Influence of a Deep Learning Noise Reduction on the CT Values, Image Noise and Characterization of Kidney and Ureter Stones
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. CT Acquisition
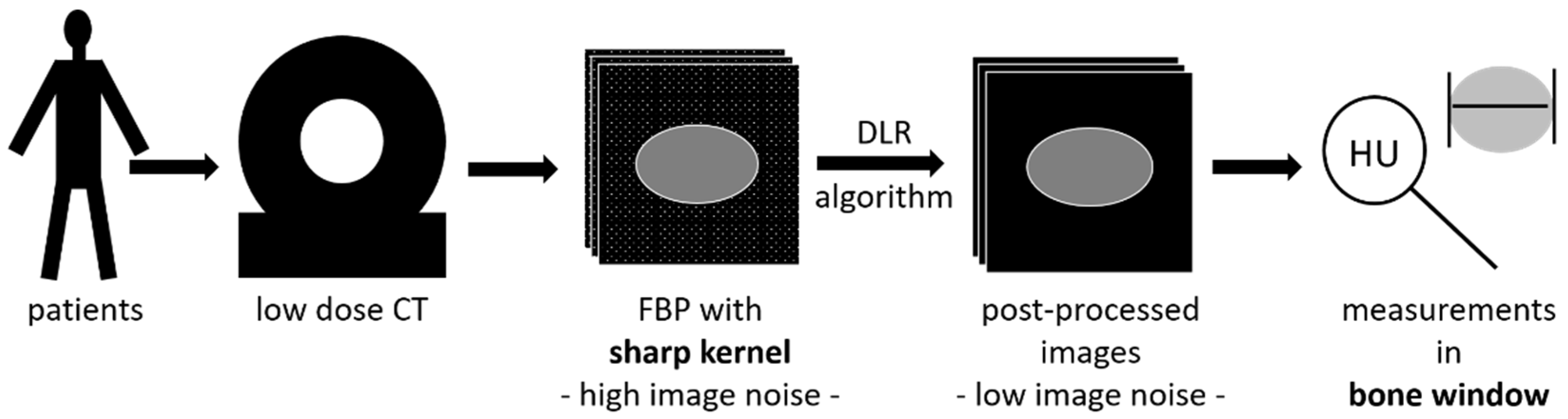
2.3. Image Reconstruction
- (a)
- B30f: Filtered back-projection with a B30f kernel for soft tissue presentation.
- (b)
- B70f: Filtered back-projection with a B70f kernel for bone or lung presentation.
- (c)
- I30f: Iterative reconstruction (SAFIRE (Siemens Healthineers, Forchheim, Germany)) with an I30f kernel for soft tissue presentation.
- (d)
