Mapping Evidence of Self-Sampling to Diagnose Sexually Transmitted Infections in Women: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Identifying the Research Question
2.2. Identifying Relevant Studies
- Peer-reviewed journal articles;
- Studies presenting evidence on self-sampling interventions for STIs;
- Studies presenting evidence on self-sampling in women for STI diagnosis;
- Studies of all designs with relevant information; and
- Studies focussing on the type, acceptability, feasibility, and effectiveness of self-sampling.
- Focused on self-sampling interventions for HIV only; and
- Only presented evidence of specimens collected by healthcare workers for STI diagnosis.
2.3. Selection of Studies
2.4. Data Charting
2.5. Quality Appraisal of Included Articles
2.6. Collating, Summarising, and Reporting Results
3. Results
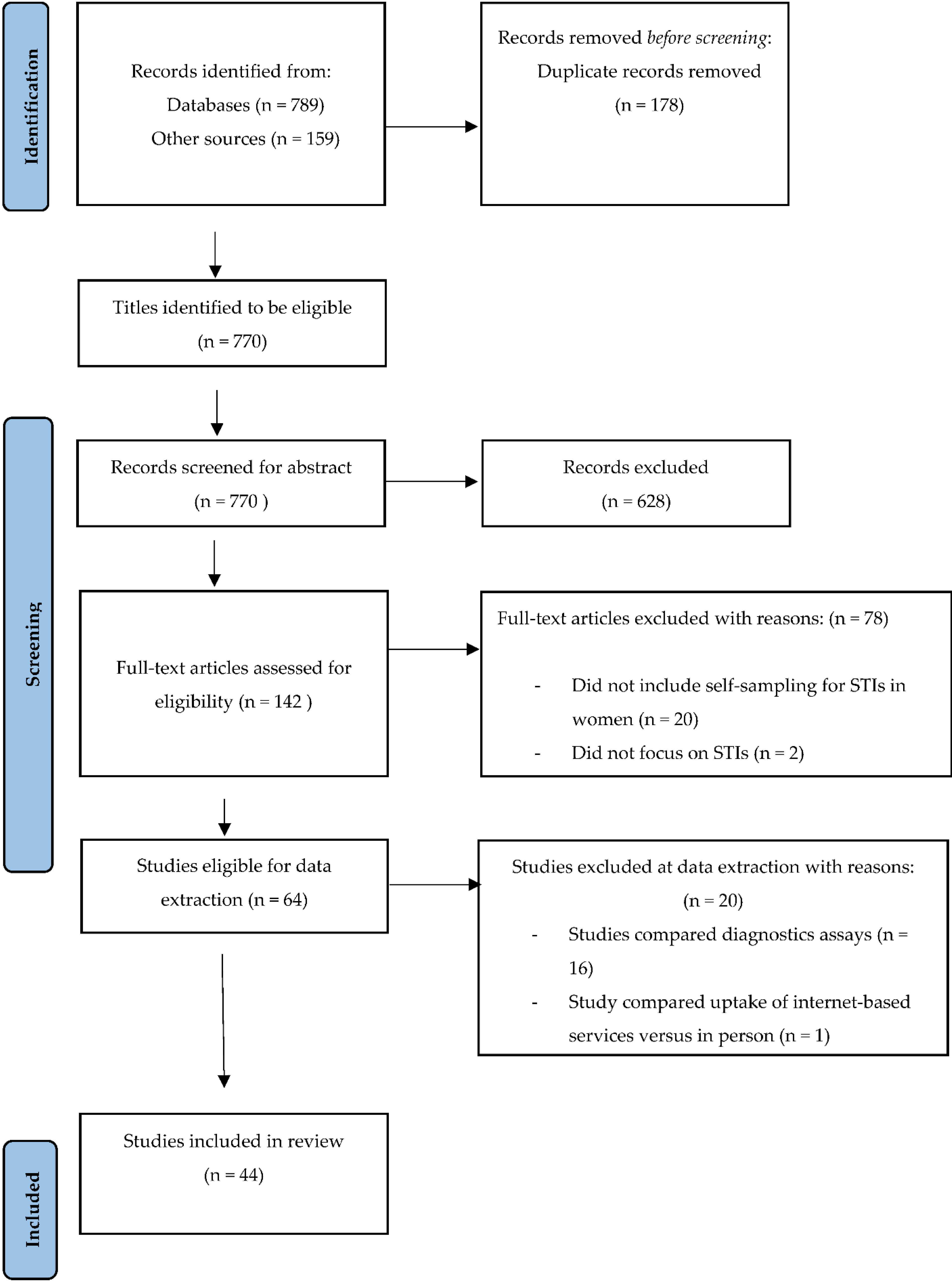
3.1. Screening Results
3.2. Quality Appraisal
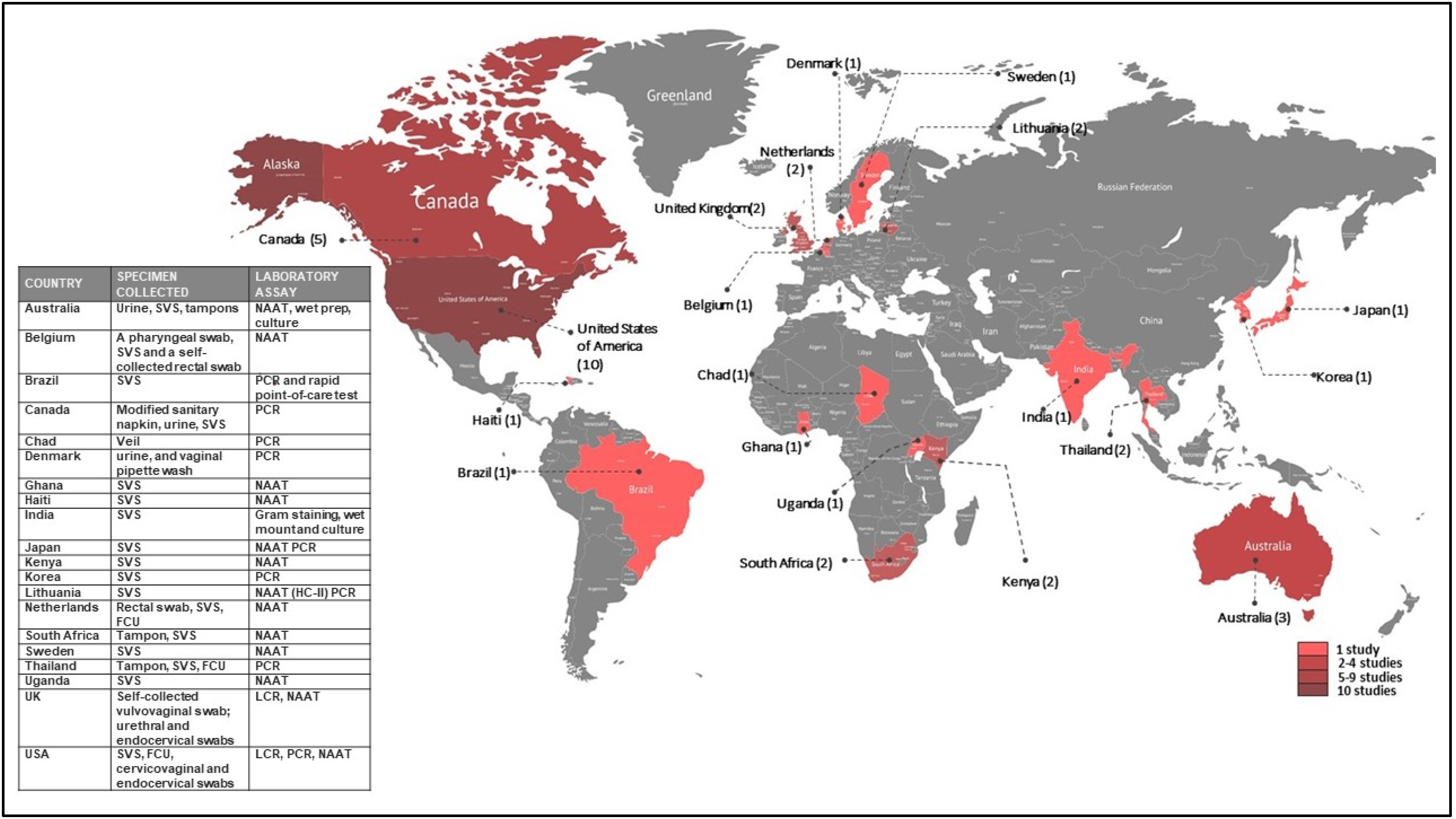
3.3. Characteristics of Studies
3.4. Summary of Findings
3.4.1. Feasibility, Acceptance, and Ease of Self-Sampling Interventions
3.4.2. Types of Self-Sampled Specimens
3.4.3. Diagnostic Accuracy in Self-Collected Specimens
3.4.4. Agreement between Physician-Collected and Self-Sampled Specimens
3.4.5. Pooled Specimens for STI Diagnosis
3.4.6. Self-Testing of Self-Collected Specimens
4. Discussion
4.1. Strengths and Limitations
4.2. Implications for Practice
4.3. Recommendations for Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC2 | Aptima Combo 2 |
| ATV | Aptima Trichomonas vaginalis |
| AHPV | Aptima Human Papilloma Virus |
| BV | Bacterial vaginosis |
| CT | Chlamydia trachomatis |
| CI | Confidence interval |
| CIN-II | Cervical intraepithelial lesion |
| FSW | Female sex workers |
| FCU | First-catch urine |
| HC-II | Hybrid Capture II |
| HSIL | High-grade squamous intraepithelial lesion |
| HR-HPV | High-risk human papilloma virus |
| HIC | High-Income Country |
| HPV | Human Papilloma Virus |
| LMIC | Low- and middle-income countries |
| LCR | Ligase chain reaction |
| MG | Mycoplasma genitalium |
| mPCR/RLB | Multiplex polymerase chain reaction/reverse line blot |
| NG | Neisseria gonorrhoea |
| NPV | Negative predictive value |
| NAAT | Nucleic Acid Amplification Test |
| P-VSCT | Physician-collected specimen collection and transport |
| POCT | Point-of-care testing |
| PPV | Positive predictive value |
| POC | Point-of-Care |
| PCR | Polymerase Chain Reaction |
| PC-VS | Patient-collected vaginal swab |
| PRISMA-ScR | Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for Scoping Reviews |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-analyses |
| STI | Sexually Transmitted infections |
| SCT | Self-collection and transport |
| S-VSCT | Self-collected specimen collection and transport |
| SIS | Single intravaginal swab |
| SVS | Self-collected vaginal swab |
| TV | Trichomonas vaginalis |
| USA | United States of America |
| UK | United Kingdom |
| VVC | Vulvovaginal Candida |
References
- World Health Organization. Report on Global Sexually Transmitted Infection Surveillance; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Panchanadeswaran, S.; Johnson, S.C.; Mayer, K.H.; Srikrishnan, A.K.; Sivaran, S.; Zelaya, C.E.; Go, V.F.; Solomon, S.; Bentley, M.E.; Celentano, D.D. Gender differences in the prevalence of sexually transmitted infections and genital symptoms in an urban setting in southern India. Sex. Transm. Infect. 2006, 82, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Organización Mundial de la Salud (Ginebra, Suiza), Światowa Organizacja Zdrowia, World Health Organization; UNAIDS. Guidelines for the Management of Sexually Transmitted Infections; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Otieno, F.O.; Ndivo, R.; Oswago, S.; Ondiek, J.; Pals, S.; McLellan-Lemal, E.; Chen, R.T.; Chege, W.; Gray, K.M. Evaluation of syndromic management of sexually transmitted infections within the Kisumu Incidence Cohort Study. Int. J. STD AIDS 2014, 25, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Paudyal, P.; Llewellyn, C.; Lau, J.; Mahmud, M.; Smith, H. Obtaining self-samples to diagnose curable sexually transmitted infections: A systematic review of patients’ experiences. PLoS ONE 2015, 10, e0124310. [Google Scholar] [CrossRef] [PubMed]
- Nodjikouambaye, Z.A.; Compain, F.; Sadjoli, D.; Mboumba Bouassa, R.S.; Péré, H.; Veyer, D.; Robin, L.; Adawaye, C.; Tonen-Wolyec, S.; Moussa, A.M.; et al. Accuracy of Curable Sexually Transmitted Infections and Genital Mycoplasmas Screening by Multiplex Real-Time PCR Using a Self-Collected Veil among Adult Women in Sub-Saharan Africa. Infect. Dis. Obstet. Gynecol. 2019, 2019, 8639510. [Google Scholar] [CrossRef]
- Ogale, Y.; Yeh, P.T.; Kennedy, C.E.; Toskin, I.; Narasimhan, M. Self-collection of samples as an additional approach to deliver testing services for sexually transmitted infections: A systematic review and meta-analysis. BMJ Glob. Health 2019, 4, e001349. [Google Scholar] [CrossRef]
- Lunny, C.; Taylor, D.; Hoang, L.; Wong, T.; Gilbert, M.; Lester, R.; Krajden, M.; Ogilvie, G. Self-Collected versus Clinician-Collected Sampling for Chlamydia and Gonorrhea Screening: A Systemic Review and Meta-Analysis. PLoS ONE 2015, 10, e0132776. [Google Scholar]
- Mbatha, J.N.; Galappaththi-Arachchige, H.N.; Mtshali, A.; Taylor, M.; Ndhlovu, P.D.; Kjetland, E.F.; Baay, M.F.D.; Mkhize-Kwitshana, Z.L. Self-sampling for human papillomavirus testing among rural young women of KwaZulu-Natal, South Africa. BMC Res. Notes 2017, 10, 702. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Sector Strategy on Sexually Transmitted Infections 2016–2021: Toward Ending STIs; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Garrett, N.J.; McGrath, N.; Mindel, A. Advancing STI Care in Low/Middle-Income Countries: Has STI Syndromic Management Reached Its Use-by Date? BMJ Publishing Group Ltd.: London, UK, 2017; pp. 4–5. [Google Scholar]
- Chesson, H.W.; Mayaud, P.; Aral, S.O. Sexually transmitted infections: Impact and cost-effectiveness of prevention. In Disease Control Priorities, Major Infectious Diseases; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2017; p. 6. [Google Scholar]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef]
- Peters, M.; Godfrey, C.; McInerney, P.; Soares, C.; Khalil, H.; Parker, D. The Joanna Briggs Institute Reviewers’ Manual 2015: Methodology for JBI Scoping Reviews; The Joanna Briggs Institute: Adelaide, Australia, 2015. [Google Scholar]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Bethel, A.C.; Rogers, M.; Abbott, R. Use of a search summary table to improve systematic review search methods, results, and efficiency. J. Med. Libr. Assoc. JMLA 2021, 109, 97. [Google Scholar] [CrossRef] [PubMed]
- Morris, B.J.; Rose, B.R. Cervical screening in the 21st century: The case for human papillomavirus testing of self-collected specimens. Clin. Chem. Lab. Med. 2007, 45, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.; Gilchrist, J.; Portillo, E.; Smieja, M.; Toor, R.; Chernesky, M. Comparison of dacron and nylon-flocked self-collected vaginal swabs and urine for the detection of Trichomonas vaginalis using analyte-specific reagents in a transcription-mediated amplification assay. Sex. Transm. Infect. 2012, 88, 160–162. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.M.; Raymond, M.; Noll, W.W.; Belloni, D.R.; Duncan, L.T.; Cole, B.F. Tampon samplings with longer cervicovaginal cell exposures are equivalent to two consecutive swabs for the detection of high-risk human papillomavirus. Sex. Transm. Dis. 2002, 29, 628–636. [Google Scholar] [CrossRef]
