Correlation between Results of Semi-Quantitative and Quantitative Tests for Hepatitis B Virus Surface Antigen among Patients Achieving Viral Suppression with Antiviral Treatment
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Laboratory Assays
2.3. Statistical Analysis
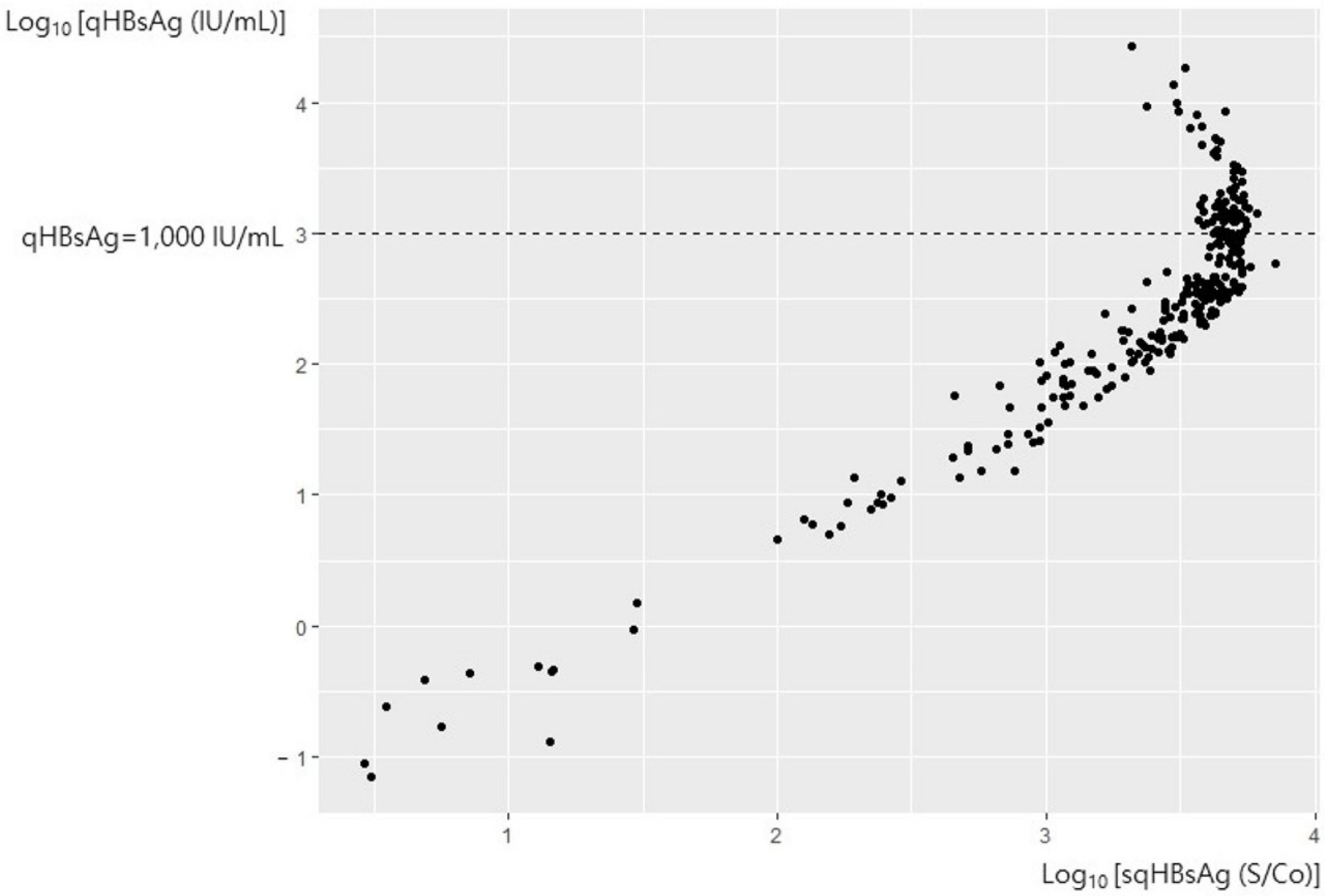
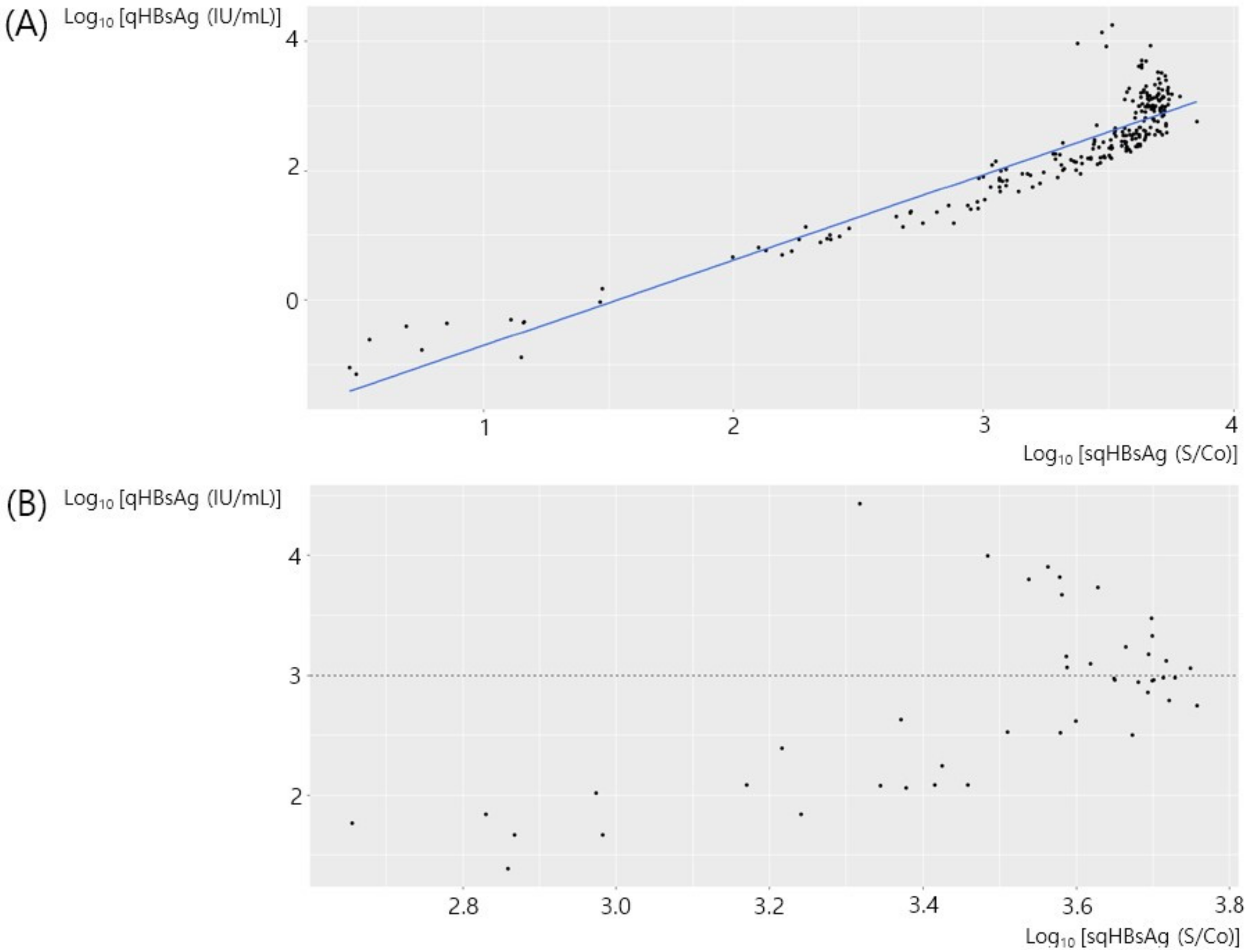
3. Results
3.1. All Samples
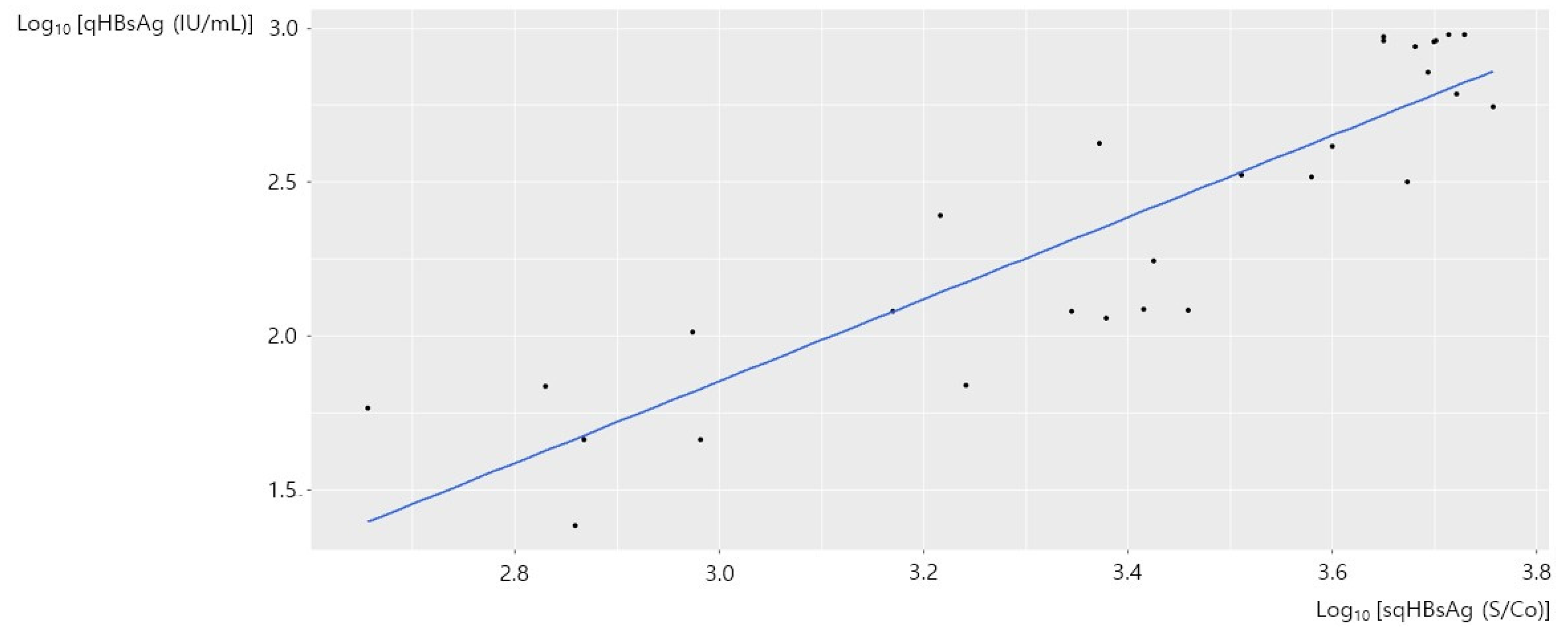
3.2. Correlation of sqHBsAg and qHBsAg in HBeAg-Negative Chronic Hepatitis B
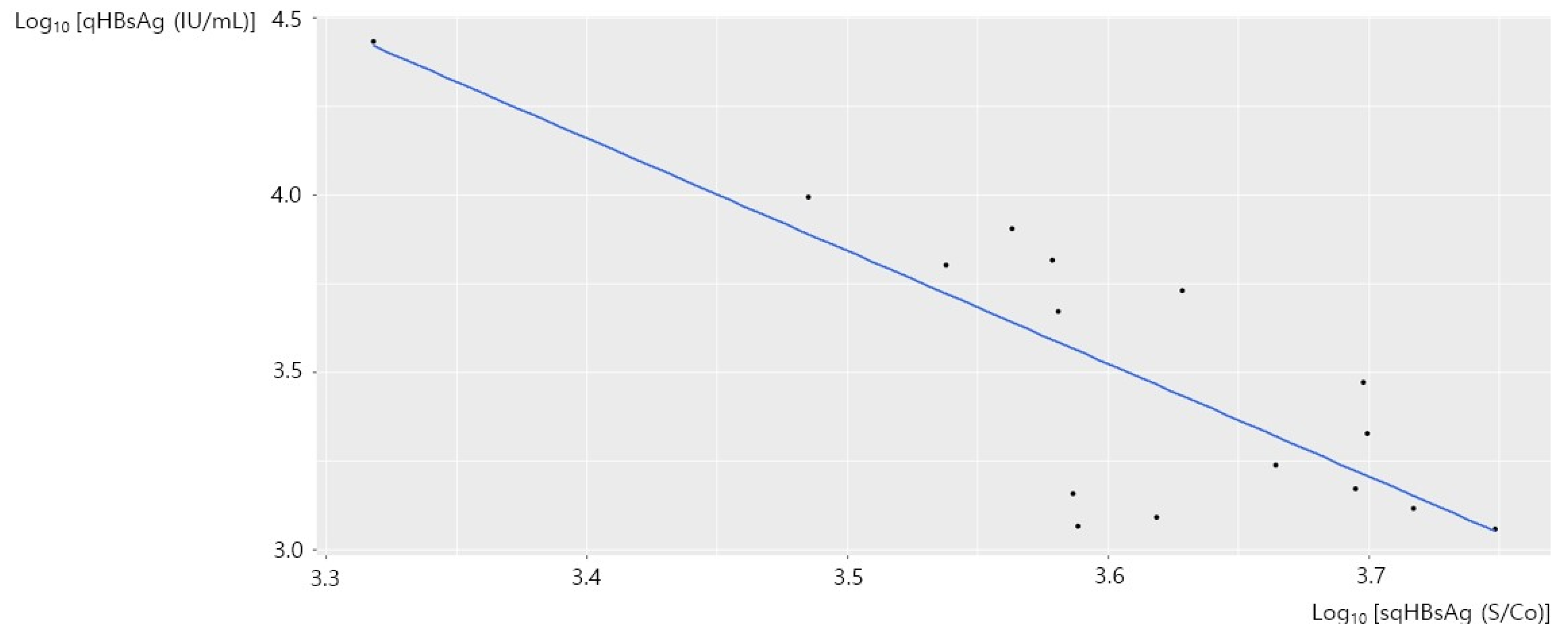
3.3. Correlation of sqHBsAg and qHBsAg in HBeAg-Positive Chronic Hepatitis B
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J. Hepatol. 2012, 57, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Liaw, Y.F.; Kao, J.H.; Piratvisuth, T.; Chan, H.L.; Chien, R.N.; Liu, C.J.; Gane, E.; Locarnini, S.; Lim, S.-G.; Han, K.-H.; et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: A 2012 update. Hepatol. Int. 2012, 6, 531–561. [Google Scholar] [CrossRef] [PubMed]
- Lok, A.S.; McMahon, B.J. Chronic hepatitis B: Update 2009. Hepatology 2009, 50, 661–662. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.L.; Wong, V.W.; Tse, A.M.; Tse, C.; Chim, A.M.; Chan, H.; Wong, G.L.; Sung, J.J. Serum hepatitis B surface antigen quantitation can reflect hepatitis B virus in the liver and predict treatment response. Clin. Gastroenterol. Hepatol. 2007, 5, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.-C.; Liu, C.-J.; Yang, H.-C.; Su, T.-H.; Wang, C.-C.; Chen, C.-L.; Hsu, C.-A.; Kuo, S.F.-T.; Liu, C.-H.; Chen, P.-J.; et al. Serum hepatitis B surface antigen levels help predict disease progression in patients with low hepatitis B virus loads. Hepatology 2013, 57, 441–450. [Google Scholar] [CrossRef]
