Choroidal Thickness in a Hyperopic Pediatric Population
Abstract
:1. Introduction
2. Materials and Methods
- 0–18 months old: the rapid postnatal phase where the axial length increases by 3.7–3.8 mm;
- 2–5 years old: slower phase, decreased growth velocity (1.1–1.2 mm);
- 6–18 years old: slow juvenile phase where the eye attains emmetropia.
Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kulp, M.T. Uncorrected hyperopia and preschool early literacy: Results of the Vision in Preschoolers-Hyperopia in Preschoolers (VIP-HIP) study. Ophthalmology 2016, 123, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H. Global and regional estimates of prevalence of refractive errors: Systematic review and meta-analysis. J. Curr. Ophthalmol. 2018, 30, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Castagno, V.D. Hyperopia: A meta-analysis of prevalence and a review of associated factors among school-aged children. BMC Ophthalmol. 2014, 14, 163. [Google Scholar] [CrossRef]
- Uretmen, O. Oculometric features of hyperopia in children with accommodative refractive esotropia. Acta Ophthalmol. Scand. 2003, 81, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Wen, G. Prevalence of myopia, hyperopia, and astigmatism in non-hispanic white and Asian children: Multi-ethnic pediatric eye disease study. Ophthalmology 2013, 120, 2109–2116. [Google Scholar] [CrossRef]
- Borchert, M. Risk factors for Hyperopia and Myopia in Preschool Children: The Multi-Ethnic Pediatric Eye Disease and Baltimore Pediatric Eye Disease Studies. Ophthalmology 2011, 118, 1966–1973. [Google Scholar] [CrossRef]
- Fan, Q. Childhood gene-environment interactions and age-dependent effects of genetic variants associated with refractive error and myopia: The CREAM Consortium. Sci. Rep. 2016, 6, 25853. [Google Scholar] [CrossRef]
- Harb, E.N. Origins of Refractive Errors: Environmental and Genetic Factors. Annu. Rev. Vis. Sci. 2019, 5, 47–72. [Google Scholar] [CrossRef]