- Chernesky, M.; Jang, D.; Gilchrist, J.; Elit, L.; Lytwyn, A.; Smieja, M.; Dockter, J.; Getman, D.; Reid, J.; Hill, C. Evaluation of a new APTIMA specimen collection and transportation kit for high-risk human papillomavirus E6/E7 messenger RNA in cervical and vaginal samples. Sex. Transm. Dis. 2014, 41, 365–368. [Google Scholar] [CrossRef]
- Li, J.; Jang, D.; Gilchrist, J.; Smieja, M.; Ewert, R.; MacRitchie, C.; Chernesky, M. Comparison of flocked and aptima swabs and two specimen transport media in the aptima combo 2 assay. J. Clin. Microbiol. 2014, 52, 3808–3809. [Google Scholar] [CrossRef][Green Version]
- Lockhart, A.; Psioda, M.; Ting, J.; Campbell, S.; Mugo, N.; Kwatampora, J.; Chitwa, M.; Kimani, J.; Gakure, A.; Smith, J.S. Prospective Evaluation of Cervicovaginal Self- and Cervical Physician Collection for the Detection of Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, and Mycoplasma genitalium Infections. Sex. Transm. Dis. 2018, 45, 488–493. [Google Scholar] [CrossRef]
- Ostergaard, L.; Moller, J.K.; Andersen, B.; Olesen, F. Diagnosis of urogenital Chlamydia trachomatis infection in women based on mailed samples obtained at home: Multipractice comparative study. BMJ 1996, 313, 1186–1189. [Google Scholar] [CrossRef]
- Stewart, C.M.; Schoeman, S.A.; Booth, R.A.; Smith, S.D.; Wilcox, M.H.; Wilson, J.D. Assessment of self taken swabs versus clinician taken swab cultures for diagnosing gonorrhoea in women: Single centre, diagnostic accuracy study. BMJ 2012, 345, e8107. [Google Scholar] [CrossRef]
- Verougstraete, N.; Verbeke, V.; De Cannière, A.-S.; Simons, C.; Padalko, E.; Coorevits, L. To pool or not to pool? Screening of Chlamydia trachomatis and Neisseria gonorrhoeae in female sex workers: Pooled versus single-site testing. Sex. Transm. Infect. 2020, 96, 417–421. [Google Scholar] [CrossRef]
- Van De Wijgert, J.; Altini, L.; Jones, H.; De Kock, A.; Young, T.; Williamson, A.L.; Hoosen, A.; Coetzee, N. Two methods of self-sampling compared to clinician sampling to detect reproductive tract infections in Gugulethu, South Africa. Sex. Transm. Dis. 2006, 33, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Van Der Pol, B.; Taylor, S.; Liesenfeld, O.; Williams, J.; Hook, E. Vaginal swabs are the optimal specimen for detection of genital Chlamydia trachomatis or Neisseria gonorrhoeae using the Cobas 4800 CT/NG test. Sex. Transm. Dis. 2013, 40, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Winer, R.L.; Feng, Q.; Hughes, J.P.; Yu, M.; Kiviat, N.B.; O’Reilly, S.A.; Koutsky, L.A. Concordance of self-collected and clinician-collected swab samples for detecting human papillomavirus DNA in women 18 to 32 years of age. Sex. Transm. Dis. 2007, 34, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Ting, J.; Mugo, N.; Kwatampora, J.; Hill, C.; Chitwa, M.; Patel, S.; Gakure, H.; Kimani, J.; Schoenbach, V.J.; Poole, C.; et al. High-risk human papillomavirus messenger RNA testing in physician- and self-collected specimens for cervical lesion detection in high-risk women, Kenya. Sex. Transm. Dis. 2013, 40, 584–589. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakayama, H.; Sagiyama, K.; Haraoka, M.; Yoshida, H.; Hagiwara, T.; Akazawa, K.; Naito, S. Evaluation of a new amplified enzyme immunoassay (EIA) for the detection of Chlamydia trachomatis in male urine, female endocervical swab, and patient obtained vaginal swab specimens. J. Clin. Pathol. 2000, 53, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, S.N.; Fairley, C.K.; Chen, S.; Giouzeppos, O.; Paterson, B.; Bowden, F.J.; Garland, S.M. Evaluation of Patient-Administered Tampon Specimens forChlamydia trachomatisandNeisseria gonorrhoeae. Sex. Transm. Dis. 2000, 27, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Shafer, M.A.; Moncada, J.; Boyer, C.B.; Betsinger, K.; Flinn, S.D.; Schachter, J. Comparing first-void urine specimens, self-collected vaginal swabs, and endocervical specimens to detect Chlamydia trachomatis and Neisseria gonorrhoeae by a nucleic acid amplification test. J. Clin. Microbiol. 2003, 41, 4395–4399. [Google Scholar] [CrossRef]
- Safaeian, M.; Kiddugavu, M.; Gravitt, P.E.; Ssekasanvu, J.; Murokora, D.; Sklar, M.; Serwadda, D.; Wawer, M.J.; Shah, K.V.; Gray, R. Comparability of self-collected vaginal swabs and physician-collected cervical swabs for detection of human papillomavirus infections in Rakai, Uganda. Sex. Transm. Dis. 2007, 34, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Rompalo, A.M.; Gaydos, C.A.; Shah, N.; Tennant, M.; Crotchfelt, K.A.; Madico, G.; Quinn, T.C.; Daniel, R.; Shah, K.V.; Gaydos, J.C.; et al. Evaluation of use of a single intravaginal swab to detect multiple sexually transmitted infections in active-duty military women. Clin. Infect. Dis. 2001, 33, 1455–1461. [Google Scholar] [CrossRef][Green Version]
- Obiri-Yeboah, D.; Adu-Sarkodie, Y.; Djigma, F.; Hayfron-Benjamin, A.; Abdul, L.; Simpore, J.; Mayaud, P. Self-collected vaginal sampling for the detection of genital human papillomavirus (HPV) using care HPV among Ghanaian women. BMC Women’s Health 2017, 17, 86. [Google Scholar] [CrossRef]
- McLarty, J.W.; Williams, D.L.; Loyd, S.; Hagensee, M.E. Cervical Human Papillomavirus Testing with Two Home Self-Collection Methods Compared With a Standard Clinically Collected Sampling Method. Sex. Transm. Dis. 2019, 46, 670–675. [Google Scholar] [CrossRef] [PubMed]
- MacMillan, S.; McKenzie, H.; Flett, G.; Templeton, A. Feasibility of patient-collected vulval swabs for the diagnosis of Chlamydia trachomatis in a family planning clinic: A pilot study. Br. J. Fam. Plann. 2000, 26, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Levy, V.; Blackmore, C.S.; Klausner, J.D. Self-collection of specimens for nucleic acid-based diagnosis of pharyngeal, cervicovaginal, urethral, and rectal Neisseria gonorrhoeae and Chlamydia trachomatis infections. In Diagnosis of Sexually Transmitted Diseases; Springer: Berlin/Heidelberg, Germany, 2012; pp. 407–418. [Google Scholar]
- Kucinskiene, V.; Juseviciute, V.; Valiukeviciene, S.; Milasauskiene, Z.; Unemo, M.; Domeika, M. Home sampling and pooling of vaginal samples are effective tools for genetic screening of Chlamydia trachomatis among high school female students in Lithuania. Scand. J. Infect. Dis. 2008, 40, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Knox, J.; Tabrizi, S.N.; Miller, P.; Petoumenos, K.; Law, M.; Chen, S.; Garland, S.M. Evaluation of self-collected samples in contrast to practitioner-collected samples for detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis by polymerase chain reaction among women living in remote areas. Sex. Transm. Dis. 2002, 29, 647–654. [Google Scholar] [CrossRef]
- Kim, M.H.; Jung, H.J.; Park, S.I.; Kim, B.J. Self-obtained vaginal samples for HPV DNA testing to detect HPV-related cervical disease. Int. J. Gynaecol. Obstet. 2021, 154, 127–132. [Google Scholar] [CrossRef]
- Khan, Z.; Bhargava, A.; Mittal, P.; Bharti, R.; Puri, P.; Khunger, N.; Bala, M. Evaluation of reliability of self-collected vaginal swabs over physician-collected samples for diagnosis of bacterial vaginosis, candidiasis and trichomoniasis, in a resource-limited setting: A cross-sectional study in India. BMJ Open 2019, 9, e025013. [Google Scholar] [CrossRef] [PubMed]
