Assessment of the Accuracy in Measuring the Enamel Thickness of Maxillary Incisors with Optical Coherence Tomography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
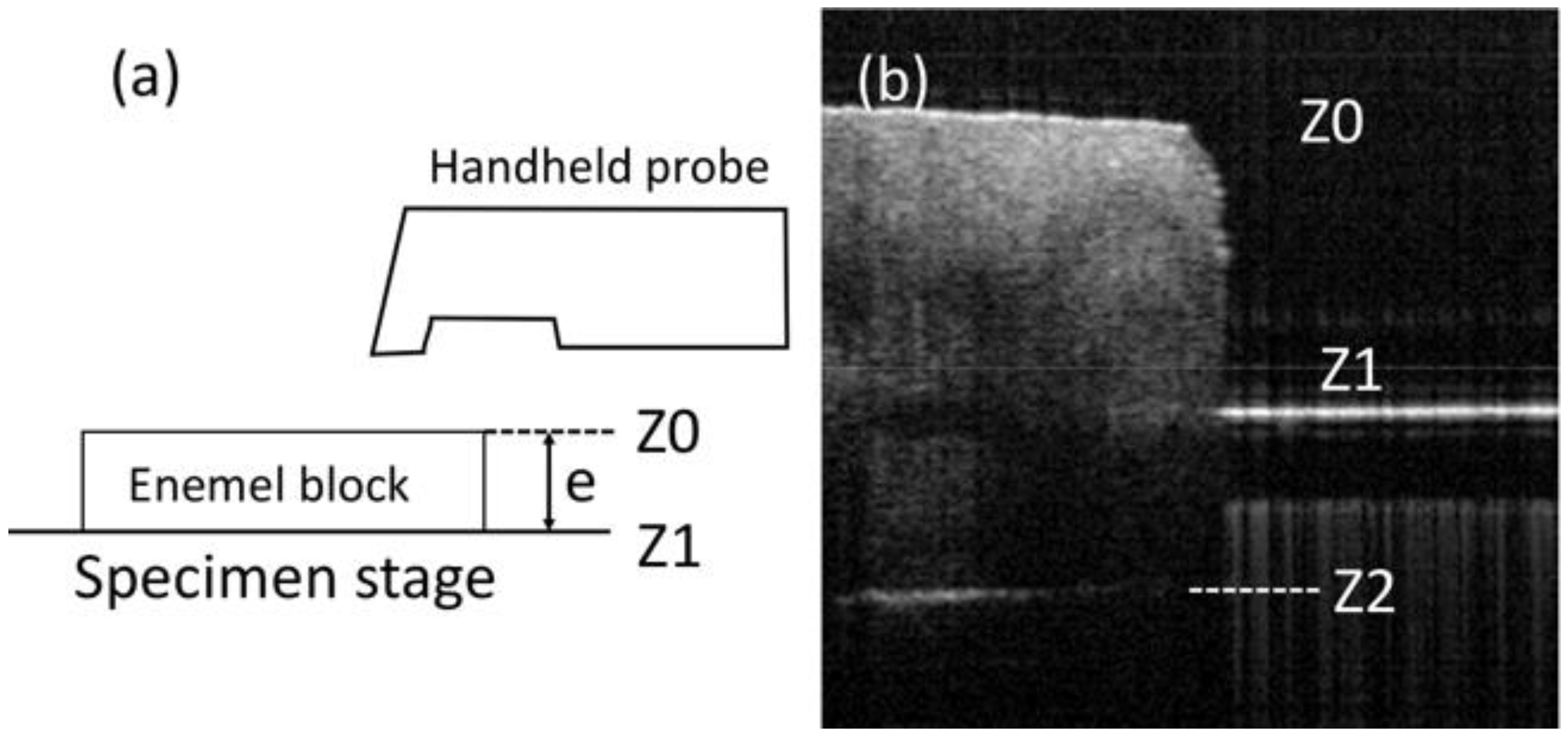
2.2. SS-OCT Imaging
2.3. OCT Image Analysis
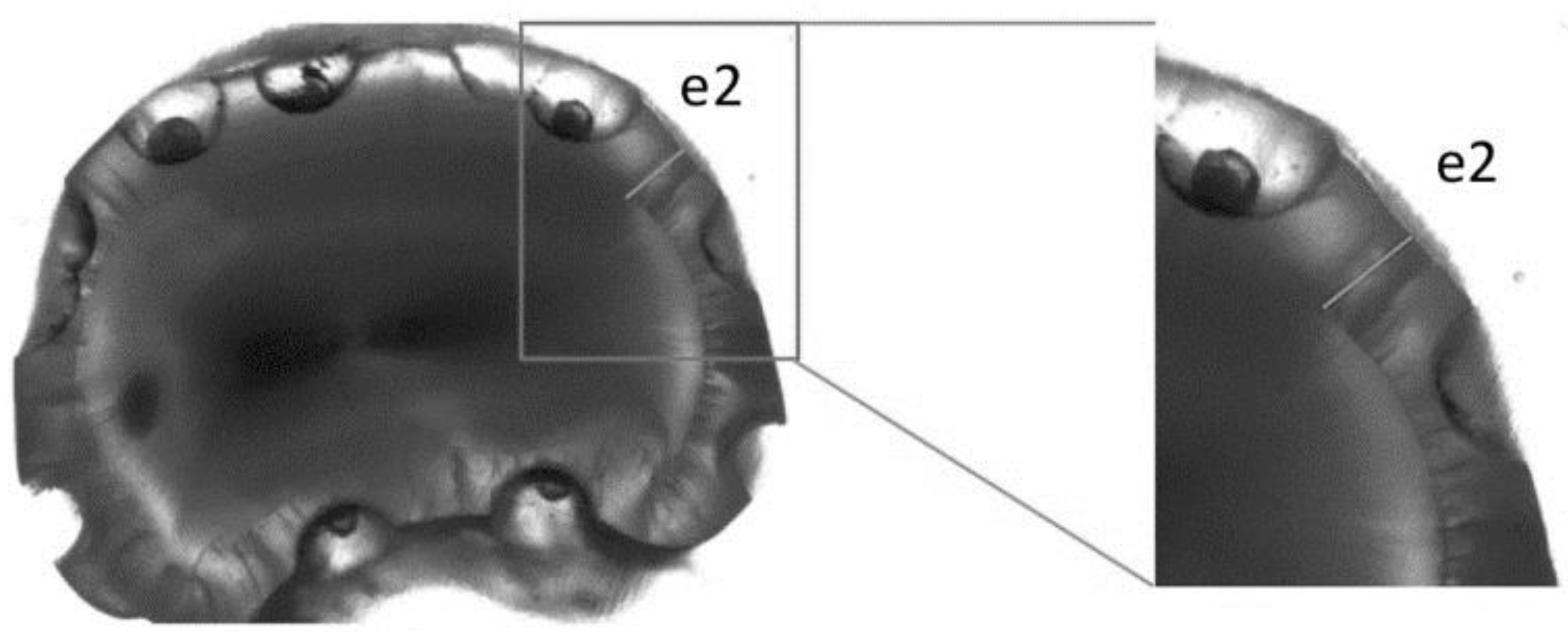
2.4. Light Microscopy Measurements
2.5. Statistical Analysis
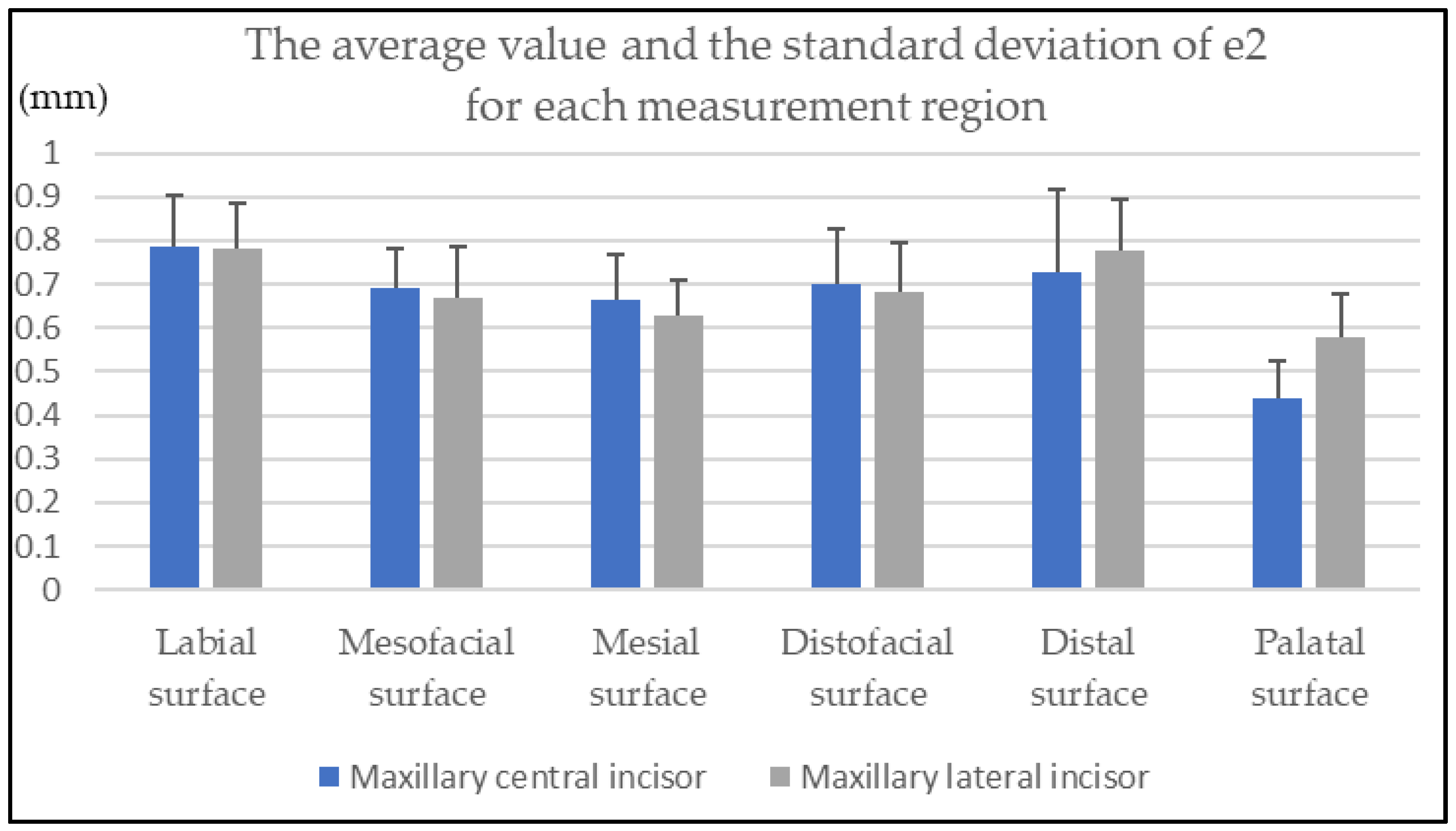
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A



References
- AlJazairy, Y.H. Survival Rates for Porcelain Laminate Veneers: A Systematic Review. Eur. J. Dent. 2020, 15, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Arif, R.; Dennison, J.B.; Garcia, D.; Yaman, P. Retrospective evaluation of the clinical performance and longevity of porcelain laminate veneers 7 to 14 years after cementation. J. Prosthet. Dent. 2019, 122, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Stangel, I.; Ellis, T.H.; Sacher, E. Adhesion to Tooth Structure Mediated by Contemporary Bonding Systems. Dent. Clin. N. Am. 2007, 51, 677–694. [Google Scholar] [CrossRef] [PubMed]
- De Munck, J.; Van Landuyt, K.; Peumans, M.; Poitevin, A.; Lambrechts, P.; Braem, M.; Van Meerbeek, B. A Critical Review of the Durability of Adhesion to Tooth Tissue: Methods and Results. J. Dent. Res. 2005, 84, 118–132. [Google Scholar] [CrossRef]
- Xing, W.; Jiang, T.; Ma, X.; Liang, S.; Wang, Z.; Sa, Y.; Wang, Y. Evaluation of the esthetic effect of resin cements and try-in pastes on ceromer veneers. J. Dent. 2010, 38, e87–e94. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, E.; Bolay, Ş.; Hickel, R.; Ilie, N. Shear bond strength of porcelain laminate veneers to enamel, dentine and enamel–dentine complex bonded with different adhesive luting systems. J. Dent. 2013, 41, 97–105. [Google Scholar] [CrossRef]
- Burke, F.J.T. Survival Rates for Porcelain Laminate Veneers with Special Reference to the Effect of Preparation in Dentin: A Literature Review. J. Esthet. Restor. Dent. 2012, 24, 257–265. [Google Scholar] [CrossRef]
