Assessment of Neck Muscle Shear Modulus Normalization in Women with and without Chronic Neck Pain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Normalization
2.3. Statistical Analysis
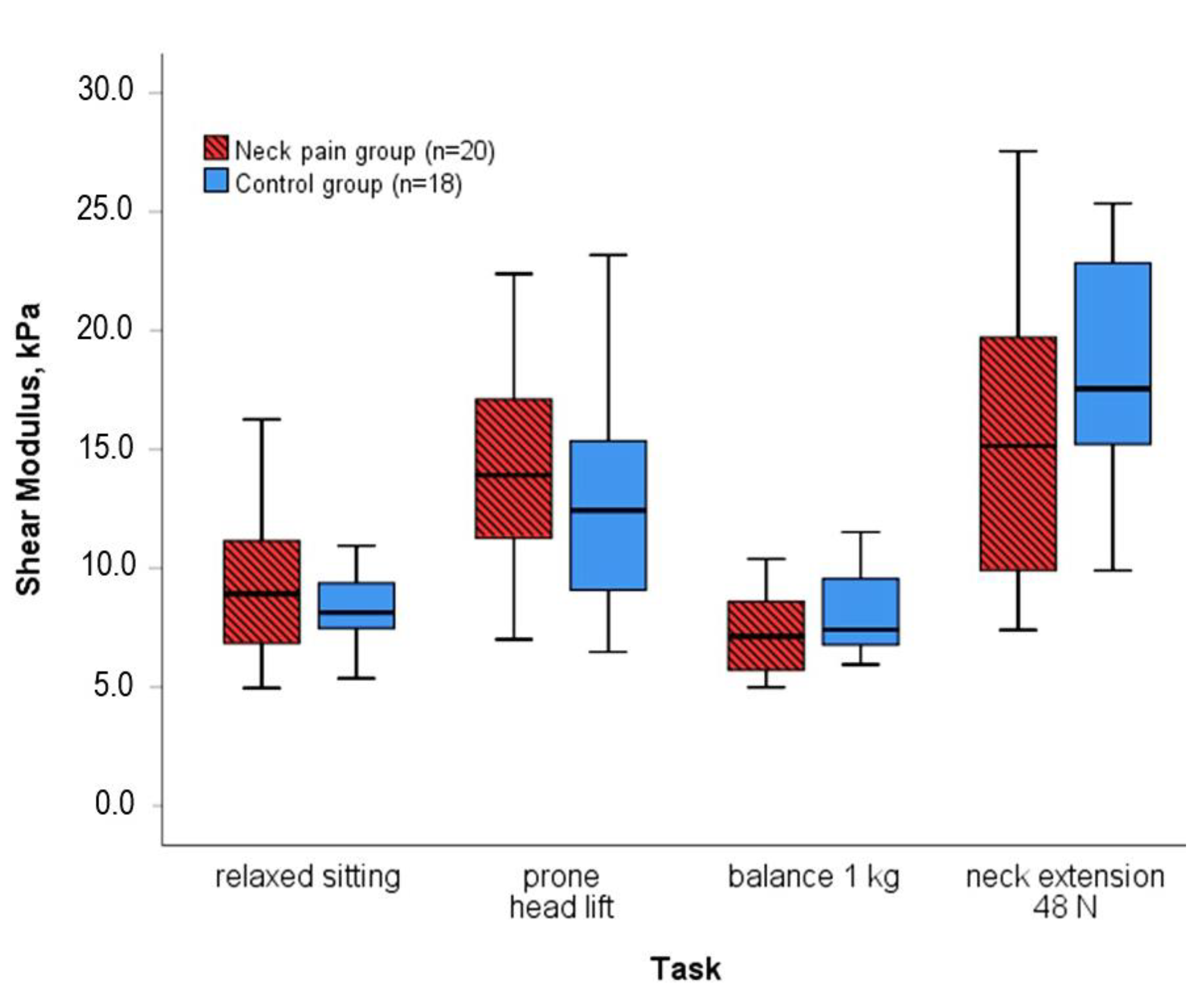
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Raw Shear Modulus, kPa | Shear Modulus % of Relaxed Sitting | Shear Modulus % of Head Lift | Shear Modulus % of Balancing 1 kg | Shear Modulus % of Extension at 48 N | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neck Pain | Control | Neck Pain | Control | Neck Pain | Control | Neck Pain | Control | Neck Pain | Control | |
| Relaxed sitting | 8.9 (4.6) | 8.1 (2.3) | 65 (25) | 69 (33) | 121 (68) | 102 (38) | 58 (40) | 48 (13) | ||
| p-value | 0.740 | 0.593 | 0.093 | 0.149 | ||||||
| Extension at 12 N | 9.3 (8.5) | 10.7 (5.7) | 115 (48) | 124 (37) | 74 (45) | 80 (29) | 145 (86) | 122 (71) | 74 (35) | 63 (25) |
| p-value | 0.573 | 0.426 | 0.290 | 0.496 | 0.099 | |||||
| Extension at 24 N | 10.8 (8.2) | 13.7 (9.9) | 134 (57) | 162 (90) | 86 (41) | 95 (38) | 171 (96) | 168 (102) | 81 (21) | 75 (14) |
| p-value | 0.317 | 0.196 | 0.093 | 0.828 | 0.217 | |||||
| Extension at 36 N | 14.8 (12.0) | 14.7 (8.1) | 157 (96) | 182 (85) | 109 (69) | 123 (77) | 195 (150) | 198 (90) | 96 (36) | 90 (13) |
| p-value | 0.496 | 0.377 | 0.149 | 0.740 | 0.158 | |||||
| Extension at 48 N | 15.1 (9.9) | 17.6 (8.5) | 173 (109) | 207 (56) | 108 (48) | 141 (63) | 225 (169) | 218 (120) | ||
| p-value | 0.149 | 0.149 | 0.017 * | 0.919 | ||||||
| 30° Rotation at 24 N | 12.7 (7.8) | 15.6 (9.0) | 143 (73) | 186 (63) | 94 (52) | 120 (57) | 204 (138) | 169 (96) | 95 (34) | 87 (34) |
| p-value | 0.377 | 0.167 | 0.093 | 0.534 | 0.696 | |||||
| Office stress | 16.6 (11.1) | 16.3 (10.4) | 187 (95) | 194 (85) | 129 (72) | 138 (69) | 234 (154) | 233 (119) | 117 (92) | 92 (42) |
| p-value | 0.998 | 0.478 | 0.317 | 0.496 | 0.099 | |||||
| Balancing 1 kg | 7.1 (3.2) | 7.4 (3.3) | 83 (39) | 98 (35) | 53 (35) | 74 (41) | 45 (43) | 46 (29) | ||
| p-value | 0.141 | 0.093 | 0.105 | 0.919 | ||||||
| Prone head lift | 13.9 (6.3) | 12.4 (6.5) | 153 (59) | 145 (71) | 189 (124) | 136 (105) | 93 (46) | 71 (32) | ||