- Geelen, T.H.; Rossen, J.W.; Beerens, A.M.; Poort, L.; Morré, S.A.; Ritmeester, W.S.; van Kruchten, H.E.; van de Pas, M.M.; Savelkoul, P.H. Performance of cobas® 4800 and m2000 real-time™ assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in rectal and self-collected vaginal specimen. Diagn. Microbiol. Infect. Dis. 2013, 77, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Husman, C.; DeSilva, L.; Chang, R.; Peralta, L. Evaluation of self-collected vaginal swab, first void urine, and endocervical swab specimens for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae in adolescent females. J. Pediatr. Adolesc. Gynecol. 2008, 21, 355–360. [Google Scholar] [CrossRef]
- Falk, L.; Coble, B.I.; Mjornberg, P.A.; Fredlund, H. Sampling for Chlamydia trachomatis infection—A comparison of vaginal, first-catch urine, combined vaginal and first-catch urine and endocervical sampling. Int. J. STD AIDS 2010, 21, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Des Marais, A.C.; Zhao, Y.; Hobbs, M.M.; Barclay, L.; Brewer, N.T.; Smith, J.S. Home Self-Collection by Mail to Test for Human Papillomavirus and Sexually Transmitted Infections. Obstet. Gynecol. 2018, 132, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Domeika, M.; Drulyte, O. Use of PCR for the detection of genital Chlamydia trachomatis infection on self-obtained mailed vaginal samples. Acta Obstet. Gynecol. Scand. 2000, 79, 570–575. [Google Scholar] [PubMed]
- Chandeying, V.; Lamlertkittikul, S.; Skov, S. A comparison of first-void urine, self-administered low vaginal swab, self-inserted tampon, and endocervical swab using PCR tests for the detection of infection with Chlamydia trachomatis. Sex. Health 2003, 1, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Chernesky, M.; Jang, D.; Gilchrist, J.; Hatchette, T.; Poirier, A.; Flandin, J.F.; Smieja, M.; Ratnam, S. Head-to-head comparison of second-generation nucleic acid amplification tests for detection of Chlamydia trachomatis and Neisseria gonorrhoeae on urine samples from female subjects and self-collected vaginal swabs. J. Clin. Microbiol. 2014, 52, 2305–2310. [Google Scholar] [CrossRef] [PubMed]
- Boggan, J.C.; Walmer, D.K.; Henderson, G.; Chakhtoura, N.; McCarthy, S.H.; Beauvais, H.J.; Smith, J.S. Vaginal self-sampling for HPV infection as a primary cervical cancer screening tool in a Haitian population. Sex. Transm. Dis. 2015, 42, 655. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.; Dan, J.; Gilchrist, J.; Luinstra, K.; Li, J.; Smieja, M.; Chernesky, M.A. Ease, Comfort, and Performance of the HerSwab Vaginal Self-Sampling Device for the Detection of Chlamydia trachomatis and Neisseria gonorrhoeae. Sex. Transm. Dis. 2016, 43, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Alary, M.; Poulin, C.; Bouchard, C.; Fortier, M.; Murray, G.; Gingras, S.; Aubé, M.; Morin, C. Evaluation of a modified sanitary napkin as a sample self-collection device for the detection of genital chlamydial infection in women. J. Clin. Microbiol. 2001, 39, 2508–2512. [Google Scholar] [CrossRef] [PubMed]
- Holland-Hall, C.; Wiesenfeld, H.; Murray, P. Self-collected vaginal swabs for the detection of multiple sexually transmitted infections in adolescent girls. J. Pediatr. Adolesc. Gynecol. 2002, 15, 307–313. [Google Scholar] [CrossRef]
- Wiesenfeld, H.C.; Heine, R.P.; Rideout, A.; Macio, I.; DiBiasi, F.; Sweet, R.L. The vaginal introitus: A novel site for Chlamydia trachomatis testing in women. Am. J. Obstet. Gynecol. 1996, 174, 1542–1546. [Google Scholar] [CrossRef]
- Chernesky, M.; Jang, D.; Gilchrist, J.; Randazzo, J.; Elit, L.; Lytwyn, A.; Smieja, M.; Reid, J.; Hill, C. Ease and comfort of cervical and vaginal sampling for Chlamydia trachomatis and Trichomonas vaginalis with a new Aptima specimen collection and transportation kit. J. Clin. Microbiol. 2014, 52, 668–670. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Karwalajtys, T.; Howard, M.; Sellors, J.; Kaczorowski, J. Vaginal self sampling versus physician cervical sampling for HPV among younger and older women. Sex. Transm. Infect. 2006, 82, 337–339. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bialasiewicz, S.; Whiley, D.M.; Buhrer-Skinner, M.; Bautista, C.; Barker, K.; Aitken, S.; Gordon, R.; Muller, R.; Lambert, S.B.; Debattista, J.; et al. A novel gel-based method for self-collection and ambient temperature postal transport of urine for PCR detection of Chlamydia trachomatis. Sex. Transm. Infect. 2009, 85, 102–105. [Google Scholar] [CrossRef] [PubMed]
- van Dommelen, L.; Dukers-Muijrers, N.; van Tiel, F.H.; Brouwers, E.E.; Hoebe, C.J. Evaluation of one-sample testing of self-obtained vaginal swabs and first catch urine samples separately and in combination for the detection of Chlamydia trachomatis by two amplified DNA assays in women visiting a sexually transmitted disease clinic. Sex. Transm. Dis. 2011, 38, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.E.; Lippman, S.A.; Caiaffa-Filho, H.H.; Young, T.; van de Wijgert, J.H. Performance of a rapid self-test for detection of Trichomonas vaginalis in South Africa and Brazil. J. Clin. Microbiol. 2013, 51, 1037–1039. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, G.S.; Patrick, D.M.; Schulzer, M.; Sellors, J.W.; Petric, M.; Chambers, K.; White, R.; FitzGerald, J.M. Diagnostic accuracy of self-collected vaginal specimens for human papillomavirus compared to clinician collected human papillomavirus specimens: A meta-analysis. Sex. Transm. Infect. 2005, 81, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Pritt, B.S. Home Self-collection and Specimen Pooling: Tools for Convenient and Economical Detection of Sexually Transmitted Infections. Clin. Infect. Dis. 2020, 73, e3194–e3195. [Google Scholar] [CrossRef] [PubMed]
- Flowers, P.; Pothoulaki, M.; Vojt, G.; Mapp, F.; Owusu, M.W.; Estcourt, C.; Cassell, J.; Saunders, J. Understanding the barriers and facilitators to using self-sampling packs for sexually transmitted infections and blood borne viruses: Thematic analyses supporting intervention optimisation. medRxiv 2020. [Google Scholar] [CrossRef]
- Desa, U. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: San Francisco, CA, USA, 2016. [Google Scholar]
- Dize, L.; West, S.; Mkocha, H.; Quinn, T.; Gaydos, C. Evaluation of pooled ocular and vaginal swabs by the Cepheid GeneXpert CT/NG assay for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae compared to the GenProbe Aptima Combo 2 Assay. Diagn. Microbiol. Infect. Dis. 2015, 81, 102–104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- World Health Organization. World Health Day 2019: Campaign Essentials; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Habel, M.A.; Leichliter, J.S.; Dittus, P.J.; Spicknall, I.H.; Aral, S.O. Heterosexual Anal and Oral Sex in Adolescents and Adults in the United States, 2011–2015. Sex. Transm. Dis. 2018, 45, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Schwebke, J.R.; Gaydos, C.A.; Davis, T.; Marrazzo, J.; Furgerson, D.; Taylor, S.N.; Smith, B.; Bachmann, L.H.; Ackerman, R.; Spurrell, T.; et al. Clinical evaluation of the Cepheid Xpert TV assay for detection of Trichomonas vaginalis with prospectively collected specimens from men and women. J. Clin. Microbiol. 2018, 56, e01091-17. [Google Scholar] [CrossRef] [PubMed]