- Tseng, T.C.; Liu, C.J.; Yang, H.C.; Su, T.H.; Wang, C.C.; Chen, C.L.; Kuo, S.F.; Liu, C.-H.; Chen, P.-G.; Chen, D.-S.; et al. High levels of hepatitis B surface antigen increase risk of hepatocellular carcinoma in patients with low HBV load. Gastroenterology 2012, 142, 1140–1149.e3. [Google Scholar] [CrossRef]
- Martinot-Peignoux, M.; Lapalus, M.; Asselah, T.; Marcellin, P. HBsAg quantification: Useful for monitoring natural history and treatment outcome. Liver Int. 2014, 34 (Suppl. S1), 97–107. [Google Scholar] [CrossRef]
- Jaroszewicz, J.; Calle Serrano, B.; Wursthorn, K.; Deterding, K.; Schlue, J.; Raupach, R.; Flisiak, R.; Bock, C.-Y.; Manns, M.P.; Wedemeyer, H.; et al. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: A European perspective. J. Hepatol. 2010, 52, 514–522. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.J.; Ahn, S.H.; Lee, J.; Park, Y.; Kim, H.S. Correlation between quantitative serum HBsAg and HBV DNA test in Korean patients who showed high level of HBsAg. J. Clin. Pathol. 2010, 63, 1027–1031. [Google Scholar] [CrossRef]
- Kwon, H.W.; Lee, H.Y.; Kim, S.G.; Kim, W.; Jung, Y.J.; Kang, K.W.; Chung, J.K.; Lee, M.C.; Lee, D.S. Quantitative measurement of serum hepatitis B surface antigen using an immunoradiometric assay in chronic hepatitis B. Nucl. Med. Mol. Imaging 2011, 45, 15–20. [Google Scholar] [CrossRef][Green Version]
- Deguchi, M.; Yamashita, N.; Kagita, M.; Asari, S.; Iwatani, Y.; Tsuchida, T.; Iinuma, K.; Mushahwar, I.K. Quantitation of hepatitis B surface antigen by an automated chemiluminescent microparticle immunoassay. J. Virol. Methods 2004, 115, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.L.