- Piemjai, M.; Arksornnukit, M. Compressive Fracture Resistance of Porcelain Laminates Bonded to Enamel or Dentin with Four Adhesive Systems. J. Prosthodont. 2007, 16, 457–464. [Google Scholar] [CrossRef]
- Hua, J.; Chen, S.-K.; Kim, Y. Refining Enamel Thickness Measurements from B-Mode Ultrasound Images. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 440–443. [Google Scholar] [CrossRef] [Green Version]
- Tagtekin, D.A.; Öztürk, F.; Lagerweij, M.; Hayran, O.; Stookey, G.; Yanikoglu, F. Thickness Measurement of Worn Molar Cusps by Ultrasound. Caries Res. 2005, 39, 139–143. [Google Scholar] [CrossRef]
- Ślak, B.; Ambroziak, A.; Strumban, E.; Maev, R.G. Enamel thickness measurement with a high frequency ultrasonic transducer-based hand-held probe for potential application in the dental veneer placing procedure. Acta Bioeng. Biomech. 2011, 13, 65–70. [Google Scholar]
- Fujimoto, J.G. Optical coherence tomography for ultrahigh resolution in vivo imaging. Nat. Biotechnol. 2003, 21, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Podoleanu, A.G. Optical coherence tomography. J. Microsc. 2012, 247, 209–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machoy, M.E.; Koprowski, R.; Szyszka-Sommerfeld, L.; Safranow, K.; Gedrange, T.; Woźniak, K. Optical coherence tomography as a non-invasive method of enamel thickness diagnosis after orthodontic treatment by 3 different types of brackets. Adv. Clin. Exp. Med. 2019, 28, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, J.; Machoy, M.; Koprowski, R.; Safranow, K.; Gedrange, T.; Woźniak, K. Enamel Thickness before and after Orthodontic Treatment Analysed in Optical Coherence Tomography. BioMed Res. Int. 2017, 2017, 8390575. [Google Scholar] [CrossRef]
- Nakajima, Y.; Shimada, Y.; Miyashin, M.; Takagi, Y.; Tagami, J.; Sumi, Y. Noninvasive cross-sectional imaging of incomplete crown fractures (cracks) using swept-source optical coherence tomography. Int. Endod. J. 2012, 45, 933–941. [Google Scholar] [CrossRef]