- Hesse, E.A.; Patton, S.A.; Huppert, J.S.; Gaydos, C.A. Using a rapid communication approach to improve a POC Chlamydia test. IEEE Trans. Biomed. Eng. 2011, 58, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Van Der Pol, B.; Williams, J.A.; Taylor, S.N.; Cammarata, C.L.; Rivers, C.A.; Body, B.A.; Nye, M.; Fuller, D.; Schwebke, J.R.; Barnes, M.; et al. Detection of Trichomonas vaginalis DNA by use of self-obtained vaginal swabs with the BD ProbeTec Qx assay on the BD Viper system. J. Clin. Microbiol. 2014, 52, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Tucker, J.D.; Bien, C.H.; Peeling, R.W. Point-of-care testing for sexually transmitted infections: Recent advances and implications for disease control. Curr. Opin. Infect. Dis. 2013, 26, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W. Applying new technologies for diagnosing sexually transmitted infections in resource-poor settings. Sex. Transm. Infect. 2011, 87, ii28–ii30. [Google Scholar] [CrossRef] [PubMed]
- Huppert, J.S.; Taylor, R.G.; St Cyr, S.; Hesse, E.A.; Reed, J.L. Point-of-care testing improves accuracy of STI care in an emergency department. Sex. Transm. Infect. 2013, 89, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.H.; Dang, A.K.; Vu, G.T.; Nguyen, C.T.; Le, T.H.T.; Truong, N.T.; Hoang, C.L.; Tran, T.T.; Tran, T.H.; Pham, H.Q.; et al. Lack of knowledge about sexually transmitted diseases (STDs): Implications for STDs prevention and care among dermatology patients in an urban city in Vietnam. Int. J. Environ. Res. Public Health 2019, 16, 1080. [Google Scholar] [CrossRef]
- Zizza, A.; Guido, M.; Recchia, V.; Grima, P.; Banchelli, F.; Tinelli, A. Knowledge, information needs and risk perception about HIV and sexually transmitted diseases after an education intervention on Italian high school and university students. Int. J. Environ. Res. Public Health 2021, 18, 2069. [Google Scholar] [CrossRef]
- Mahlangu, P.T.; KNzaumvila, D.; Ramochele-Ngwenya, M.M.; HMabuza, L. Knowledge, Attitudes, and Beliefs of Childbearing Women at a District Hospital in South Africa Regarding Sexually Transmitted Infections. Open Public Health J. 2021, 14, 399–408. [Google Scholar] [CrossRef]
- Nigussie, T.; Yosef, T. Knowledge of sexually transmitted infections and its associated factors among polytechnic college students in Southwest Ethiopia. Pan Afr. Med. J. 2020, 37, 68. [Google Scholar] [CrossRef]
- Pinto, C.N.; Niles, J.K.; Kaufman, H.W.; Marlowe, E.M.; Alagia, D.P.; Chi, G.; Van Der Pol, B. Impact of the COVID-19 Pandemic on Chlamydia and Gonorrhea Screening in the US. Am. J. Prev. Med. 2021, 61, 386–393. [Google Scholar] [CrossRef]
| Criteria | Determinants | Description |
|---|---|---|
| Population | Women | Women of sexual reproductive age |
| Concept | Self-sampling interventions |
|
| Context | STIs | STIs in women excluding Human Immunodeficiency Virus (HIV). |
| Date | Database | Keywords | Number of Results Retrieved |
|---|---|---|---|
| 14 July 2021 | Scopus | (TITLE-ABS-KEY (sampling OR sample OR “self sampling” OR “self sample” OR “sti testing” OR “sti diagnosis” OR “sexually transmitted infections test*” OR “self-collect*” OR “sexually transmitted disease testing*”) AND TITLE-ABS-KEY (“Specimen Handling”) AND TITLE-ABS-KEY (“Sexually Transmitted Disease*” OR “sexually transmitted infection*”) AND TITLE-ABS-KEY (wom*n OR female* OR girl*) AND NOT TITLE-ABS-KEY (aids OR “HIV Infections” OR hiv OR “human immunodeficiency virus” OR “acquired immunodeficiency syndrome”)) | 117 |
| 15 July 2022 | Cochrane | (sampling OR sample OR “self sampling” OR “self sample” OR “sti testing” OR “sti diagnosis” OR “sexually transmitted infections test*” OR “self-collect*” OR “sexually transmitted disease testing*”):ti,ab,kw (Word variations have been searched) | 26 |
| 19 July 2021 | PubMed | (((sampling[tw] OR sample[tw] OR “self sampling”[tw] OR “self sample”[tw] OR “sti testing”[tw] OR “sti diagnosis”[tw] OR “sexually transmitted infections test*”[tw] OR “self-collect*”[tw] OR “sexually transmitted disease testing*”[tw] AND (female[Filter])) AND (“Specimen Handling/methods”[Mesh] OR “Specimen Handling”[tw] AND (female[Filter]))) AND (“Sexually Transmitted Diseases, Bacterial”[Mesh] OR “Sexually Transmitted Diseases, Viral”[Mesh] OR “sexually transmitted infection*”[tw] OR “sexually transmitted disease*”[tw])) NOT (“HIV Infections”[Mesh] OR “HIV Infections”[tw]) | 213 |
| 19 July 2022 | Web of Science | ((((ALL=(sampling OR sample OR “self sampling” OR “self sample” OR “sti testing” OR “sti diagnosis” OR “sexually transmitted infections test*” OR “self-collect*” OR “sexually transmitted disease testing*”)) AND ALL=(“Sexually Transmitted Disease*” OR “sexually transmitted infection*” OR STI OR STD)) AND ALL=(wom*n OR female* OR girl*)) AND ALL=(“Specimen Handling” or “Specimen Collection” OR Specimen)) NOT ALL=(aids OR “HIV Infections” OR hiv OR “human immunodeficiency virus” OR “acquired immunodeficiency syndrome”) | 311 |
| 21 July 2022 | Medline (EBSCO) | (((ALL=(sampl* OR “self sampl*” OR “sti test*” OR “sti diagnosis” OR “sexually transmitted infections test*” OR “self-collect*” OR “sexually transmitted disease test*”))) AND ALL=( ) NOT ALL=(″) | 140 |
| Author | Country | Aim | Population and Sample Size | Self-Sampling Intervention | Diagnostic Test | Key Findings |
|---|---|---|---|---|---|---|
| Weisenfeld et al., 1996 [55] | USA | Agreement between physician-collected specimens and self-sampling in patients with urogenital CT. | n = 300 of which 200 self-samples and 100 samples from a pilot study | Vaginal introitus swab | Amplicor CT test | Vaginal introitus swabs, provider-collected to detect urogenital CT: sensitivity = 92% (95% coefficient of variation (CI), 83 to 100). Sensitivity of vaginal introitus swabs was greater than PCR, culture or enzyme immunoassay of the cervix or urethra. Self-sampling, PCR: sensitivity = 81%. Urine samples, PCR: sensitivity = 73%. |
| Ostergaard et al., 1996 [24] | Denmark | Self-sampling to collect urogenital samples at home, mailed to the laboratory for CT deoxyribonucleic acid (DNA) analysis. Diagnostic efficacy was compared to provider- collected urethral and endocervical swabs. | n = 222 aged 18–25 years | First-catch urine (FCU), vaginal pipette wash | Amplicor PCR | Prevalence of CT = 11.2% (23/205 women). Self-sampling, PCR: Sensitivity = 96%, specificity = 92.9%. Self-sampling, LCR: Sensitivity = 100%, specificity = 99.5%. Provider-collected: Sensitivity = 91%, specificity = 100%. |
| Tanaka et al., 2000 [31] | Japan | Compare vaginal swabs obtained by providers and self-sampling to screen for CT infection. | Group 1 = 193 men, 187 women Group 2 = 91 high-risk sex workers | Vaginal swab, FCU, endocervical sample | New generation amplified immunoassay IDEIA PCE chlamydia kit and PCR | Male urine samples and female endocervical swabs: IDEIA PCE performed similarly to the Amplicor PCR. Relative sensitivity of IDEIA (79.3%), IDEIA PCE (91.4%), and Amplicor PCR (100%) on male first-void urine specimens. Relative sensitivities of IDEIA (85%), IDEIA PCE (95%), and Amplicor PCR (100%) on female endocervical specimens. Self-sampled vaginal swabs (SVS), IDEIA PCE: positivity rate = 25.2%. Clinician-collected vaginal specimens, IDEIA PCE: positivity rate = 23.1%. Clinician-collected endocervical swabs, PCR and IDEIA PCE, positivity rate = 27.5%. |