| p-value | 0.393 | 0.593 | 0.105 | 0.017 * | ||||||
References
- Collaborators, U.B.o.D. The State of US Health, 1990-2010: Burden of Diseases, Injuries, and Risk Factors. JAMA 2013, 310, 591–608. [Google Scholar] [CrossRef] [Green Version]
- Vasseljen, O.; Woodhouse, A.; Bjørngaard, J.H.; Leivseth, L. Natural course of acute neck and low back pain in the general population: The HUNT study. Pain 2013, 154, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Cote, P.; Cassidy, J.D.; Carroll, L.J.; Kristman, V. The annual incidence and course of neck pain in the general population: A population-based cohort study. Pain 2004, 112, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Côté, P.; van der Velde, G.; Cassidy, J.D.; Carroll, L.J.; Hogg-Johnson, S.; Holm, L.W.; Carragee, E.J.; Haldeman, S.; Nordin, M.; Hurwitz, E.L.; et al. The Burden and Determinants of Neck Pain in Workers: Results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. J. Manipulative Physiol. Ther. 2009, 32, S70–S86. [Google Scholar] [CrossRef]
- MacDermid, J.C.; Walton, D.M.; Bobos, P.; Lomotan, M.; Carlesso, L. A Qualitative Description of Chronic Neck Pain has Implications for Outcome Assessment and Classification. Open Orthop. J. 2016, 10, 746. [Google Scholar] [CrossRef] [Green Version]
- Takasawa, E.; Yamamoto, A.; Kobayashi, T.; Tajika, T.; Shitara, H.; Ichinose, T.; Mieda, T.; Iizuka, Y.; Iizuka, H.; Takagishi, K. Characteristics of neck and shoulder pain in the Japanese general population. J. Orthop. Sci. 2015, 20, 403–409. [Google Scholar] [CrossRef]
- Dieterich, A.V.; Andrade, R.J.; Le Sant, G.; Falla, D.; Petzke, F.; Hug, F.; Nordez, A. Shear wave elastography reveals different degrees of passive and active stiffness of the neck extensor muscles. Eur. J. Appl. Physiol. 2017, 117, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.S.M.; Jakubowski, K.L.; Spear, S.C.; Rymer, W.Z. Muscle material properties in passive and active stroke-impaired muscle. J. Biomech. 2019, 83, 197–204. [Google Scholar] [CrossRef]