- Makishi, P.; Shimada, Y.; Sadr, A.; Tagami, J.; Sumi, Y. Non-destructive 3D imaging of composite restorations using optical coherence tomography: Marginal adaptation of self-etch adhesives. J. Dent. 2011, 39, 316–325. [Google Scholar] [CrossRef]
- Choma, M.A.; Sarunic, M.V.; Yang, C.; Izatt, J.A. Sensitivity advantage of swept source and Fourier domain optical coherence tomography. Opt. Express 2003, 11, 2183–2189. [Google Scholar] [CrossRef] [Green Version]
- Leitgeb, R.; Hitzenberger, C.K.; Fercher, A.F. Performance of fourier domain vs. time domain optical coherence tomography. Opt. Express 2003, 11, 889–894. [Google Scholar] [CrossRef]
- Poneros, J.M.; Brand, S.; Bouma, B.E.; Tearney, G.J.; Compton, C.C.; Nishioka, N.S. Diagnosis of specialized intestinal metaplasia by optical coherence tomography. Gastroenterology 2001, 120, 7–12. [Google Scholar] [CrossRef]
- Wang, Y.; Bower, B.A.; Izatt, J.A.; Tan, O.; Huang, D. Retinal blood flow measurement by circumpapillary Fourier domain Doppler optical coherence tomography. J. Biomed. Opt. 2008, 13, 064003. [Google Scholar] [CrossRef] [Green Version]
- Welzel, J. Optical coherence tomography in dermatology: A review. Ski. Res. Technol. 2001, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.-S.; Ho, Y.-C.; Lee, S.-Y.; Chuang, C.-C.; Tsai, J.-C.; Lin, K.-F.; Sun, C.-W. Dental Optical Coherence Tomography. Sensors 2013, 13, 8928–8949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colston, B.W.; Everett, M.J.; Da Silva, L.B.; Otis, L.L.; Stroeve, P.; Nathel, H. Imaging of hard- and soft-tissue structure in the oral cavity by optical coherence tomography. Appl. Opt. 1998, 37, 3582–3585. [Google Scholar] [CrossRef]
- Katkar, R.A.; Tadinada, S.A.; Amaechi, B.T.; Fried, D. Optical Coherence Tomography. Dent. Clin. N. Am. 2018, 62, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Sadr, A.; Shimada, Y.; Tagami, J.; Sumi, Y. Validation of swept source optical coherence tomography (SS-OCT) for the diagnosis of smooth surface caries in vitro. J. Dent. 2013, 41, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Ozawa, N.; Tagami, J.; Sumi, Y. Swept-source optical coherence tomography as a new tool to evaluate defects of resin-based composite restorations. J. Dent. 2011, 39, 543–548. [Google Scholar] [CrossRef]