| Tabrizi et al., 2000 [32] | Australia | Evaluate two commercial amplification systems detecting CT and NG from tampon specimens | n = 400 tampon specimens | Tampon specimens | In-house PCR assay, Abbott LCR, Roche cobas® Amplicor | Detection of CT, commercial assays similar to in-house PCR (p = 0.68, p = 0.73). Detection of NG, in-house PCR superior to Abbott LCR (p = 0.0001) but similar to Roche PCR (p = 0.11). Roche PCR and LCR similar detection of CT. LCR testing of extracted DNA did not increase sensitivity. |
| Domeika et al., 2000 [48] | Lithuania | Using self-sampled and mailed specimens to detect genital CT | n = 94 | Vaginal introital sample | PCR (AMPLICOR CT, Roche Diagnostic Systems, Inc., Branchburg, N) | CT, self-sampling, PCR vs. cell culture: Sensitivity = 100% Vaginal samples, PCR: Sensitivity = 100%, >PCR and cell culture on cervical samples. Single vaginal sampling, PCR: Sensitivity = 100%. Self-samples, mailed vaginal specimens are feasible for PCR-testing for genital CT. Self-sampling would help to reach a section of the population in which pelvic examination and cervical sampling are not routinely performed. |
| Macmillan et al., 2000 [38] | UK | The feasibility of using self-sampled vulval swabs, instead of FCU to diagnose female genital CT infection in a family planning population. | n = 103 younger than 25 years old | vulval swab, urine | LCR | Prevalence of CT = 11.7%. Vulval swabs had 100% sensitivity, 100% specificity, and 100% Positive Predictive Value (PPV) and Negative Predictive Value (NPV). FCU had 91.7% sensitivity, 100% specificity, and PPV = 100% and NPV = 98.9%. Women found both tests to be acceptable. |
| Rompalo et al., 2001 [35] | USA | Evaluate a single intra- vaginal swab (SIS) for simultaneous detection of NG, CT, Trachomatis vaginalis (TV), and HPV infections among military women on active duty. | n = 793 | Intravaginal swab (a Dacron SIS from the AMPLICOR collection kit) | A combination test that uses PCR combined with DNA probe hybridization in a colorimetric detection assay. | NG culture: sensitivity = 70.8%, specificity = 100%. NG PCR: sensitivity = 95.8%, specificity = 97.8%. CT enzyme immunoassay: sensitivity = 72.8%, specificity = 90%. CT PCR: sensitivity = 94.6%, specificity = 99.3%. Self-sampling with an SIS accurately detects multiple STIs. |
| Alary et al., 2001 [53] | Canada | Evaluate a modified sanitary napkin as a self-sampling device to detect CT infection in women. Self-sampled specimens vs. endocervical and FCU from the same women. | n = 246 | Modified sanitary napkin, FCU | cobas® Amplicor PCR | Modified sanitary napkin, PCR: sensitivity = 93.1% (95% CI, 83.3 to 98.1%), specificity = 98.9% (95% CI, 97.4 to 99.6%). FCU, PCR: sensitivity = 81.0% (95% CI, 68.6 to 90.1%), specificity = 100% (95% CI, 99.2 to 100%). Modified sanitary napkin: PPV = 91.5% (54 of 59), NPV = 99.1% (447 of 451). Urine samples: PPV = 100% (47 of 47), NPV = 97.6% (451 of 462). Modified sanitary napkins may be an effective non-invasive device for self- sampling to detect urogenital CT infection. |
| Harper et al., 2002 [20] | USA | Compare the detection of high-risk HPV using tampons with longer exposure times in the cervicovaginal vault vs. self-sampling swabs. Women’s acceptance of sampling with a tampon for longer periods. | n = 103 aged 16 years and older. | Tampon | PCR | 309 tampons vs. 618 self-sampled swabs, 83% were returned. Among women, the 10-s tampon detected fewer with normal histology and high-risk HPV (HR-HPV) relative to swabs (p = 0.0412). The 1 h, 4 h, and overnight tampons had similar detection rates to swabs. In women with cervical intraepithelial neoplasma (CIN), tampons and swabs similarly identified HR-HPV. |
| Holland-Hall et al., 2002 [54] | USA | The use of self-sampling to screen female adolescent detainees for three organisms in a setting where speculum exams are not feasible. | Sample size not indicated | Vaginal swab | PCR | Self-sampling and endocervical testing yielded similar results for NG (K: 0.614, p = 0.001), CT (K: 0.865, p = 0.001). Self-sampling and vaginal microscopy yielded similar results for TV (K: 0.627, p = 0.001). All participants supported the practice of self-sampling using a vaginal swab. All participants stated willingness to perform self-testing in between their regular pelvic exams. |
| Knox et al., 2002 [41] | Australia | Compared FCU, SVS, self-sampled tampon and practitioner-collected endocervical swab specimens to detect NG, CT and TV. | n = 318 | Vaginal swab, urine, tampon, endocervical swab | Culture, wet prep and Nucleic Acid Amplification Test (NAAT) PCR | Detection rate, PCR: CT = 11.5%, NG = 11.8%, TV = 24.6%. PCR significantly more sensitive than microscopy and culture in detecting NG and TV. CT, PCR: Sensitivity, tampons = 100%; FCU = 72.7% NG, PCR: Sensitivity, tampons = 97.2%, endocervical swab = 92.6%, self-sampled swab = 71.9%, FCU = 31.2%. Sensitivity of urine PCR for detecting NG improved with freezing of urine specimens and shorter transport time. TV, PCR: Sensitivity, tampons = 100%, TV = 87.7%. |
| Chandeying et al., 2003 [49] | Thailand | Compared several specimen types to detect CT infection. Assess the acceptability of self-sampling. | n = 953 | urine, vaginal swab, tampon | PCR | CT prevalence = 17.6% amongst female sex workers (FSWs) and 5.7% amongst outpatient women. Acceptability: Tampon = 72.6%, self-sampled vaginal swab = 74.2%. In FSWs: Sensitivity, tampon = 95.9%, SVS = 89.2%, more sensitive than either urine or endocervical swabs. In outpatient women: Sensitivity, endocervical swabs = 100%, tampons and SVS = 85.7%. Specificity was >98% for all sampling methods for both groups. |
| Shafer et al., 2003 [33] | USA | Compare FCU, self-collected vaginal swabs and physician-collected endocervical specimens to detect CT and NG in a large cohort of young women upon entering the military. | n = 2157 | FCU and vaginal swab | NAAT—LCR | SVS: best detection of CT and NG. CT, detection rate: FCU = 72%, endocervical specimen = 64%, FCU/vaginal swab = 94. Women preferred self-sampling to routine pelvic examinations. |
| Ogilvie et al., 2005 [61] | n/a | Meta-analysis comparing the accuracy of patient-collected vaginal specimens with clinician- collected specimens for detecting HPV-DNA. | n = 106 studies | Multiple specimen types, Dacron, cotton swab, cytobrush, tampons | PCR, Hybrid Capture II (HCII) | Self-sampling vs. clinician-collected specimens: sensitivity = 0.74, specificity = 0.88. Self-sampling in referral settings: sensitivity = 0.81, specificity = 0.90. Tampons offered sensitivity between 0.67–0.94 (n = 4 studies). PCR and HC-II offered similar sensitivity. |
| Karwalajtys et al., 2006 [57] | Canada | Agreement physician obtained cervical and SVS to detect HPV DNA. Women’s preferences for collection method according to age | n = 543 women aged 15 to 49 years and a group of 50 years and older | SVS | HC-II assay for carcinogenic HPV | n = 307 women, aged 15–49 years. Prevalence of HPV: vaginal swabs = 20.8% (64/307), cervical specimens = 17.6% (54/307). Prevalence of HPV, women older than 50 years, vaginal swabs = 9.9% (15/152), cervical specimens = 8.6% (13/152). Vaginal swabs vs. cervical specimens: Agreement ƙ = 0.54 (younger women) and ƙ = 0.37 (older women) (both p < 0.001), indicating fair agreement. Nearly half of women preferred self- sampling or had no preference. |