- Koo, T.K.; Hug, F. Factors that influence muscle shear modulus during passive stretch. J. Biomech. 2015, 48, 3539–3542. [Google Scholar] [CrossRef] [Green Version]
- Nordez, A.; Hug, F. Muscle shear elastic modulus measured using supersonic shear imaging is highly related to muscle activity level. J. Appl. Physiol. 2010, 108, 1389–1394. [Google Scholar] [CrossRef]
- Romero-Morales, C.; Bravo-Aguilar, M.; Ruiz-Ruiz, B.; Almazán-Polo, J.; López-López, D.; Blanco-Morales, M.; Téllez-González, P.; Calvo-Lobo, C. Current advances and research in ultrasound imaging to the assessment and management of musculoskeletal disorders. Dis. Mon. 2020, 67, 101050. [Google Scholar] [CrossRef]
- Eby, S.F.; Cloud, B.A.; Brandenburg, J.E.; Giambini, H.; Song, P.; Chen, S.; LeBrasseur, N.K.; An, K.-N. Shear wave elastography of passive skeletal muscle stiffness: Influences of sex and age throughout adulthood. Clin. Biomech. 2015, 30, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Hvedstrup, J.; Tørring Kolding, L.; Ashina, M.; Winther Schytz, H. Increased neck muscle stiffness in migraine patients with ictal neck pain: A shear wave elastography study. Cephalgia 2020, 40, 565–574. [Google Scholar] [CrossRef]
- Heizelmann, A.; Tasdemir, S.; Schmidberger, J.; Gräter, T.; Kratzer, W.; Grüner, B. Measurements of the trapezius and erector spinae muscles using virtual touch imaging quantification ultrasound-Elastography: A cross section study. BMC Musculoskelet. Disord. 2017, 18, 370. [Google Scholar] [CrossRef]
- Franchi-Abella, S.; Elie, C.; Correas, J.M. Ultrasound elastography: Advantages, limitations and artefacts of the different techniques from a study on a phantom. Diagn. Interv. Imaging 2013, 94, 497–501. [Google Scholar] [CrossRef] [Green Version]
- Linek, P. The importance of body mass normalisation for ultrasound measurements of the morphology of oblique abdominis muscles: The effect of age, gender, and sport practice. Folia Morphol. 2018, 77, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Besomi, M.; Hodges, P.W.; Clancy, E.A.; Van Dieën, J.; Hug, F.; Lowery, M.; Merletti, R.; Søgaard, K.; Wrigley, T.; Besier, T.; et al. Consensus for experimental design in electromyography (CEDE) project: Amplitude normalization matrix. J. Electromyogr. Kinesiol. 2020, 53, 102438. [Google Scholar] [CrossRef]
