Effectiveness of Evaluation of Adenoid Hypertrophy in Children by Flexible Nasopharyngoscopy Examination (FNE), Proposed Schema of Frequency of Examination: Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Participants
2.2. Inclusion and Exclusion Criteria
2.3. Study Methods
2.4. Endoscopy
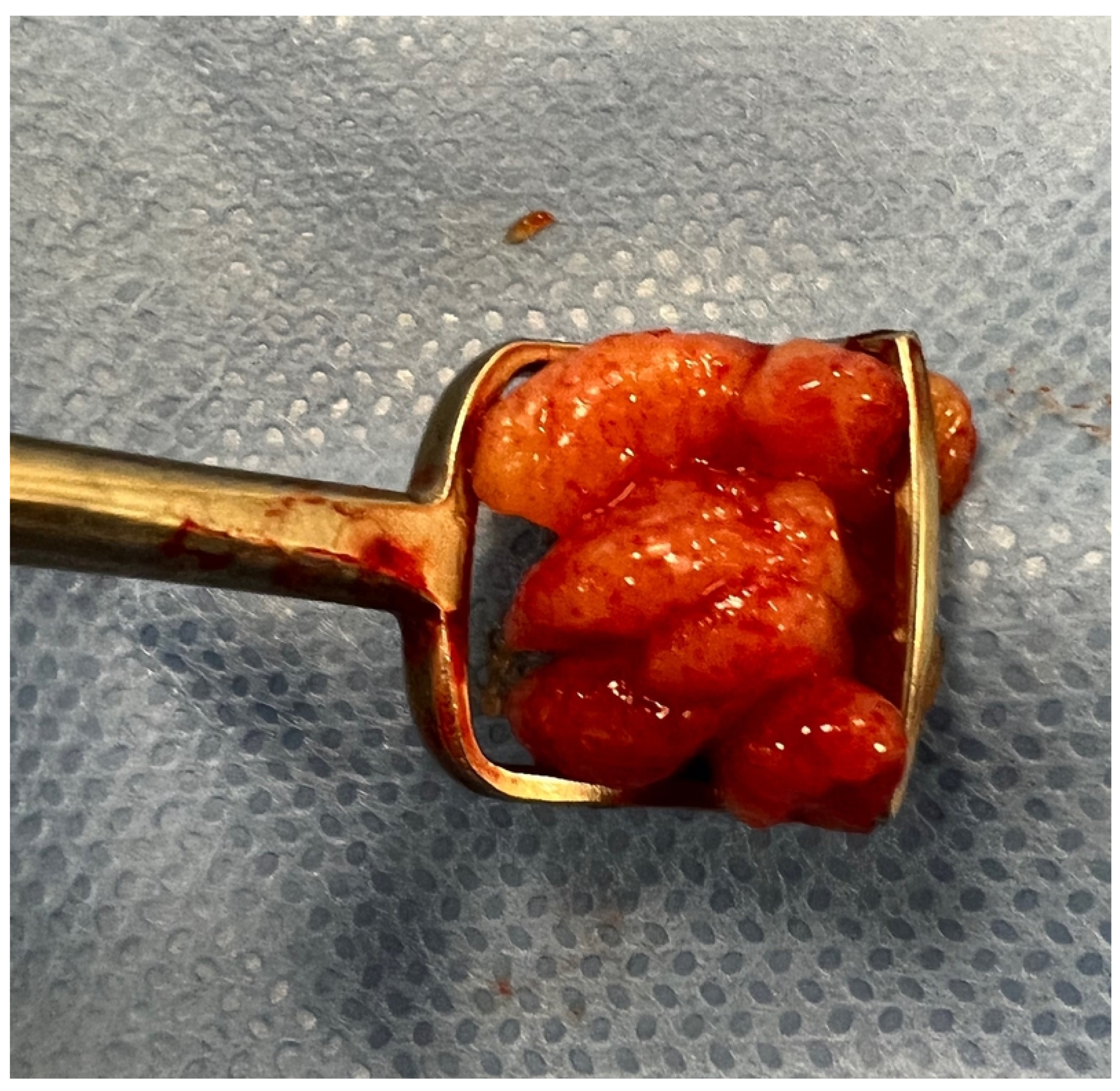
2.5. Surgery
2.6. Statistical Analysis
2.7. Ethics
3. Results
3.1. Association of A/C Ratio with Operative Adenoid Size
3.1.1. Patients’ Characteristics
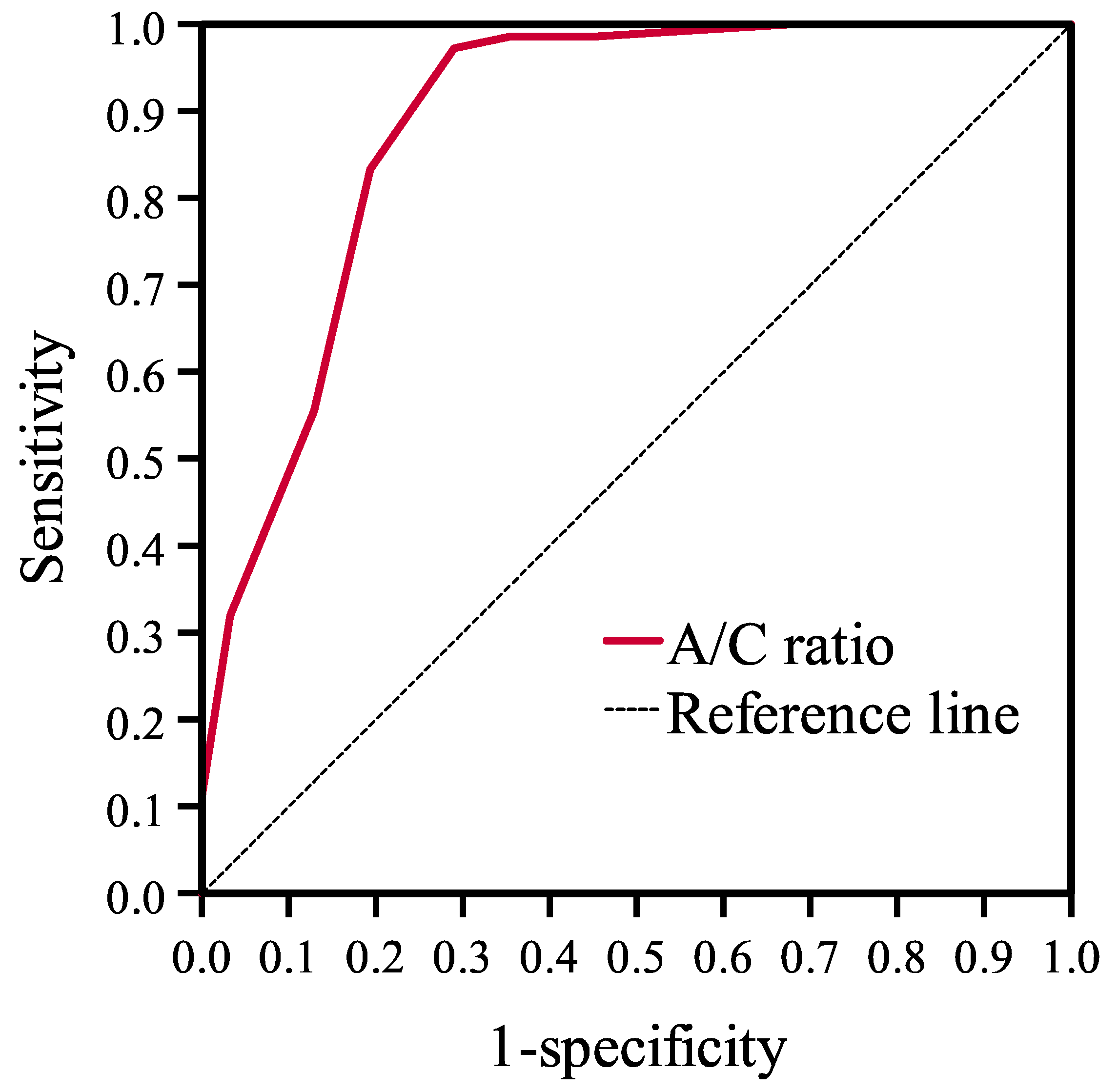
3.1.2. Diagnostic Value of A/C Ratio
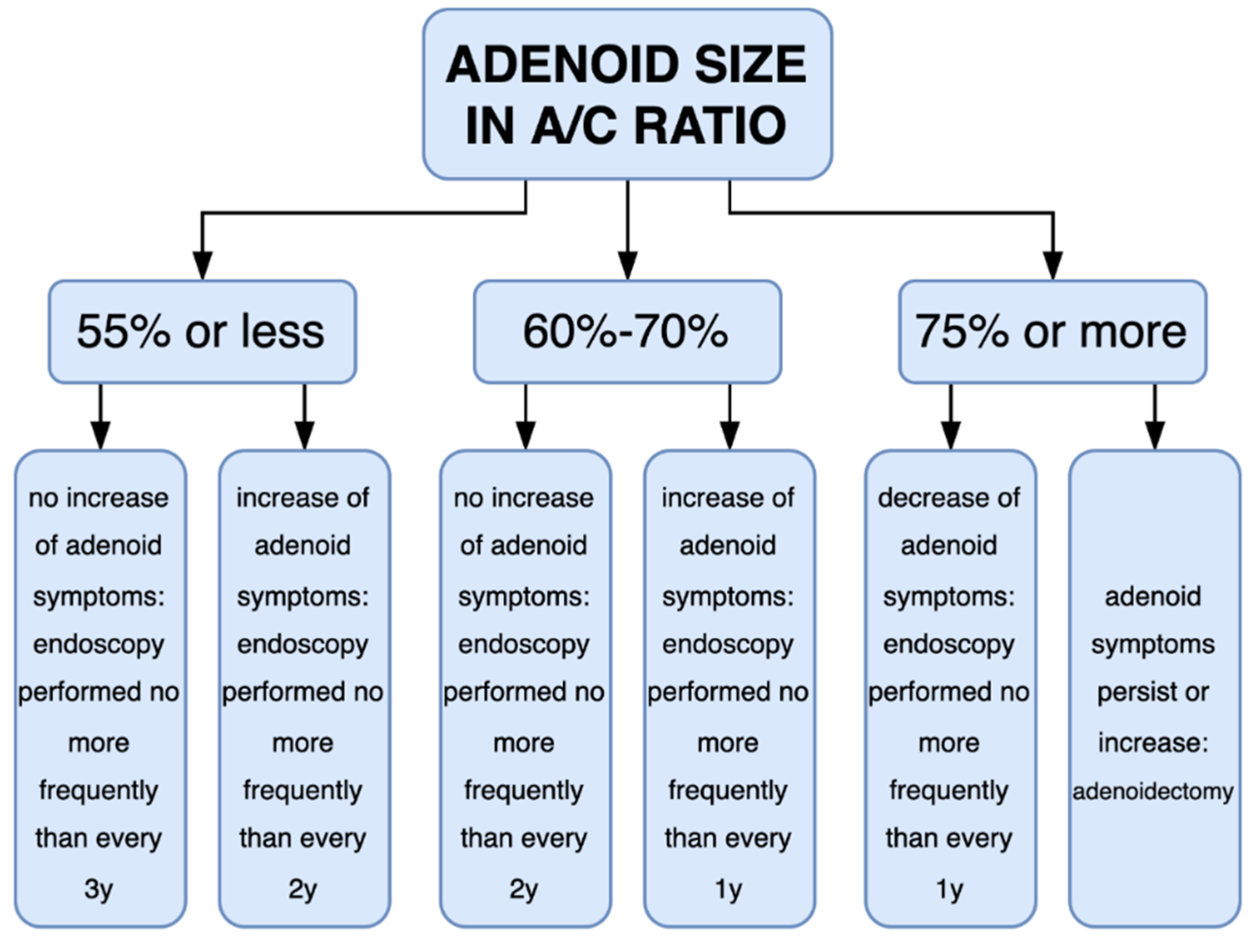
3.2. Volatility of the A/C Ratio over Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Qiao, X.; Qi, S.; Zhang, X.; Li, S. Effect of adenoid hypertrophy on the upper airway and craniomaxillofacial region. Transl. Pediatr. 2021, 10, 2563–2572. [Google Scholar] [CrossRef]
- Di Mauro, P.; Cocuzza, S.; Maniaci, A.; Ferlito, S.; Rasà, D.; Anzivino, R.; Vicini, C.; Iannella, G.; La Mantia, I. The effect of adenotonsillectomy on children’s behavior and cognitive performance with obstructive sleep apnea syndrome: State of the art. Children 2021, 8, 921. [Google Scholar] [CrossRef] [PubMed]
- Purnell, P.R.; Ramadan, J.H.; Ramadan, H.H. Can symptoms differentiate between chronic adenoiditis and chronic rhinosinusitis in pediatric patients. Ear Nose Throat J. 2019, 98, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Ruben, R.J. The adenoid: Its history and a cautionary tale. Laryngoscope 2017, 127 (Suppl. 2), S13–S28. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Lee, D.H.; Lee, N.S.; Won, Y.S.; Yoon, H.R.; Suh, B.D. Size assessment of adenoid and nasopharyngeal airway by acoustic rhinometry in children. J. Laryngol. Otol. 1999, 113, 899–905. [Google Scholar] [CrossRef]
- Patel, A.; Brook, C.D.; Levi, J.R. Comparison of adenoid assessment by flexible endoscopy and mirror exam. Int. J. Pediatr. Otorhinolaryngol. 2020, 134, 110073. [Google Scholar] [CrossRef]