- Sumi, Y.; Ozawa, N.; Nagaosa, S.; Minakuchi, S.; Umemura, O. Application of optical coherence tomography (OCT) to nondestructive inspection of dentures. Arch. Gerontol. Geriatr. 2011, 53, 237–241. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Zhang, L.; Xue, L.; Tian, J. Simultaneous refractive index and thickness measurements of bio tissue by optical coherence tomography. J. Biomed. Opt. 2002, 7, 628–632. [Google Scholar] [CrossRef]
- Uhlhorn, S.R.; Borja, D.; Manns, F.; Parel, J.-M. Refractive index measurement of the isolated crystalline lens using optical coherence tomography. Vis. Res. 2008, 48, 2732–2738. [Google Scholar] [CrossRef] [Green Version]
- Fujita, R.; Komada, W.; Nozaki, K.; Miura, H. Measurement of the remaining dentin thickness using optical coherence tomography for crown preparation. Dent. Mater. J. 2014, 33, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Meng, Z.; Yao, X.S.; Yao, H.; Liang, Y.; Liu, T.; Li, Y.; Wang, G.; Lan, S. Measurement of the refractive index of human teeth by optical coherence tomography. J. Biomed. Opt. 2009, 14, 034010–034014. [Google Scholar] [CrossRef] [PubMed]
- Hariri, I.; Sadr, A.; Shimada, Y.; Tagami, J.; Sumi, Y. Effects of structural orientation of enamel and dentine on light attenuation and local refractive index: An optical coherence tomography study. J. Dent. 2012, 40, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, Y.; Murayama, R.; Kurokawa, H.; Miyazaki, M.; Mihata, Y.; Kmaguchi, S. Influence of tooth-surface hydration conditions on optical coherence-tomography imaging. J. Dent. 2011, 39, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Hahn, P.; Gustav, M.; Hellwig, E. An in vitro assessment of the strength of porcelain veneers dependent on tooth preparation. J. Oral Rehabil. 2000, 27, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Hong, N.; Yang, H.; Li, J.; Wu, S.; Li, Y. Effect of Preparation Designs on the Prognosis of Porcelain Laminate Veneers: A Systematic Review and Meta-Analysis. Oper. Dent. 2017, 42, E197–E213. [Google Scholar] [CrossRef]