- Yang, J.F.; Winter, D.A. Electromyography amplitude normalisation methods: Improving their sensitivity as diagnostic tools in gait analysis. Arch. Phys. Med. Rehabil. 1984, 65, 517–521. [Google Scholar]
- Burden, A. How should we normalize electromyograms obtained from healthy participants? What we have learned from over 25 years of research. J. Electromyogr. Kinesiol. 2010, 20, 1023–1035. [Google Scholar] [CrossRef]
- Ettinger, L.; Weiss, J.; Shapiro, M.; Karduna, A. Normalization to Maximal Voluntary Contraction is Influenced by Subacromial Pain. J. Appl. Biomech. 2016, 32, 433–440. [Google Scholar] [CrossRef]
- Marras, W.S.; Davis, K.G.; Maronitis, A.B. A non-MVC EMG normalization technique for the trunk musculature: Part 2. Validation and use to predict spinal loads. J. Electromyogr. Kinesiol. 2001, 11, 11–18. [Google Scholar] [CrossRef]
- Brandenburg, J.E.; Eby, S.F.; Song, P.; Kingsley-Berg, S.; Bamlet, W.; Sieck, G.C.; An, K.N. Quantifying passive muscle stiffness in children with and without cerebral palsy using ultrasound shear wave elastography. Dev. Med. Child Neurol. 2016, 58, 1288–1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dieterich, A.V.; Yavuz, U.S.; Petzke, F.; Nordez, A.; Falla, D. Neck muscle stiffness measured with shear wave elastography in women with chronic nonspecific neck pain. J. Orthop. Sports Phys. Ther. 2020, 50, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Falla, D.; Jull, G.; Edwards, S.; Koh, K.; Rainoldi, A. Neuromuscular efficiency of the sternocleidomastoid and anterior scalene muscles in patients with chronic neck pain. Disabil. Rehabil. 2004, 26, 712–717. [Google Scholar] [CrossRef]
- Liu, K.H.; Bhatia, K.; VChu, W.; He, L.T.; Leung, S.F.; Ahuja, A.T. Shear Wave Elastography-A New Quantitative Assessment of Post-Irradiation Neck Fibrosis. Ultraschall Med. 2015, 36, 348–354. [Google Scholar] [CrossRef]