- Ysunza, A.; Pamplona, M.C.; Ortega, J.M.; Prado, H. Video fluoroscopy for evaluating adenoid hypertrophy in children. Int. J. Pediatr. Otorhinolaryngol. 2008, 72, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Zicari, A.M.; Magliulo, G.; Rugiano, A.; Ragusa, G.; Celani, C.; Carbone, M.P.; Occasi, F.; Duse, M. The role of rhinomanometry after nasal decongestant test in the assessment of adenoid hypertrophy in children. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 352–356. [Google Scholar] [CrossRef]
- Hoppe, H.; Thoeny, H.C.; Dinkel, H.P.; Zbären, P.; Vock, P. Virtual laryngoscopy and multiplanar reformats with multirow detector CT for detection and grading of upper airway stenosis. Rofo 2002, 174, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Torretta, S.; Marchisio, P.; Esposito, S.; Cappadona, M.; Fattizzo, M.; Pignataro, L. Diagnostic accuracy of the nasal obstruction index in detecting adenoid hypertrophy in children without allergy. Int. J. Pediatr. Otorhinolaryngol. 2011, 7, 57–61. [Google Scholar] [CrossRef]
- Wang, D.Y.; Bernheim, N.; Kaufman, L.; Clement, P. Assessment of adenoid size in children by fibreoptic examination. Clin. Otolaryngol. Allied Sci. 1997, 22, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.A.; Jackson, C.M.; Paterson, D.G.; Scammon, S.E. The measurement of the body in childhood. In The Measurement of Man; University of Minnesota Press: Minneapolis, MN, USA, 1930. [Google Scholar]
- Yamada, H.; Sawada, M.; Higashino, M.; Abe, S.; El-Bialy, T.; Tanaka, E. Longitudinal morphological changes in the adenoids and tonsils in Japanese school children. J. Clin. Med. 2021, 10, 4956. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Manabe, A.; Yang, S.S.; Yoon, H.S.; Kanda, E.; Ono, T. Patterns of adenoid and tonsil growth in Japanese children and adolescents: A longitudinal study. Sci. Rep. 2018, 8, 17088. [Google Scholar] [CrossRef]