-Y.; Thompson, A.; Martinot-Peignoux, M.; Piratvisuth, T.; Cornberg, M.; Brunetto, M.R.; Tillmann, H.L.; Kao, J.-H.; Jia, J.-D.; Wedemeyer, H.; et al. Hepatitis B surface antigen quantification: Why and how to use it in 2011—A core group report. J. Hepatol. 2011, 55, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Yao, C.Y. Rapid and quantitative detection of hepatitis B virus. World J. Gastroenterol. 2015, 21, 11954–11963. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.C.; Hsu, C.W.; Gu, P.W.; Yeh, C.T.; Lin, S.M.; Chiu, C.T. Comparison of the elecsys HBsAg II assay and the architect assay for quantification of hepatitis B surface antigen in chronic hepatitis B patients. Biomed. J. 2015, 38, 250–256. [Google Scholar]
- Wursthorn, K.; Jaroszewicz, J.; Zacher, B.J.; Darnedde, M.; Raupach, R.; Mederacke, I.; Cornberg, M.; Manns, M.P.; Wedemeyer, H. Correlation between the Elecsys HBsAg II assay and the Architect assay for the quantification of hepatitis B surface antigen (HBsAg) in the serum. J. Clin. Virol. 2011, 50, 292–296. [Google Scholar] [CrossRef]
- Mühlbacher, A.; Weber, B.; Bürgisser, P.; Eiras, A.; Cabrera, J.; Louisirirotchanakul, S.; Tiller, F.-W.; Kim, H.-S.; Helden, J.V.; Bossi, V.; et al. Multicenter study of a new fully automated HBsAg screening assay with enhanced sensitivity for the detection of HBV mutants. Med. Microbiol. Immunol. 2008, 197, 55–64. [Google Scholar] [CrossRef]
- Kim, M.A.; Kim, S.U.; Sinn, D.H.; Jang, J.W.; Lim, Y.S.; Ahn, S.H.; Shim, J.J.; Seo, Y.S.; Baek, Y.H.; Kim, S.G.; et al. Discontinuation of nucleos(t)ide analogues is not associated with a higher risk of HBsAg seroreversion after antiviral-induced HBsAg seroclearance: A nationwide multicentre study. Gut 2020, 69, 2214–2222. [Google Scholar] [CrossRef]