- Creze, M.; Nordez, A.; Soubeyrand, M.; Rocher, L.; Maître, X.; Bellin, M.F. Shear wave sonoelastography of skeletal muscle: Basic principles, biomechanical concepts, clinical applications, and future perspectives. Skeletal Radiol. 2018, 47, 457–471. [Google Scholar] [CrossRef]
- Davis, L.C.; Baumer, T.G.; Bey, M.J.; van Holsbeeck, M. Clinical utilization of shear wave elastography in the musculoskeletal system. Ultrasonography 2019, 38, 2–12. [Google Scholar] [CrossRef]
- Bouillard, K.; Nordez, A.; Hodges, P.W.; Cornu, C.; Hug, F. Evidence of changes in load sharing during isometric elbow flexion with ramped torque. J. Biomech. 2012, 45, 1424–1429. [Google Scholar] [CrossRef]
- French, H.P.; Huang, X.; Cummiskey, A.; Meldrum, D.; Malone, A. Normalisation method can affect gluteus medius electromyography results during weight bearing exercises in people with hip osteoarthritis (OA): A case control study. Gait Posture 2015, 41, 470–475. [Google Scholar] [CrossRef]
- Benoit, D.L.; Lamontagne, M.; Cerulli, G.; Liti, A. The clinical significance of electromyography normalisation techniques in subjects with anterior cruciate ligament injury during treadmill walking. Gait Posture 2003, 18, 56–63. [Google Scholar] [CrossRef]
- Allison, G.T.; Marshall, R.N.; Singer, K.P. EMG signal amplitude normalization technique in stretch-shortening cycle movements. J. Electromyogr. Kinesiol. 1993, 3, 236–244. [Google Scholar] [CrossRef]
- Szeto, G.P.J.; Straker, L.M.; O’Sullivan, P.B. Neck-shoulder muscle activity in general and task-specific resting postures of symptomatic computer users with chronic neck pain. Man. Ther. 2009, 14, 338–345. [Google Scholar] [CrossRef]
- Eby, S.F.; Song, P.; Chen, S.; Chen, Q.; Greenleaf, J.F.; An, K.-N. Validation of shear wave elastography in skeletal muscle. J. Biomech. 2013, 46, 2381–2387. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, N.; Hirata, K.; Kanehisa, H.; Yoshitake, Y. Validity of Measurement of Shear Modulus by Ultrasound Shear Wave Elastography in Human Pennate Muscle. PLoS ONE 2015, 10, e0124311. [Google Scholar] [CrossRef] [Green Version]