- Handelman, C.S.; Osborne, G. Growth of the nasopharynx and adenoid development from one to eighteen years. Angle Orthod. 1976, 46, 243–259. [Google Scholar] [CrossRef]
- Boleslavská, J.; Kopřivová, H.; Komínek, P. Is it important to evaluate the size of adenoid vegetations? Otorinolaryng. Foniat. Prague 2006, 55, 133–138. (In Czech) [Google Scholar]
- Shaalan, H.F. What is the right size of the adenoid curette? J. Laryngol. Otol. 2003, 117, 796–800. [Google Scholar] [CrossRef]
- Baldassari, C.M.; Choi, S. Assessing adenoid hypertrophy in children: X-ray or nasal endoscopy? Laryngoscope 2014, 124, 1509–1510. [Google Scholar] [CrossRef]
- Major, M.P.; Saltaji, H.; El-Hakim, H.; Witmans, M.; Major, P.; Flores-Mir, C. The accuracy of diagnostic tests for adenoid hypertrophy: A systematic review. J. Am. Dent. Assoc. 2014, 145, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Mlynarek, A.; Tewfik, M.A.; Hagr, A.; Manoukian, J.J.; Schloss, M.D.; Tewfik, T.L.; Choi-Rosen, J. Lateral neck radiography versus direct video rhinoscopy in assessing adenoid size. J. Otolaryngol. 2004, 33, 360–365. [Google Scholar] [CrossRef]
- Caylakli, F.; Hizal, E.; Yilmaz, I.; Yilmazer, C. Correlation between adenoid-nasopharynx ratio and endoscopic examination of adenoid hypertrophy: A blind, prospective clinical study. Int. J. Pediatr Otorhinolaryngol. 2009, 73, 1532–1535. [Google Scholar] [CrossRef]
- Yıldırım, Y.S.; Apuhan, T.; Aksoy, F.; Veyseller, B.; Ozturan, O. Is transnasal endoscopic examination necessary before and after adenoidectomy? Indian J. Otolaryngol. Head Neck Surg. 2014, 66 (Suppl. 1), 257–260. [Google Scholar] [CrossRef][Green Version]