- Gish, R.G.; Locarnini, S.A. Chronic hepatitis B: Current testing strategies. Clin. Gastroenterol. Hepatol. 2006, 4, 666–676. [Google Scholar] [CrossRef]
- Lok, A.S.; McMahon, B.J. Chronic hepatitis B. Hepatology 2007, 45, 507–539. [Google Scholar] [CrossRef]
- Blumberg, B.S.; Sutnick, A.I.; London, W.T.; Millman, I. Australia antigen and hepatitis. N. Engl. J. Med. 1970, 283, 349–354. [Google Scholar] [CrossRef][Green Version]
- Brunetto, M.R.; Moriconi, F.; Bonino, F.; Lau, G.K.; Farci, P.; Yurdaydin, C.; Piratvisuth, T.; Luo, K.; Wang, Y.; Hadziyannis, S.; et al. Hepatitis B virus surface antigen levels: A guide to sustained response to peginterferon alfa-2a in HBeAg-negative chronic hepatitis B. Hepatology 2009, 49, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Moucari, R.; Korevaar, A.; Lada, O.; Martinot-Peignoux, M.; Boyer, N.; Mackiewicz, V.; Dauvergne, A.; Cardoso, A.C.; Asselah, T.; Nicolas-Chanoine, M.H.; et al. High rates of HBsAg seroconversion in HBeAg-positive chronic hepatitis B patients responding to interferon: A long-term follow-up study. J. Hepatol. 2009, 50, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.C.; Kao, J.H. Clinical utility of quantitative HBsAg in natural history and nucleos(t)ide analogue treatment of chronic hepatitis B: New trick of old dog. J. Gastroenterol. 2013, 48, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Louisirirotchanakul, S.; Khupulsup, K.; Akraekthalin, S.; Chan, K.P.; Saw, S.; Aw, T.C.; Cho, D.H.; Shin, M.-G.; Lim, J. Comparison of the technical and clinical performance of the Elecsys HBsAg II assay with the Architect, AxSym, and Advia Centaur HBsAg screening assays. J. Med. Virol. 2010, 82, 755–762. [Google Scholar] [CrossRef]