| Labial Surface | Mesiofacial Surface | Mesial Surface | Distofacial Surface | Distal Surface | Palatal Surface | |
|---|---|---|---|---|---|---|
| H1 | 0.02 | 0.05 | 0.03 | 0.05 | 0.06 | 0.03 |
| H2 | 0.02 | 0.04 | 0.02 | 0.03 | 0.02 | 0.04 |
| H3 | 0.04 | 0.05 | 0.05 | 0.07 | 0.05 | 0.03 |
| H4 | 0.02 | 0.03 | 0.06 | 0.04 | 0.06 | 0.04 |
| H5 | 0.04 | 0.03 | - | 0.04 | - | 0.04 |
| Labial Surface | Mesiofacial Surface | Mesial Surface | Distofacial Surface | Distal Surface | Palatal Surface | |
|---|---|---|---|---|---|---|
| H1 | 0.04 | 0.03 | 0.08 | 0.04 | 0.05 | 0.06 |
| H2 | 0.07 | 0.05 | 0.07 | 0.07 | 0.04 | 0.06 |
| H3 | 0.03 | 0.05 | 0.05 | 0.02 | 0.05 | 0.04 |
| H4 | 0.05 | 0.04 | 0.05 | 0.03 | 0.04 | 0.05 |
| H5 | 0.03 | 0.03 | - | 0.03 | - | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyagi, H.; Oki, K.; Tsukiyama, Y.; Ayukawa, Y.; Koyano, K. Assessment of the Accuracy in Measuring the Enamel Thickness of Maxillary Incisors with Optical Coherence Tomography. Diagnostics 2022, 12, 1634. https://doi.org/10.3390/diagnostics12071634
Miyagi H, Oki K, Tsukiyama Y, Ayukawa Y, Koyano K. Assessment of the Accuracy in Measuring the Enamel Thickness of Maxillary Incisors with Optical Coherence Tomography. Diagnostics. 2022; 12(7):1634. https://doi.org/10.3390/diagnostics12071634
Chicago/Turabian StyleMiyagi, Hiroshi, Kyosuke Oki, Yoshihiro Tsukiyama, Yasunori Ayukawa, and Kiyoshi Koyano. 2022. "Assessment of the Accuracy in Measuring the Enamel Thickness of Maxillary Incisors with Optical Coherence Tomography" Diagnostics 12, no. 7: 1634. https://doi.org/10.3390/diagnostics12071634
APA StyleMiyagi, H., Oki, K., Tsukiyama, Y., Ayukawa, Y., & Koyano, K. (2022). Assessment of the Accuracy in Measuring the Enamel Thickness of Maxillary Incisors with Optical Coherence Tomography. Diagnostics, 12(7), 1634. https://doi.org/10.3390/diagnostics12071634