| Van de Wijgert et al., 2006 [27] | South Africa | Self-sampling using vaginal swabs or tampons compared to physician- obtained swabs | n = 450 | Tampon, vaginal swab | cobas® Amplicor CT/NG test, TV by MDM culture, bacterial vaginosis (BV) by Nugent scoring of a Gram-stain slide, 22 Candida species by Sabdex culture, and high-risk HPV types by the Digene HC-II for hrHPV DNA Test. | Self-sampling (tampons and swabs): satisfactory validity for NG, CT, BV, and Candida species. Self-sampling (swabs): satisfactory validity for HR-HPV. Self-sampling was not suitable for diagnosing TV by culture. Self-sampling was feasible and acceptable, but some women preferred speculum examinations, which allowed the clinician to view the vagina and cervix. |
| Morris and Rose 2007 [18] | Not indicated | HPV detection as primary cervical cancer screening | Sample size not indicated | Tampons, vaginal swabs | PCR NAAT | PCR tests for HPV show high test sensitivity and reliability PCR tests for HPV could be adopted as a stand-alone test, and, if positive, other tests such as p16INK4a or cytology could be used to increase specificity. Women can self-sample and send samples to laboratories Self-sampling is convenient and easy. Suited to the lifestyles and busy schedules of the modern woman. |
| Kucinskiene et al., 2007 [40] | Lithuania | The utility of self-sampling and pooling of samples for screening for CT among sexually active students. | n = 424 | Vaginal swabs | Digene HC-II CT/NG Test | CT was present in 30 (5.6%) of 533 vaginal samples. Out of the 177 pools (three samples per pool), 29 pools were positive for CT/NG. 26 positive pools contained at least one positive CT sample and two contained two positive CT samples. The remaining CT/NG positive pool was only positive for NG. HC-II, pooled vaginal samples: Sensitivity = 100%, specificity = 100%. 30 (7.1%) sexually active students (20–24 years old, n = 424) tested positive for CT. Prevalence in high schools ranged from 0 to 1%. Prevalence in college students was as high as 14.2%. |
| Winer et al., 2007 [29] | USA | SVS vs. physician-collected cervical vs. physician-collected vulvovaginal swabs in women. Compared ability of mailed samples and in-clinic self-collected samples to detect HPV DNA. | n = 374 | Vaginal swab | HPV PCR analysis | HPV detection: physician-collected cervical/vulvovaginal > clinician-collected vulvovaginal > self-sampled vaginal > clinician-collected cervical Agreement between sampling modalities: women (25 to 30 years) = 86.5–95.7% (κ 0.65–0.92); women (18 to 25 years) = 94.9–98.8% (κ 0.84–0.96). |
| Safaeian et al., 2007 [34] | Uganda | Compare SVS and physician-collected cervical swabs in their ability to detect HPV DNA. | n = 2223 | Vaginal swab | HC-II determined carcinogenic HPV. PCR to determine HPV genotypes. | More than 86% of women complied with self-sampling, only 51% accepted a pelvic examination. HR-HPV, prevalence = 19% (self-sampling and physician-collected samples) Self-sampling vs. physician-collected sampling: agreement = 92% (κ = 0.75), HIV-positive (ƙ = 0.71), HIV-negative (ƙ = 0.75). |
| Fang et al., 2008 [45] | USA | Concordance of two self-sampling methods (FCU vs. vaginal swab) and provider-collected endocervical samples for detecting CT and NG | n = 350 aged 12–18 years | FCU and self-sampled vaginal swabs | BDProbeTec ET Amplified DNA Assay | n = 342 adolescents CT positivity rate = 26.6 per 100 women NG positivity rate = 11.7 per 100 women Vaginal swab: Sensitivity, CT = 97.3%, NG = 100% FCU: Sensitivity, CT = 89.2%, NG = 88.6% Provider-collected sample (PES): Sensitivity, CT = 90.1%, NG = 95.5% Specificities: 94.7%~99.7% for CT and NG. Agreement, CT: SVS vs. PES (ƙ = 0.89), SVS vs. FCU (ƙ = 0.88) and PES vs. FCU (ƙ = 0.91) (p < 0.0001) Agreement, NG: SVS vs. PES (ƙ = 0.91), SVS vs. FCU (ƙ = 0.87) and PES vs. FCU (ƙ = 0.91) (p < 0.0001). |
| Bialasiewicz et al., 2009 [58] | Australia | A novel, super-absorbent polymer-based method for self-collection and ambient temperature transport of urine. Evaluate ability to detect CT. | 52 urine specimens | Urine | PCR for CT (cobas® TaqMan 48 rtPCR) | Gel-based urine sample vs. neat urine: Sensitivity = 94.6–100%, specificity = 100% No PCR inhibition or reduced analytical sensitivity using gel-based samples. |
| Falk et al., 2010 [46] | Sweden | Sensitivity of self-sampled vaginal specimens, FCU, self-sampled specimens/FCU and endocervical specimens to detect genital CT in asymptomatic women. | n = 318 | Vaginal swab, FCU, endocervical specimens | cobas® Amplicor CT Test, LightMix 480HT PCR OLBIOL GmbH, (Berlin, Germany) on a LightCycler 480 | 172 of 318 women tested positive for CT. 19 (16.8%) of asymptomatic women (n = 113) had discordant tests (FCU vs. self-sampling) and 7 (12.1%) of symptomatic women (n = 58) had discordant tests (FCU vs. self-sampling). CT, sensitivity: endocervical specimens = 97.1% (166/171), self-sampled specimens = 96.5% (165/171) and self-sampled vaginal/FCU specimens = 95.3% (163/171), FCU = 87.7% (150/171), which was significantly lower. |
| van Dommelen et al., 2011 [59] | The Netherlands | Performance of SVS/FCU combination compared FCU or vaginal swabs alone. | n = 791 | SVS, first-catch urine (FCU) | NAAT: Strand Displacement Amplification (SDA) assay and PCR | CT detection rate: SVS = 94% (89%–99%), FCU = 90% (84%–96%), SVS/FCU = 94% (89%–99%) (NAAT by SDA and PCR) Detection rates were similar across sample types. SVS vs. FCU, agreement = 98% (p = 0.61) SVS vs. SVS/FCU, agreement = 99% (p = 1) FCU vs. SVS/FCU, agreement = 98.8% (p = 0.51) |
| Stewart et al., 2012 [25] | UK | Accuracy of self-sampled vulvovaginal swabs vs. clinician-taken urethral and endocervical swabs for detecting NG in women attending a sexual health clinic in an urban setting | n = 3973 older than 16 years | Self-sampled vulvovaginal swab | NAAT— Aptima Combo 2 (AC2) | Culture: sensitivity = 81% Clinician taken endocervical NAATs: sensitivity = 96% Self-sampled vulvovaginal NAATs: sensitivity = 99% AC2 tests were significantly more sensitive than culture (p < 0.001). Endocervical vs. vulvovaginal swabs: No difference. Therefore, the specificities and PPV of all tests in all sites were 100%, and NPV of all tests were 99% or greater. Culture: sensitivity = 84%. Clinician-taken endocervical AC2: sensitivity = 100%. Self-sampled vulvovaginal swab AC2: sensitivity = 100%. AC2 assays were significantly more sensitive than culture (p = 0.004) for both endocervical and endocervical swabs. |
| Levy et al., 2012 [39] | Not indicated | Specimen collection and test characteristics of NAATs at different anatomical sites. | Sample size not indicated | Self-collection: urethra, cervicovaginal, rectum and pharynx. | NAATs | NG/CT detection: urine samples for men, self-sampled vaginal swabs in women. |
| Jang et al., 2012 [19] | Not indicated | Compare SVS and FCU to diagnose TV | n = 530 | Dacron swab taken from an APTIMA collection kit, nylon-flocked swab, FCU | Transcription-mediated amplification analyte-specific reagents using a cutoff of 50 000 relative light units. | Only seven of 75 women infected with TV reported symptoms. Self-sampling: Sensitivity = 97.2%, specificity = 97.6% FCU: Sensitivity = 41.7%, specificity = 100%. Dacron swab: Sensitivity = 92.3%, specificity = 98.8%. Flocked-nylon swab: Sensitivity 92.3%, specificity = 99.2%. |