- Thompson, A.J.; Nguyen, T.; Iser, D.; Ayres, A.; Jackson, K.; Littlejohn, M.; Slavin, J.; Bowden, S.; Gane, E.J.; Abbott, W.; et al. Serum hepatitis B surface antigen and hepatitis B e antigen titers: Disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology 2010, 51, 1933–1944. [Google Scholar] [CrossRef]
- Mak, L.Y.; Seto, W.K.; Fung, J.; Yuen, M.F. Use of HBsAg quantification in the natural history and treatment of chronic hepatitis B. Hepatol. Int. 2020, 14, 35–46. [Google Scholar] [CrossRef]
- Yang, R.; Cui, L.; Liu, Y.; Cong, X.; Fei, R.; Wu, S.; Wei, L. A hook-effect-free homogeneous light-initiated chemiluminescence assay: Is it reliable for screening and the quantification of the hepatitis B surface antigen? Ann. Transl. Med. 2020, 8, 606. [Google Scholar] [CrossRef]
- Song, B.C.; Cui, X.J.; Kim, H. Hepatitis B virus genotypes in Korea: An endemic area of hepatitis B virus infection. Intervirology 2005, 48, 133–137. [Google Scholar] [CrossRef]
- Lee, J.M.; Ahn, S.H.; Chang, H.Y.; Shin, J.E.; Kim, D.Y.; Sim, M.K.; Hong, S.P.; Chung, H.J.; Kim, S.O.; Han, K.H.; et al. Reappraisal of HBV genotypes and clinical significance in Koreans using MALDI-TOF mass spectrometry. Korean J. Hepatol. 2004, 10, 260–270. [Google Scholar]
| HBeAg (+) | ||||||||
| sqHBsAg (S/Co) | 100 | 500 | 1000 | 2000 | 3000 | 4000 | 1291.89 | 7286.87 |
| qHBsAg (IU/mL) | 3.32 | 28.27 | 71.11 | 178.91 | 306.91 | 450.09 | 100 | 1000 |
| HBeAg (−) | ||||||||
| sqHBsAg (S/Co) | 100 | 500 | 1000 | 2000 | 3000 | 4000 | 1108.73 | 6319.54 |