- Chino, K.; Takahashi, H. Influence of pennation angle on measurement of shear wave elastography: In vivo observation of shear wave propagation in human pennate muscle. Physiol. Meas. 2018, 39, 115003. [Google Scholar] [CrossRef]
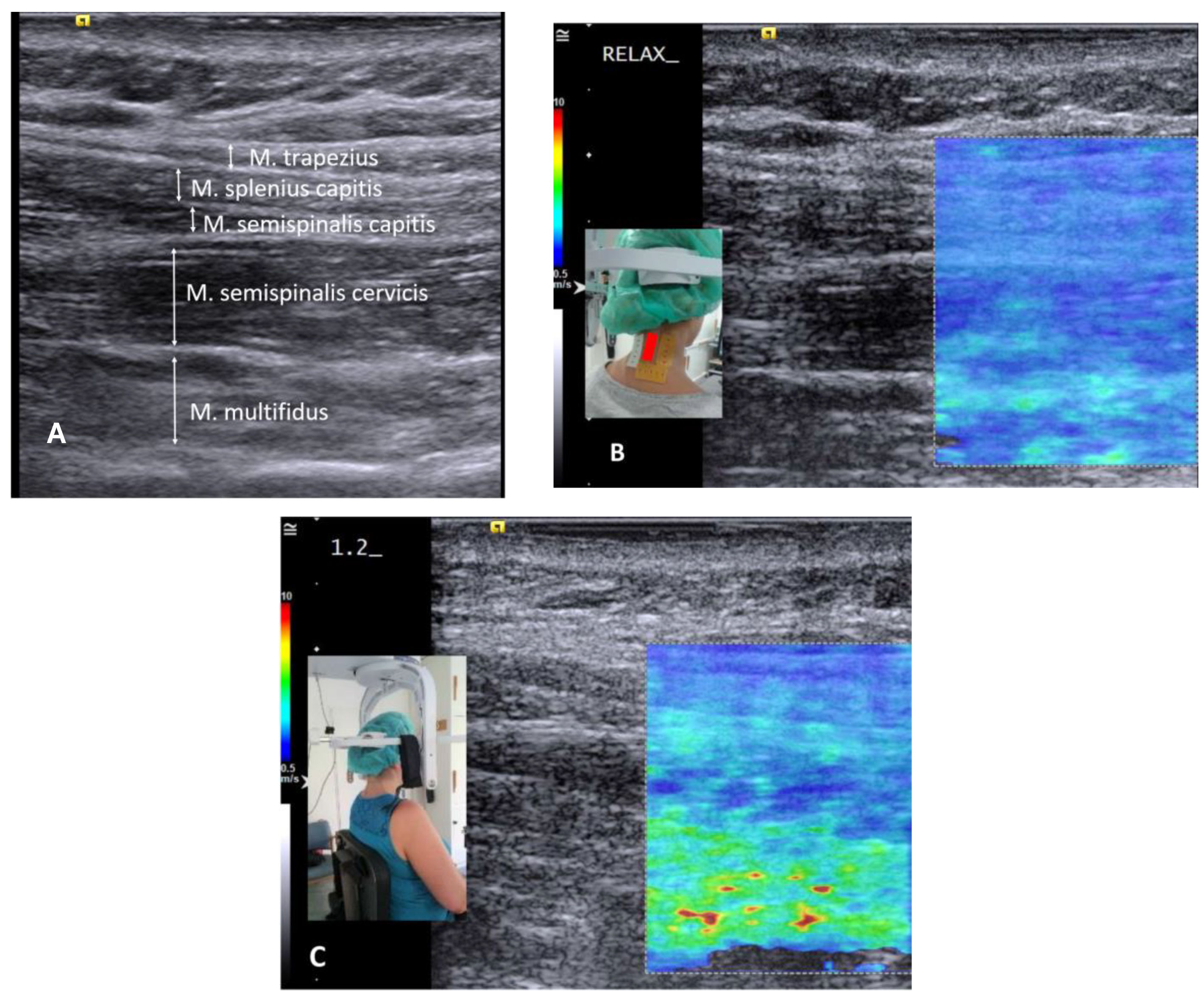

| Raw Shear Modulus, kPa | Shear Modulus % of Relaxed Sitting | Shear Modulus % of Head Lift | Shear Modulus % of Balancing 1 kg | Shear Modulus % of Extension at 48 N | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neck Pain | Control | Neck Pain | Control | Neck Pain | Control | Neck Pain | Control | NeckPain | Control | |
| Shear modulus median (IQR) | 13.1 (6.4) | 13.6 (5.6) | 139.6 (58.5) | 155.9 (43.5) | 94.2 (34.4) | 107.8 (31.8) | 188.9 (83.0) | 174.4 (62.2) | 89.1 (30.7) | 76.5 (19.6) |
| Coefficient IQR/median | 0.49 | 0.41 | 0.42 | 0.28 | 0.37 | 0.29 | 0.44 | 0.36 | 0.34 | 0.26 |
| Result for the neck pain group | 3.3% lower stiffness | 10.5% lower normalized stiffness | 12.6% lower normalized stiffness | 8.3% higher normalized stiffness | 16.6% higher normalized stiffness | |||||
| Mann-Whitney p-value | 0.654 | 0.317 | 0.059 | 0.251 | 0.082 | |||||
| T-Test p-value (if applicable) | n.a. | 0.242 | n.a. | 0.362 | 0.035 * | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dieterich, A.V.; Yavuz, U.Ş.; Petzke, F.; Nordez, A. Assessment of Neck Muscle Shear Modulus Normalization in Women with and without Chronic Neck Pain. Diagnostics 2022, 12, 1791. https://doi.org/10.3390/diagnostics12081791
Dieterich AV, Yavuz UŞ, Petzke F, Nordez A. Assessment of Neck Muscle Shear Modulus Normalization in Women with and without Chronic Neck Pain. Diagnostics. 2022; 12(8):1791. https://doi.org/10.3390/diagnostics12081791
Chicago/Turabian StyleDieterich, Angela V., Utku Şükrü Yavuz, Frank Petzke, and Antoine Nordez. 2022. "Assessment of Neck Muscle Shear Modulus Normalization in Women with and without Chronic Neck Pain" Diagnostics 12, no. 8: 1791. https://doi.org/10.3390/diagnostics12081791
APA StyleDieterich, A. V., Yavuz, U. Ş., Petzke, F., & Nordez, A. (2022). Assessment of Neck Muscle Shear Modulus Normalization in Women with and without Chronic Neck Pain. Diagnostics, 12(8), 1791. https://doi.org/10.3390/diagnostics12081791