| Jones et al., 2013 [60] | Brazil, South Africa | Evaluated the XenoStrip TV test, now the OSOM Trichomonas rapid test in two developing countries. Compared home- and clinic-based screenings. The home arm required two self-sampled vaginal swabs. | Sample size not indicated, Women aged 14–25 in South Africa.Women aged 18 to 40 years in Brazil | SVS | PCR and rapid point-of-care test (POCT) | Specificity for self-testing using the rapid TV test was high in both settings. South Africa: sensitivity = 83.3%; Brazil: sensitivity = 68.4% (non-significant, z test p = 0.2). Pooled sensitivity = 76.7% (95% CI, 61.4 to 88.2%). Pooled specificity = 99.1% (95% CI, 98.2 to 99.6%). Self-sample, PCR: specificity = 99.1%, 95% CI, 98.2 to 99.6%), sensitivity = 76.7%; 95% CI, 61.4 to 88.2%). Sensitivity was higher among symptomatic women (87.5%; 95% CI, 47.3 to 99.7%) than asymptomatic women (80%; CI, 51.9 to 95.7%). |
| Geelen et al., 2013 [44] | Nether-lands | Clinical performance of rectal and self-sampled vaginal swabs for detecting of CT and NG | n = 921 | Rectal swab, self-sampled vaginal swabs | Roche cobas® 4800 CT/NG assay and Abbott m2000 real-time™ CT/NG | Rectal swabs: High concordance rates for detecting CT and NG ( ≥ 96%) using the cobas® 4800 and the Abbot m2000 real-time™ assay. κ coefficients > 0.75, indicating excellent agreement. Self-sampled vaginal swabs: High concordance rate (≥99%) using the cobas® 4800 and Abbot m2000 real-time™ assays for detecting CT and NG. |
| Ting et al., 2013 [30] | Kenya | Compare APTIMA HR-HPV mRNA testing of physician-collected and self-sampled specimens for detecting high-grade cervical lesions in high-risk FSWs in Kenya. Identify risk factors for HR-HPV mRNA in our population of FSWs | n = 350 aged 18 to 49 years | self-sampled specimen using the APTIMA Cervical Specimen Collection and Transport cytobrush | Aptima HPV (AHPV), AC2, Aptima TV (ATV) | Prevalence: hrHPV mRNA, physician collected samples = 30%, self-sampled specimens = 29%. Prevalence high-grade squamous intraepithelial lesion (HSIL) = 4% (n = 15). HSIL, HR-HPV testing: Sensitivity, physician-collected samples = 86% (95% CI, 62%–98%), self-sampled specimens = 79% (95% CI, 55–95%). HSIL, HR-HPV testing: Specificity, physician-collected samples = 73% (95% CI, 68%–79%), self-samples specimens = 75% (95% CI, 70%–79%). Risk factors for HPV: age < 30 years, TV or Mycoplasma genitalium (MG) infection, more than eight years of educational cbasattainment. |
| Van Der Pol et al., 2013 [28] | USA | Patient infection status derived from vaginal swab specimens compared with other sample types | n = 4279 | FCU; a single vaginal swab, Self-collected or clinician-collected using the cobas® collection kit | NAAT, cobas® CT/NG (c4800) Test (Roche Diagnostics, Indianapolis, IN) performed on the cobas® 4800 system | Detection rates: CT = 248, NG = 65 CT, self-collected vs. other samples, agreement = 98.8% to 99.2%, ƙ = 0.88 NG, Self-collected vs. other samples, agreement = 99.8% to 99,9%, ƙ = 0.92 |
| Chernesky et al., 2014 [21] | Canada | Compared self-sampled cervical collection and transportation (SCT) samples to PreservCyt and SurePath cervical samples | n = 580 | Self- collected vaginal sample using SCT | Aptima HPV assay, a target NAAT | Cervical SCT vs. PreservCyt samples: agreement = 91.1%; J = 0.82, AHPV assay Cervical SCT vs. SurePath samples: agreement = 86.7%; J = 0.72, AHPV assay. Self-sampled vaginal SCT vs. physician- collected SCT: agreement = 84.7%; J = 0.68, p = 0.014, 3.35 times more extra positives in self-sampled vaginal SCT Self-sampled vaginal SCT vs. cervical SCT samples: agreement = 82.0%; J = 0.63, p = 0.046, similar extra positives Women found the kit easy to use and comfortable for self-sampling |
| Li et al., 2014 [22] | USA | Compare AC2 performance on combinations of vaginal swabs, transportation media and FCU samples. | n = 287 | flocked swab, Aptima vaginal swab, FCU | AC2 | 37/287 women tested positive for CT. All samples were detected by the Aptima swab, the flocked swab in the Aptima specimen transport medium and the ESwab in ESwab medium. Aptima swabs in Aptima specimen transport uniquely detected CT in three swabs. Flocked swabs in Aptima specimen transport medium uniquely detected CT in two swabs. CT, FCU: Sensitivity = 100%. |
| Chernesky et al., 2014 [56] | Canada | Compare a specimen collection and transport (SCT) kit for detecting CT and TV from SVS and physician-collected vaginal and cervical samples | n = 708 | vaginal swab using the SCT kit Self-vaginal: S-VSCT Physician- collected vaginal (P-VSCT)Physician-collected cervical: (P-CSCT) | CT: AC2, TV: ATV | 84.3% of women were comfortable with collecting specimens. 87.4% of women, 25 years and older, were comfortable with self-sampling 78.8% of women, younger than 25, were comfortable with self-sampling. CT, agreement: S-VSCT vs. P-VSCT 99.6% (ƙ = 0.93). S-VSCT vs. CSCT 99.4% (ƙ = 0.91), S-VSCT vs. PC L-Pap 99.4% (ƙ = 0.91), S-VSCT vs. P L-Pap 99.3% (ƙ = 0.88). TV, agreement: S-VSCT vs. P-VSCT 99.9% (ƙ = 0.97), S-VSCT vs. P-VSCT 99.7% (ƙ = 0.94), S-VSCT vs. PC L-Pap 99.6% (ƙ = 0.91), S-VSCT vs. SP L-Pap 98.8% (ƙ = 0.78). |
| Boggan et al., 2015 [51] | Haiti | Feasibility of HPV screening as primary testing for cervical cancer. Compare vaginal self-sampling to physician- administered cervical screening methods | n = 1845 aged between 25–65 years. | Vaginal swabs | HR-HPV genotyping using the HC-II HPV assay pool. | HR-HPV screening is a feasible tool for primary cervical cancer screening in a low-resource, Haitian population. Women volunteered to participate in vaginal self-screening for HPV. Sensitivity of HPV screening for detecting ≥CIN-II: vaginal samples = 87.5%, cervical samples = 96.9%. Cervical vs. vaginal samples: High agreement. Vaginal self-sampling sample can be implemented in this under-screened and high-risk population. |
| Arias et al., 2016 [52] | Canada | Survey opinions of young sexually active women on ease and comfort of self-sampling using HerSwab. Agreement between self-sampling and provider-collected swabs for detecting CT and NG. | n = 189 aged16–41 years | Vaginal swab collected with a HerSwab device | AC2 | Respondents (97.1%) reported that the HerSwab instructions were easy to follow. 80.9% of respondents preferred self-collection over physician collection. 79.7% (137/172) of respondents would consider self-sampling at home. 96.2% (177/184) of respondents found it easy or very easy to insert and withdraw the device. 93.4% (171/183) of respondents found it easy and very easy to turn the device handle while inside the vagina. Agreement: self-sampling vs. provider collected specimen, CT: 94.7% (90.2%–97.3%; κ = 0.64 (0.43–0.85)) Agreement: self-sampling vs. provider- collected specimen, NG: 98.4% (95.1–99.6; κ = 0.56 [0.13–1]). |
| Obiri-Yeboah et al., 2017 [36] | Ghana | The performance of self-collected cervico-vaginal samples for detecting HPV compared to clinician collection | n = 333 | vaginal swab using careHPV brush | careHPV assay | HPV: agreement between self-collected and clinician-collected samples = 94.2% (ƙ = 0.88, p ≤ 0.0001) HIV seropositive: agreement between self-collected and Clinicia-collected samples, ƙ = 0.84 (p < 0.0001) HIV seronegative: agreement between self-collected and clinician-collected samples, ƙ = 0.86 (p < 0.0001) self-collected vs. clinician-collected: sensitivity = 92.6% (95% CI: 85.3–97.0%), specificity = 95.9% (95% CI: 89.8– 98.9%). |