- Lertsburapa, K.; Schroeder, J.W.; Sullivan, C. Assessment of adenoid size: A comparison of lateral radiographic measurements, radiologist assessment, and nasal endoscopy. Int. J. Pediatr. Otorhinolaryngol. 2010, 74, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Pirilä-Parkkinen, K.; Löppönen, H.; Nieminen, P.; Tolonen, U.; Pääkkoe, E.; Pirttiniemi, P. Validity of upper airway assessment in children: A clinical, cephalometric, and MRI study. Angle Orthod. 2011, 81, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Cohen, O.; Betito, H.R.; Adi, M.; Shapira-Galitz, Y.; Halperin, D.; Lahav, Y.; Warman, M. Development of the nasopharynx: A radiological study of children. Clin. Anat. 2020, 33, 1019–1024. [Google Scholar] [CrossRef]
- Linder-Aronson, S.; Leighton, B.C. A longitudinal study of the development of the posterior nasopharyngeal wall between 3 and 16 years of age. Eur. J. Orthod. 1983, 5, 47–58. [Google Scholar] [CrossRef]
- Subtelny, J.D. Width of the nasopharynx and related anatomic structure in normal and unoperated cleft palate children. Am. J. Orthod. 1955, 41, 889–909. [Google Scholar] [CrossRef]
- Bergland, O. The bony nasopharynx. A roentgen-craniometric study. Acta Odontol. Scand. 1963, 21, 1–137. [Google Scholar]
- Papaioannou, G.; Kambas, I.; Tsaoussoglou, M.; Panaghiotopoulou-Gartagani, P.; Chrousos, G.; Kaditis, A.G. Age-dependent changes in the size of adenotonsillar tissue in childhood: Implications for sleep-disordered breathing. J. Pediatr. 2013, 162, 269–274.e4. [Google Scholar] [CrossRef]
- Handelman, C.S.; Pruzansky, S. The Size of the Adenoids in Normal and C. P. I. Children; Presented at the IADR: Washington, DC, USA, 1967. [Google Scholar]
- Wang, Y.; Jiao, H.; Mi, C.; Yang, G.; Han, T. Evaluation of adenoid hypertrophy with ultrasonography. Indian J. Pediatr. 2020, 87, 910–915. [Google Scholar] [CrossRef]
- Chisholm, E.J.; Lew-Gor, S.; Hajioff, D.; Caulfield, H. Adenoid size assessment: A comparison of palpation, nasendoscopy and mirror examination. Clin. Otolaryngol. 2005, 30, 39–41. [Google Scholar] [CrossRef]