- Zhang, Y. RPE and Choroid Mechanisms Underlying Ocular Growth and Myopia. Prog. Mol. Biol. Transl. Sci. 2015, 134, 221–240. [Google Scholar]
- Nickla, D.L. The multifunctional choroid. Prog. Retin. Eye Res. 2010, 29, 144–168. [Google Scholar] [CrossRef]
- Atchison, D.A. Relative peripheral hyperopia does not predict development and progression of myopia in children. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6162–6170. [Google Scholar] [CrossRef] [PubMed]
- Summers, J.A. Visually Induced Changes in Choroidal Cytokine Production: Implications for Ocular Growth Regulation. Investig. Ophthalmol. Vis. Sci. 2021, 56, 6162–6170. [Google Scholar]
- Banc, A. Normative data for optical coherence tomography in children: A systematic review. Eye 2021, 35, 714–738. [Google Scholar] [CrossRef]
- Hung, L.F. Vision-dependent changes in the CT of macaque monkeys. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1259–1269. [Google Scholar]
- Wildsoet, C. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res. 1995, 35, 1175–1194. [Google Scholar] [CrossRef]
- Ruiz-Moreno, J.M. Macular CT in normal pediatric population measured by swept-source optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2013, 54, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Jin, P. Choroidal and Retinal Thickness in Children with Different Refractive Status Measured by Swept-Source Optical Coherence. Am. J. Ophthalmol. 2016, 168, 164–176. [Google Scholar] [CrossRef]
- Öner, V. Influence of Hyperopia and Amblyopia on Choroidal Thickness in Children. Eur. J. Ophthalmol. 2016, 26, 623–626. [Google Scholar] [CrossRef]
- Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs—An extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology 1991, 98, 786–806. [Google Scholar] [CrossRef]
- Fujiwara, A. Enhanced depth imaging spectral-domain optical coherence tomography of subfoveal choroidal thickness in normal Japanese eyes. Jpn. J. Ophthalmol. 2012, 56, 230–235. [Google Scholar] [CrossRef]
- Lambert, S.R. Taylor & Hoyt’s Pediatric Ophthalmology and Strabismus, 3rd ed.; ElSevier: Edinburg, UK, 2017; pp. 40–46. [Google Scholar]