| de Marais et al., 2018 [47] | USA | Clinical performance of self-sampling cervico-vaginal specimens for detecting CIN-II in US women at risk of cervical cancer due to underscreening. Compare self-sampled specimens and physician-collected specimens to detect CT, NG, TV, and MG | n = 284 | Cervico-vaginal swab, using Viba brush | AHPV, AC2 assay for CT and NG, the ATV assay and the Aptima analyte-specific reagent-based assay for MG | Detection rate: 193 of 284 women were at high risk for HPV, irrespective of sampling and cytology. Self-sampling: Detected high-risk HPV in all cases of HSIL and CIN-II + TV, detection: Self-sampling = 10.2%, Physician = 10.8% MG, detection: Self-sampling = 3.3%, Physician = 5.5% CT, detection: Self-sampling = 1.1%, Physician = 2.1% NG, detection: Self-sampling = 0%, Physician = 0.5%. High-risk HPV: Self-sampling ƙ = 0.56, Physician ƙ = 0.66 TV: Self-sampling ƙ = 0.86, Physician ƙ = 0.91. MG: Self-sampling ƙ = 0.65, Physician ƙ = 0.83. Most participants understood self-collection instructions (93.6%) and were willing to use self-collection in the future (96.3%). |
| Lockhart et al., 2018 [23] | Kenya | The agreement of SCT for CT, NG, TV and MG screening using self-versus physician-collected specimens. The acceptability of self-sampling for female sex workers (FSWs) over 18 months. | ages 18 to 49 years, sample size not indicated | self-sampled cervico-vaginal sample using the Aptima Cervical Specimen Collection and Transport cytobrush | CT, NG: the Aptima Combo 2 assay TV, MG: the ATV assay | Prevalence, SCT: NG = 2.9%, CT = 5.2%, TV = 9.2%, MG = 20.1%. Prevalence, physician-collected: NG = 2.3%, CT = 3.7%, TV = 7.2%, MG = 12.9%. Agreement between samples was consistently strong (ƙ range, 0.66–1.00) for all STIs, except for MG which had a moderate agreement (ƙ range, 0.50–0.75). Most participants found self-collection easy (94%) and comfortable (89%). SCT was effective for STI screening in a clinic-based, less-developed country setting. |
| Khan et al., 2019 [43] | India | Reliability of self-sampled vaginal swabs vs. physician-collected swabs to diagnose fungal (Candida albicans or non- albicans Candida species) bacterial vaginosis (BV) and parasitic TV aetiology of vaginal discharge and prevalence of various infections and coinfections. | n = 550 | Vaginal swabs | Gram staining, wet mount, and culture | Prevalence: Bacterial vaginosis (n = 79, 14.4%), vulvovaginal candida (VVC) (n = 144, 26.2%) and TV (n = 3, 0.5%) VVC coexisted with BV in 58 (10.5%) patients. No coinfection of TV with BV or VVC. Candida albicans was isolated in 84 (58.3%) VVC cases. Self-sampling, BV: sensitivity = 91.1%, specificity = 100%, PPV = 100%, NPV = 98.5% Self-sampling, Candida albicans VVC and TV: sensitivity (100%), specificity (100%), PPV (100%) and NPV (100%). Self-sampling vs. physician, agreement: ƙ = 0.95 (BV), ƙ = 0.99 (VVC), ƙ= 1.0 (TV).With specific instructions and guidance, self-collected swabs can approximate physician-collected swabs. |
| McLarty et al., 2019 [37] | USA | Compare tampons, self- sampled vaginal swabs and physician- collected specimens to diagnose HPV. | n = 174 | Tampons, swabs (Eve Medical HerSwab) | Roche cobas® HPV method | HR-HPV prevalence = 13.5% (n = 174) All physician-collected specimens were sufficient for detecting HPV. 15 (27%) of tampon specimens were of poor quality. 1 (2%) of vaginal swabs were of poor quality. Vaginal swabs were similar to physician-collected specimens, while tampons were of poor quality. |
| Nodjikouambaye et al., 2019 [6] | Chad | Performance of a novel genital veil (V-Veil-Up Gyn Collection Device, V-Veil-Up Pharma Ltd., Nicosia, Cyprus) for self- sampling to diagnose STIs as compared to physician- collected specimens. | n = 271 | Self-sampling with veil | IVD-marked multiplex real-time PCR Allplex STI Essential Assay | Genital mycoplasmas detected in 54.2% of samples. Ureasplasma parvum detected in 42.6% of samples. Self-sampling performed similarly to physician-collected samples in detecting genital microorganisms. Sensitivity = 97% (95%CI: 92.5–99.2%), specificity = 88.0% (95%CI: 80.7–93.3%). |
| Verougstra et al., 2020 [26] | Belgium | The feasibility of molecular testing for CT and NG in pooled versus single site samples in a large cohort of FSWs. | n = 501 | a pharyngeal swab, a self-collected vaginal swab and a self-collected rectal swab | NAAT using Abbott Real Time | n = 489 patients, prevalence: CT = 6.5% (95% CI 4.5% to 9.1%), NG = 3.5% (95% CI 2.0% to 5.5%), CT and NG coinfections = 1.4% 42 patients tested positive on at least one non-pooled sample. Only five tested negative in the pooled sample. CT: Sensitivity = 94% (95% CI 79% to 99%). NG: Sensitivity = 82% (95% CI 57% to 96%). Missed pooled samples derived from single-site infections with low bacterial loads. Testing only vaginal samples would have missed 40% of CT infections and 60% of NG infections. |
| Kim et al., 2021 [42] | Korea | Do self-sampled vaginal specimens contain enough DNA to detect HPV. Compare self-sampled specimens with physician-collected cervical samples. Investigated ease, comfort and reliability of a self-sampling to obtain a vaginal sample. | n = 151 | vaginal swab—(using G+ Kit®; DocTool) | PCR: the Anyplex II HPV28 Detection assay, Real-time PCR using CFX96. | Prevalence HPV, PCR: self-sampling = 67.5%, physician-collected = 57.4%. Prevalence, high-risk (HR) HPV, PCR: self-sampling = 58.7%, physician-collected = 48.6% Sensitivity, HR HPV: self-sampling = 100% (95% CI 0.09 to 0.32) for high-grade squamous intraepithelial lesion, 78% (95% CI –0.09 to 0.13) for atypical squamous cells, 95% (95% CI –0.01 to 0.25) for low-grade squamous intraepithelial lesion. Self-sampled specimens contained enough DNA to detect HPV. Self-sampled vs. physician-collected samples had similar sensitivity and specificity. Self-sampling is feasible for detecting abnormal cervical cytology. Self-sampling is easy and reliable. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaya, Z.N.; Mapanga, W.; van Niekerk, B.; Dlangalala, T.; Kgarosi, K.; Dzobo, M.; Mulqueeny, D.; Mashamba-Thompson, T.P. Mapping Evidence of Self-Sampling to Diagnose Sexually Transmitted Infections in Women: A Scoping Review. Diagnostics 2022, 12, 1803. https://doi.org/10.3390/diagnostics12081803
Jaya ZN, Mapanga W, van Niekerk B, Dlangalala T, Kgarosi K, Dzobo M, Mulqueeny D, Mashamba-Thompson TP. Mapping Evidence of Self-Sampling to Diagnose Sexually Transmitted Infections in Women: A Scoping Review. Diagnostics. 2022; 12(8):1803. https://doi.org/10.3390/diagnostics12081803
Chicago/Turabian StyleJaya, Ziningi N., Witness Mapanga, Brian van Niekerk, Thobeka Dlangalala, Kabelo Kgarosi, Mathias Dzobo, Delarise Mulqueeny, and Tivani P. Mashamba-Thompson. 2022. "Mapping Evidence of Self-Sampling to Diagnose Sexually Transmitted Infections in Women: A Scoping Review" Diagnostics 12, no. 8: 1803. https://doi.org/10.3390/diagnostics12081803
APA StyleJaya, Z. N., Mapanga, W., van Niekerk, B., Dlangalala, T., Kgarosi, K., Dzobo, M., Mulqueeny, D., & Mashamba-Thompson, T. P. (2022). Mapping Evidence of Self-Sampling to Diagnose Sexually Transmitted Infections in Women: A Scoping Review. Diagnostics, 12(8), 1803. https://doi.org/10.3390/diagnostics12081803