- Masna, K.; Zwierz, A.; Domagalski, K.; Burduk, P. The impact of the thermal seasons on adenoid size, its mucus coverage and otitis media with effusion: A cohort study. J. Clin. Med. 2021, 10, 5603. [Google Scholar] [CrossRef] [PubMed]
- Lesinskas, E.; Drigotas, M. The incidence of adenoidal regrowth after adenoidectomy and its effect on persistent nasal symptoms. Eur. Arch Otorhinolaryngol. 2009, 266, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.; Mahadevan, M.; Douglas, R.G. Incidence and factors associated with revision adenoidectomy: A retrospective study. Int. J. Pediatr. Otorhinolaryngol. 2017, 103, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Dearking, A.C.; Lahr, B.D.; Kuchena, A.; Orvidas, L.J. Factors associated with revision adenoidectomy. Otolaryngol. Head Neck Surg. 2012, 146, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Liapi, A.; Dhanasekar, G.; Turner, N.O. Role of revision adenoidectomy in paediatric otolaryngological practice. J. Laryngol. Otol. 2006, 120, 219–221. [Google Scholar] [CrossRef]
- Demirhan, H.; Aksoy, F.; Özturan, O.; Yıldırım, Y.S.; Veyseller, B. Medical treatment of adenoid hypertrophy with “fluticasone propionate nasal drops”. Int. J. Pediatr. Otorhinolaryngol. 2010, 74, 773–776. [Google Scholar] [CrossRef]
- Zwierz, A.; Masna, K.; Domagalski, K.; Burduk, P. The long-term effects of 12-week intranasal steroid therapy on adenoid size, its mucus coverage and otitis media with effusion: A cohort study in preschool children. J. Clin. Med. 2022, 11, 507. [Google Scholar] [CrossRef]
| Characteristics | All Patients | Female | Male | p Value | |
|---|---|---|---|---|---|
| n | 108 | 43 | 65 | ||
| Gender | female | 43 (39.8%) | 43 (39.8%) | - | |
| male | 65 (60.2%) | 65 (60.2%) | |||
| Age (years) | mean ± SD | 5.2 ± 1.8 | 5.4 ± 1.9 | 5.0 ± 1.7 | 0.267 |
| median (Q25–Q75) | 5.0 (4.0–6.0) | 5.0 (4.0–6.0) | 4.5 (4.0–5.5) | ||
| Adenoid size (A/C ratio *, %) | mean ± SD | 77.5 ± 13.2 | 78.8 ± 13.4 | 76.6 ± 13.2 | 0.396 |