- Xiong, S. Changes in choroidal thickness varied by age and refraction in children and adolescents: A 1-year longitudinal study. Am. J. Ophthalmol. 2020, 213, 46–56. [Google Scholar] [CrossRef] [PubMed]

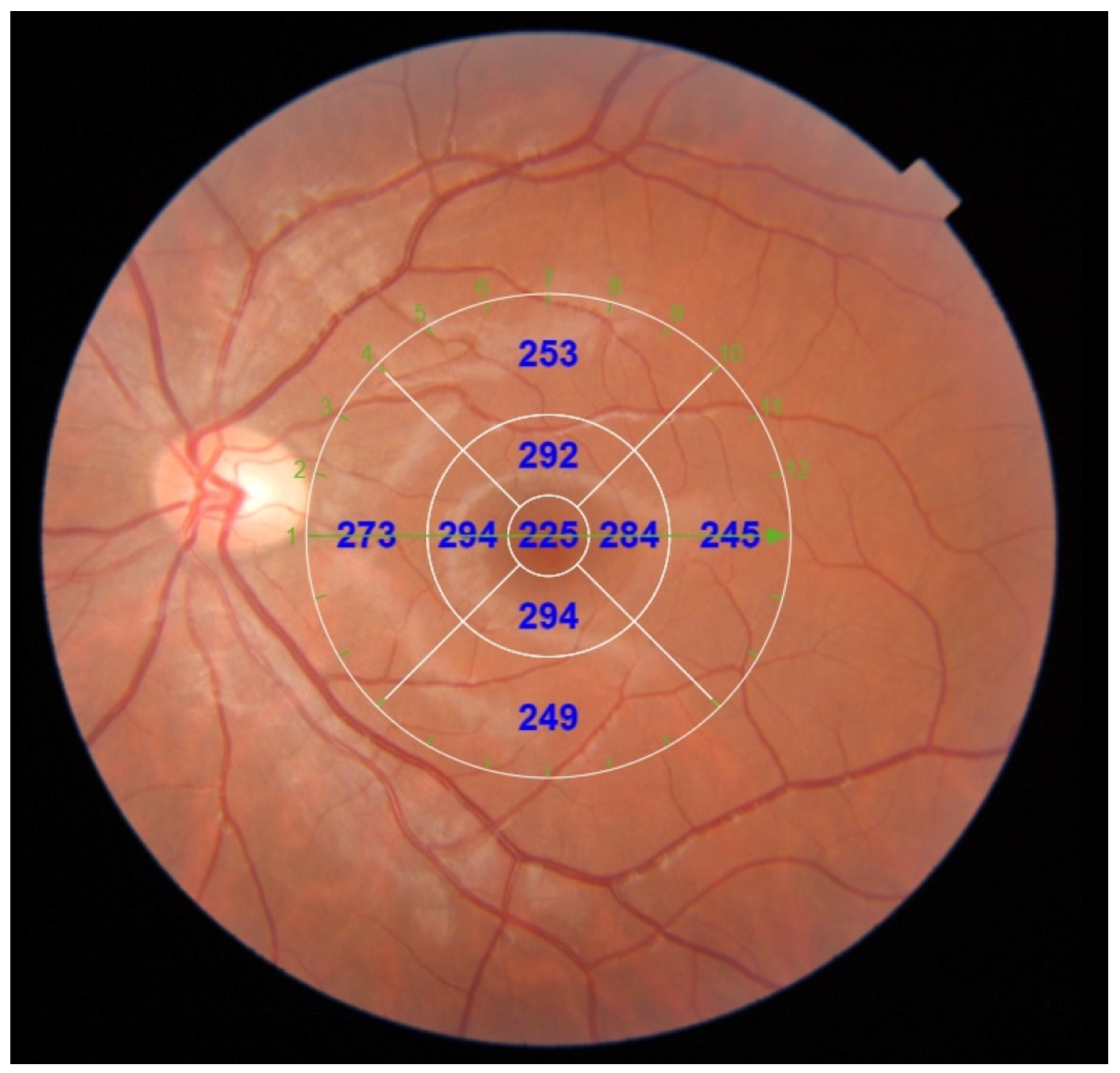
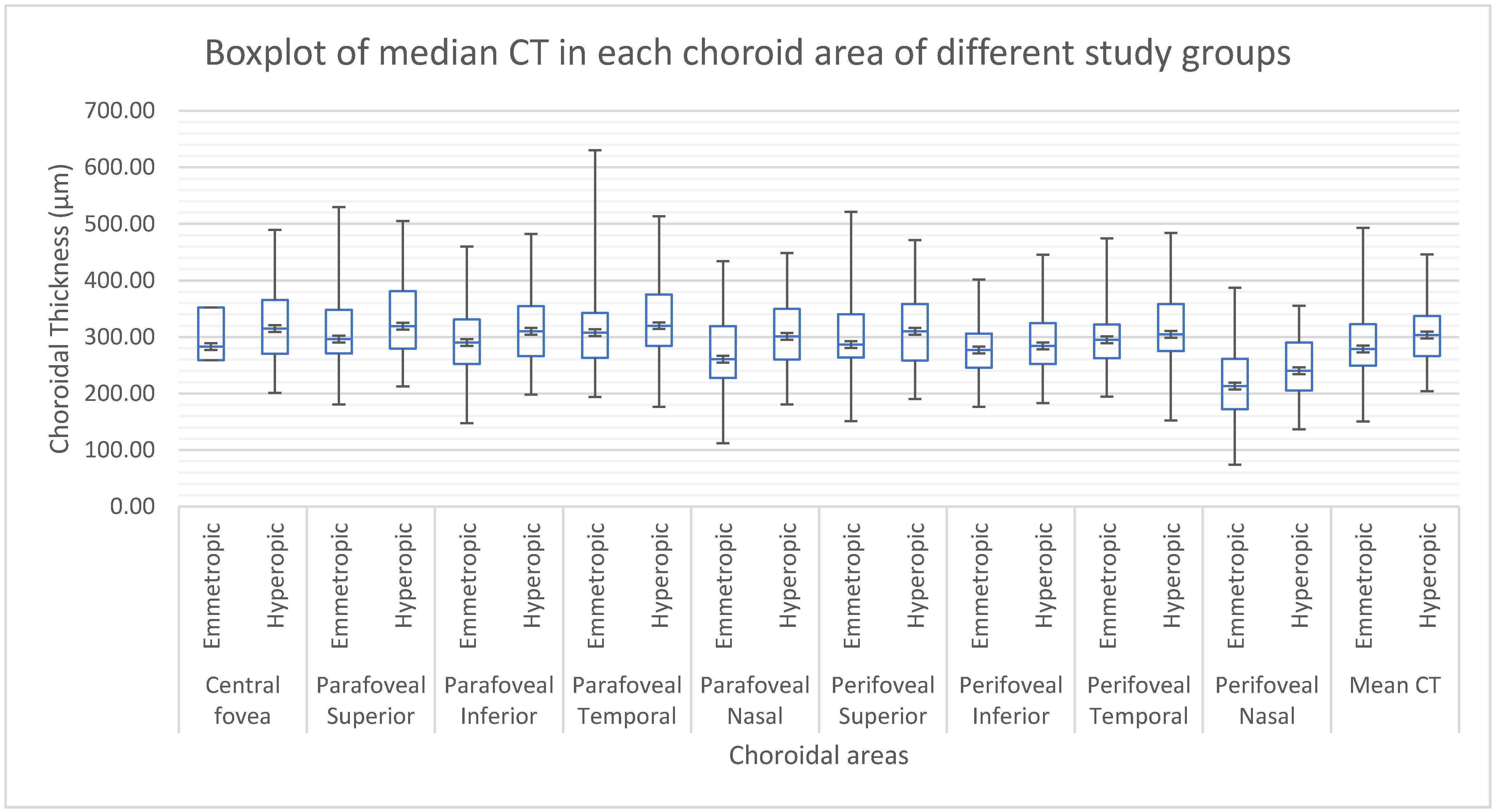

| Parameter | Hyperopic n = 62 | Emmetropic n = 66 | Mann–Whitney U | ||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| AGE | 9 | 12 | 9.5 | 13 | p = 0.682 |
| AL | 22.04 | 4.65 | 22.90 | 3.5 | p < 0.001 |
| SE | 3.88 | 7.25 | 0.75 | 1.88 | p < 0.001 |
| MEAN CT | 303.22 | 297 | 279 | 244.45 | p = 0.039 |
| Mean CT | SEX | AGE | AL | SE | ||
|---|---|---|---|---|---|---|
| Correlation coefficients | STUDY GROUP | −0.183 p = 0.039 | 0.154 p = 0.083 | 0.036 p = 0.684 | 0.486 p = < 0.001 | −0.866 p = < 0.001 |
| AGE | 0.023 p = 0.797 | 0.276 p = 0.002 | −0.049 p = 0.582 | |||
| AL | −0.298 p = 0.001 | −0.617 p = < 0.001 | ||||
| SE | 0.261 p = 0.003 |
| Mean CT | ||
|---|---|---|
| Correlation coefficients | AL | 0.274 p = 0.305 |
| SE | 0.018 p = 0.948 |
| Mean CT | ||
|---|---|---|
| Correlation coefficients | AL | −0.366 p = < 0.001 |
| SE | 0.287 p = 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerena Arévalo, V.A.; Ruiz-Moreno, J.M. Choroidal Thickness in a Hyperopic Pediatric Population. Diagnostics 2022, 12, 2330. https://doi.org/10.3390/diagnostics12102330
Gerena Arévalo VA, Ruiz-Moreno JM. Choroidal Thickness in a Hyperopic Pediatric Population. Diagnostics. 2022; 12(10):2330. https://doi.org/10.3390/diagnostics12102330
Chicago/Turabian StyleGerena Arévalo, Vanessa Antonia, and Jose Maria Ruiz-Moreno. 2022. "Choroidal Thickness in a Hyperopic Pediatric Population" Diagnostics 12, no. 10: 2330. https://doi.org/10.3390/diagnostics12102330
APA StyleGerena Arévalo, V. A., & Ruiz-Moreno, J. M. (2022). Choroidal Thickness in a Hyperopic Pediatric Population. Diagnostics, 12(10), 2330. https://doi.org/10.3390/diagnostics12102330








