Average Survival (from First Hospitalization until Death) | 95% CI for Average Survival Months | p-Value of the Comparison Test against the Life Expectancy Value (3 Months) |
|---|---|---|---|
| Control group | 16 | 6.8–25.4 months | 0.009 |
| Study group | 19 | 5–33 months | 0.035 |
| Patients | Average of the Minimal TB (mg/dL) | 95% CI | Average of the Maximal TB (mg/dL) | 95% CI |
|---|---|---|---|---|
| Control group | 6.62 | 2.5–10.7 | 9.69 | 6.1–13.2 |
| Study group | 3.48 | 1.1–5.8 | 5.88 | 1.9–9.8 |
| p | 0.028 | 0.017 | ||
| Patients | Average of the Minimal AST (U/L) | 95% CI | Average of the Maximal AST (U/L) | 95% CI |
|---|---|---|---|---|
| Control group | 105.53 | 60.7–150.3 | 172.33 | 91.5–253.17 |
| Study group | 72.75 | 29–135.7 | 105.39 | 40.6–170.15 |
| p | 0.035 | 0.243 | ||
| ALT(U/L) | ALT (U/L) | |||
| Control group | 70.46 | 43–97.9 | 175.3 | 86.3–264.3 |
| Study group | 64.12 | 24.3–103.9 | 133.12 | 22.8–244.4 |
| p | 0.77 | 0.53 | ||
| Patients | Average of the minimal urea level (mg/dL) | 95% CI | Average of the maximal urea level (mg/dL) | 95% CI |
|---|---|---|---|---|
| Control group | 39.3 | 25.12–53.47 | 53.8 | 35.7–71.8 |
| Study group | 32.5 | 28.4–36.6 | 39.3 | 33.4–45.17 |
| p | 0.393 | 0.01 | ||
| Average of the minimal creatinine level (mg/dL) | 95% CI | Average of the maximal creatinine level (mg/dL) | 95% CI | |
| Control group | 1.02 | 0.72–1.32 | 1.44 | 0.94–1.94 |
| Study group | 0.62 | 0.6–0.64 | 0.72 | 0.59–0.84 |
| p | 0.012 | 0.008 | ||
| Patients/eGFR | Average eGFR in the Control Group ± St. Dev. | Average eGFR in the Study Group ± St. Dev. | p |
|---|---|---|---|
| eGFR/minimal creatinine | 68.38 ± 31.04 | 104.28 ± 11.96 | 0.022 |
| eGFR/maximal creatinine | 48.73 ± 20.22 | 89.02 ± 11.94 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandru, V.; Ungureanu, B.S.; Stan-Ilie, M.; Oprita, R.; Balan, G.G.; Plotogea, O.-M.; Rinja, E.; Butuc, A.; Panaitescu, A.; Constantinescu, A.; et al. Efficacy of Endobiliary Radiofrequency Ablation in Preserving Survival, Performance Status and Chemotherapy Eligibility of Patients with Unresectable Distal Cholangiocarcinoma: A Case-Control Study. Diagnostics 2022, 12, 1804. https://doi.org/10.3390/diagnostics12081804
Sandru V, Ungureanu BS, Stan-Ilie M, Oprita R, Balan GG, Plotogea O-M, Rinja E, Butuc A, Panaitescu A, Constantinescu A, et al. Efficacy of Endobiliary Radiofrequency Ablation in Preserving Survival, Performance Status and Chemotherapy Eligibility of Patients with Unresectable Distal Cholangiocarcinoma: A Case-Control Study. Diagnostics. 2022; 12(8):1804. https://doi.org/10.3390/diagnostics12081804
Chicago/Turabian StyleSandru, Vasile, Bogdan Silviu Ungureanu, Madalina Stan-Ilie, Ruxandra Oprita, Gheorghe G. Balan, Oana-Mihaela Plotogea, Ecaterina Rinja, Andreea Butuc, Afrodita Panaitescu, Alexandru Constantinescu, and et al. 2022. "Efficacy of Endobiliary Radiofrequency Ablation in Preserving Survival, Performance Status and Chemotherapy Eligibility of Patients with Unresectable Distal Cholangiocarcinoma: A Case-Control Study" Diagnostics 12, no. 8: 1804. https://doi.org/10.3390/diagnostics12081804
APA StyleSandru, V., Ungureanu, B. S., Stan-Ilie, M., Oprita, R., Balan, G. G., Plotogea, O.-M., Rinja, E., Butuc, A., Panaitescu, A., Constantinescu, A., Gheonea, D. I., & Constantinescu, G. (2022). Efficacy of Endobiliary Radiofrequency Ablation in Preserving Survival, Performance Status and Chemotherapy Eligibility of Patients with Unresectable Distal Cholangiocarcinoma: A Case-Control Study. Diagnostics, 12(8), 1804. https://doi.org/10.3390/diagnostics12081804