| median (Q25–Q75) | 80.0 (75.0–85.0) | 80.0 (75.0–90.0) | 80.0 (70.0–85.0) | ||
| 40 | 1 (0.9%) | 1 (2.3%) | 0 (0.0%) | 0.505 | |
| 50 | 10 (9.3%) | 2 (4.7%) | 8 (12.3%) | ||
| 60 | 9 (8.3%) | 4 (9.3%) | 5 (7.7%) | ||
| 65 | 3 (2.8%) | 1 (2.3%) | 2 (3.1%) | ||
| 70 | 3 (2.8%) | 1 (2.3%) | 2 (3.1%) | ||
| 75 | 13 (12.0%) | 7 (16.3%) | 6 (9.2%) | ||
| 80 | 23 (21.3%) | 7 (16.3%) | 16 (24.6%) | ||
| 85 | 22 (20.4%) | 7 (16.3%) | 15 (23.1%) | ||
| 90 | 16 (14.8%) | 8 (18.6%) | 8 (12.3%) | ||
| 95 | 8 (7.4%) | 5 (11.6%) | 3 (4.6%) | ||
| Adenoid size (A/C ratio on Bolesławska scale, %) | 35–65 (B II) | 23 (21.3%) | 8 (18.6%) | 15 (23.1%) | 0.587 |
| >65 (B III) | 85 (78.7%) | 35 (81.4%) | 50 (76.9%) | ||
| Operative adenoid size, 3 categories | small | 21 (19.4%) | 8 (18.6%) | 13 (20.0%) | 0.857 |
| medium | 12 (11.1%) | 6 (14.0%) | 6 (9.2%) | ||
| large | 75 (69.4%) | 29 (67.4%) | 46 (70.8%) | ||
| Operative adenoid size, 2 categories | not large | 33 (30.6%) | 14 (32.6%) | 19 (29.2%) | 0.713 |
| large | 75 (69.4%) | 29 (67.4%) | 46 (70.8%) | ||
| Consistency between A/C ratio in Bolesławska scale and operative adenoid size ** | true | 96 (88.9%) | 37 (86.0%) | 59 (90.8%) | 0.536 |
| false | 12 (11.1%) | 6 (14.0%) | 6 (9.2%) |
| Characteristics | Operative Adenoid Size, 3 Categories | p Value 1 | p Value 2 | ||
|---|---|---|---|---|---|
| Small | Medium | Large | |||
| n | 21 | 12 | 75 | ||
| Gender | |||||
| female | 8 (38.1%) | 6 (50.0%) | 29 (38.7%) | 0.857 | 0.713 |
| male | 13 (61.9%) | 6 (50.0%) | 46 (61.3%) | ||
| Age (years) | |||||
| mean ± SD | 5.0 ± 1.5 | 4.7 ± 1.3 | 5.3 ± 1.9 | 0.559 | 0.336 |
| median (Q25–Q75) | 4.5 (4.0–6.5) | 4.3 (4.0–5.8) | 5.0 (4.0–6.0) | ||
| Adenoid size (A/C ratio, %) | |||||
| mean ± SD | 59.5 ± 13.6 | 69.6 ± 10.1 | 83.8 ± 6.8 | <0.001 3 | <0.001 |
| median (Q25–Q75) | 50.0 (50.0–65.0) | 67.5 (60.0–77.5) | 85.0 (80.0–90.0) | ||
| Adenoid size (A/C ratio on Bolesławska scale, %) | |||||