| qHBsAg (IU/mL) | 4.15 | 34.86 | 87.23 | 218.25 | 373.19 | 546.03 | 100 | 1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, G.E.; Kim, J.Y.; Shin, H.; Hong, J.H.; Hur, M.H.; Cho, H.; Park, M.K.; Choi, N.R.; Kim, J.; Lee, Y.B.; et al. Correlation between Results of Semi-Quantitative and Quantitative Tests for Hepatitis B Virus Surface Antigen among Patients Achieving Viral Suppression with Antiviral Treatment. Diagnostics 2022, 12, 1757. https://doi.org/10.3390/diagnostics12071757
Chung GE, Kim JY, Shin H, Hong JH, Hur MH, Cho H, Park MK, Choi NR, Kim J, Lee YB, et al. Correlation between Results of Semi-Quantitative and Quantitative Tests for Hepatitis B Virus Surface Antigen among Patients Achieving Viral Suppression with Antiviral Treatment. Diagnostics. 2022; 12(7):1757. https://doi.org/10.3390/diagnostics12071757
Chicago/Turabian StyleChung, Goh Eun, Ju Yeon Kim, Hyunjae Shin, Ji Hoon Hong, Moon Haeng Hur, Heejin Cho, Min Kyung Park, Na Ryung Choi, Jihye Kim, Yun Bin Lee, and et al. 2022. "Correlation between Results of Semi-Quantitative and Quantitative Tests for Hepatitis B Virus Surface Antigen among Patients Achieving Viral Suppression with Antiviral Treatment" Diagnostics 12, no. 7: 1757. https://doi.org/10.3390/diagnostics12071757
APA StyleChung, G. E., Kim, J. Y., Shin, H., Hong, J. H., Hur, M. H., Cho, H., Park, M. K., Choi, N. R., Kim, J., Lee, Y. B., Cho, E. J., Yu, S. J., Kim, Y. J., Yoon, J.-H., & Lee, J.-H. (2022). Correlation between Results of Semi-Quantitative and Quantitative Tests for Hepatitis B Virus Surface Antigen among Patients Achieving Viral Suppression with Antiviral Treatment. Diagnostics, 12(7), 1757. https://doi.org/10.3390/diagnostics12071757