| 35–65 (B II) | 16 (76.2%) | 6 (50.0%) | 1 (1.3%) | <0.001 | <0.001 |
| >65 (B III) | 5 (23.8%) | 6 (50.0%) | 74 (98.7%) | ||
| Consistency between A/C ratio on Bolesławska scale and operative adenoid size | |||||
| true | 16 (76.2%) | 6 (50.0%) | 74 (98.7%) | <0.001 | <0.001 |
| false | 5 (23.8%) | 6 (50.0%) | 1 (1.3%) | ||
| A/C Ratio | ||
|---|---|---|
| AUC | 0.894 | |
| 95% CI | 0.818–0.969 | |
| p value | <0.001 | |
| Cutoff value (%) | 75.0 | 80.0 |
| Sensitivity | 97.3% | 84.0% |
| Specificity | 72.7% | 81.8% |
| PPV | 89.0% | 91.3% |
| NPV | 92.3% | 69.2% |
| Characteristics | All Patients | |
|---|---|---|
| n | 81 | |
| Gender | female | 43 (53.1%) |
| male | 38 (46.9%) | |
| Age at the first visit (years) | mean ± SD | 3.9 ± 1.2 |
| median (Q25–Q75) | 3.5 (3.0–4.5) | |
| Number of measurements | 3 | 47 (58.0%) |
| 4 | 16 (19.8%) | |
| 5 | 15 (18.5%) | |
| 6 | 2 (2.5%) | |
| 7 | 1 (1.2%) | |
| Period of observation (years) | mean ± SD | 2.4 ± 1.2 |
| median (Q25–Q75) | 2.0 (1.5–3.0) | |
| 1–3 | 63 (77.8%) | |
| 3.5–5.5 | 18 (22.2%) | |
| First visit | mean ± SD | 65.4 ± 13.2 |
| median (Q25–Q75) | 65.0 (55.0–75.0) | |
| Bolesławska scale | <35 | 1 (1.2%) |
| 35–65 | 46 (56.8%) | |
| >65 | 34 (42.0%) | |
| Last visit | mean ± SD | 61.2 ± 16.1 |
| median (Q25–Q75) | 60.0 (50.0–75.0) | |
| Bolesławska scale | <35 | 4 (4.9%) |
| 35–65 | 46 (56.8%) | |
| >65 | 31 (38.3%) | |
| Change; first vs. last visit | decrease | 21 (25.9%) |
| no change | 17 (21.0%) | |
| increase | 43 (53.1%) | |
| ≤10 | 54 (66.7%) | |
| >10 | 27 (33.3%) | |
| ≤15 | 61 (75.3%) | |
| >15 | 20 (24.7%) | |
| Max | mean ± SD | 71.0 ± 12.3 |
| median (Q25–Q75) | 70.0 (60.0–80.0) | |
| Min | mean ± SD | 56.3 ± 14.6 |
| median (Q25–Q75) | 55.0 (50.0–70.0) | |
| Range | mean ± SD | 14.7 ± 11.2 |
| median (Q25–Q75) | 10.0 (5.0–20.0) | |
| ≤10 | 42 (51.9%) | |
| >10 | 39 (48.1%) | |
| ≤15 | 56 (69.1%) | |
| >15 | 25 (30.9%) | |
| Semi-annual continuous measurements, 1 year | n | 38 (46.9%) |
| Range | ≤10 | 28 (73.7%) |
| >10 | 10 (26.3%) | |
| ≤15 | 35 (92.1%) | |
| >15 | 3 (7.9%) | |
| Annual continuous measurements (at least one measurement per year) | n | 57 (70.4%) |
| Range | ≤10 | 34 (59.6%) |
| >10 | 23 (40.4%) | |
| ≤15 | 42 (73.7%) | |
| >15 | 15 (26.3%) | |
| Two-year period | n | 37 (45.7%) |
| Range | ≤10 | 21 (56.8%) |
| >10 | 16 (43.2%) | |
| ≤15 | 29 (78.4%) | |
| >15 | 8 (21.6%) | |
| Three-year period | n | 20 (24.7%) |
| Range | ≤10 | 7 (35.0%) |
| >10 | 13 (65.0%) | |
| ≤15 | 11 (55.0%) | |
| >15 | 9 (45.0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zwierz, A.; Domagalski, K.; Masna, K.; Burduk, P. Effectiveness of Evaluation of Adenoid Hypertrophy in Children by Flexible Nasopharyngoscopy Examination (FNE), Proposed Schema of Frequency of Examination: Cohort Study. Diagnostics 2022, 12, 1734. https://doi.org/10.3390/diagnostics12071734
Zwierz A, Domagalski K, Masna K, Burduk P. Effectiveness of Evaluation of Adenoid Hypertrophy in Children by Flexible Nasopharyngoscopy Examination (FNE), Proposed Schema of Frequency of Examination: Cohort Study. Diagnostics. 2022; 12(7):1734. https://doi.org/10.3390/diagnostics12071734
Chicago/Turabian StyleZwierz, Aleksander, Krzysztof Domagalski, Krystyna Masna, and Paweł Burduk. 2022. "Effectiveness of Evaluation of Adenoid Hypertrophy in Children by Flexible Nasopharyngoscopy Examination (FNE), Proposed Schema of Frequency of Examination: Cohort Study" Diagnostics 12, no. 7: 1734. https://doi.org/10.3390/diagnostics12071734
APA StyleZwierz, A., Domagalski, K., Masna, K., & Burduk, P. (2022). Effectiveness of Evaluation of Adenoid Hypertrophy in Children by Flexible Nasopharyngoscopy Examination (FNE), Proposed Schema of Frequency of Examination: Cohort Study. Diagnostics, 12(7), 1734. https://doi.org/10.3390/diagnostics12071734