- I70f: Iterative reconstruction (SAFIRE (Siemens Healthineers, Forchheim, Germany)) with an I70f kernel for bone or lung presentation.
- (e)
- P30f: PixelShine (AlgoMedica), version 1.2.104, using the reconstructed images of (a) with the parameters P214A8S.
- (f)
- P70f: PixelShine (AlgoMedica), version 1.2.104, using the reconstructed images of (b) with the parameters PB14A4L2.
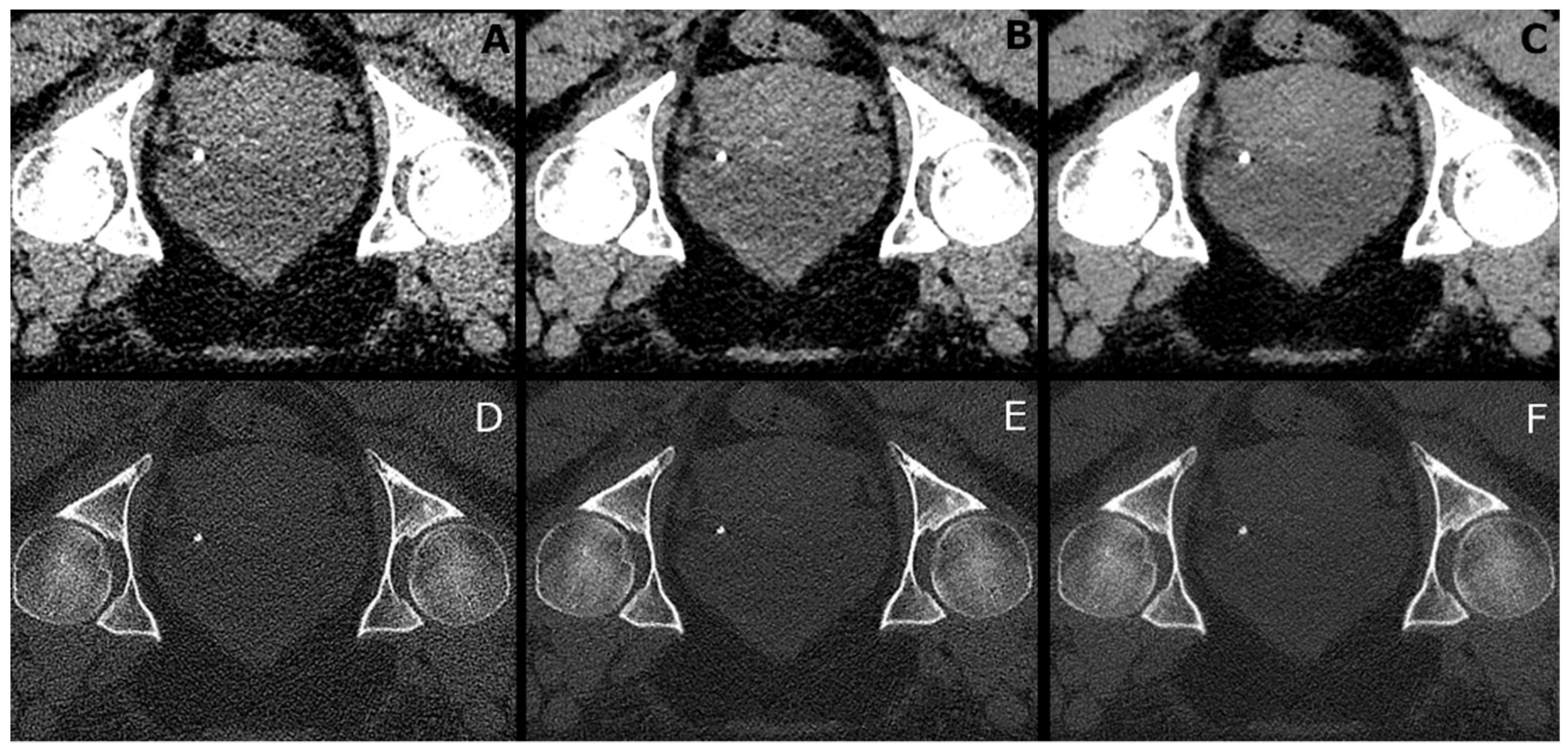
2.4. Image Analysis
2.5. Analysis of Urinary Concrements
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Stone Size
3.3. CT-Attenuation Values of Stones
3.4. Stone Composition
3.5. CT Values and Image Noise in Tissues and Air
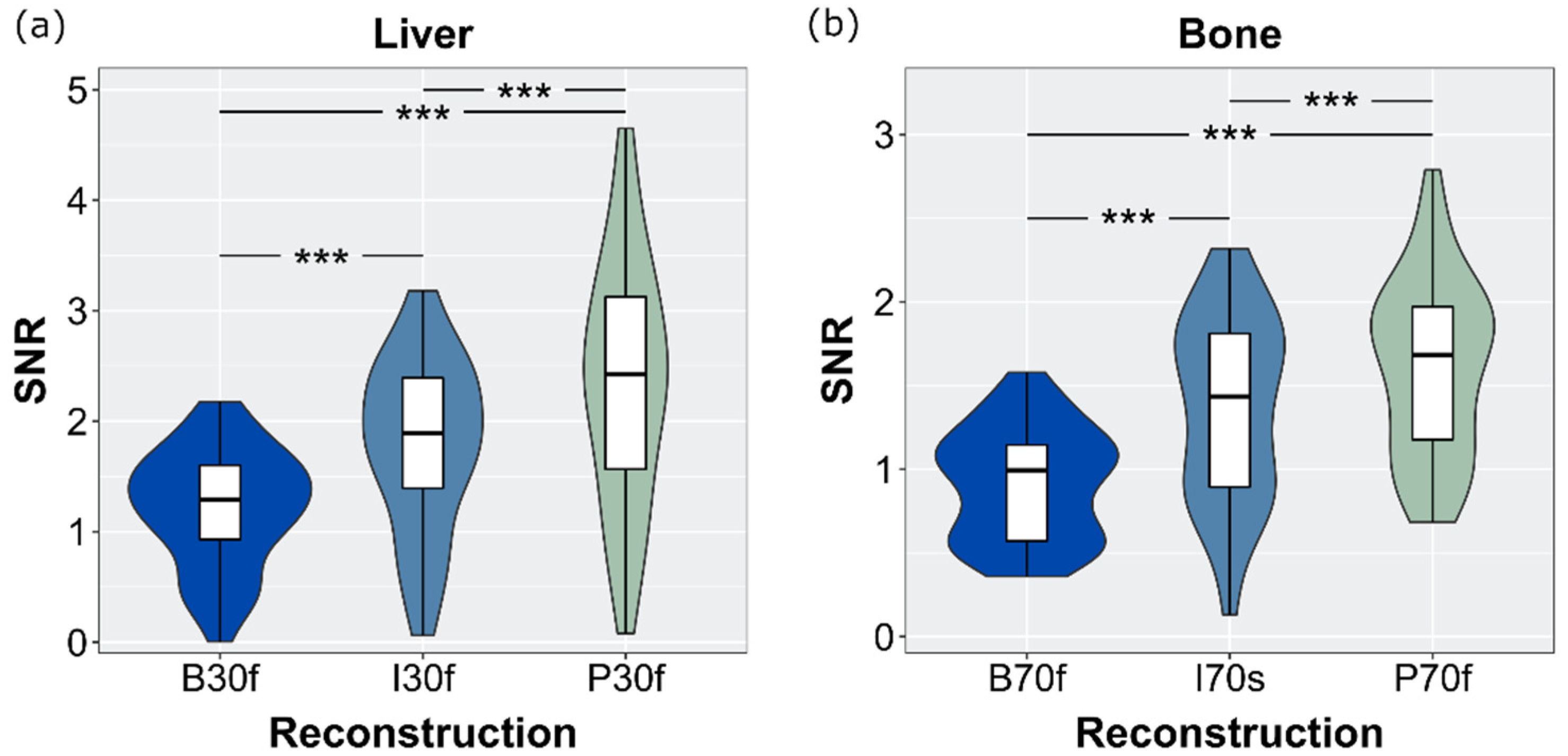
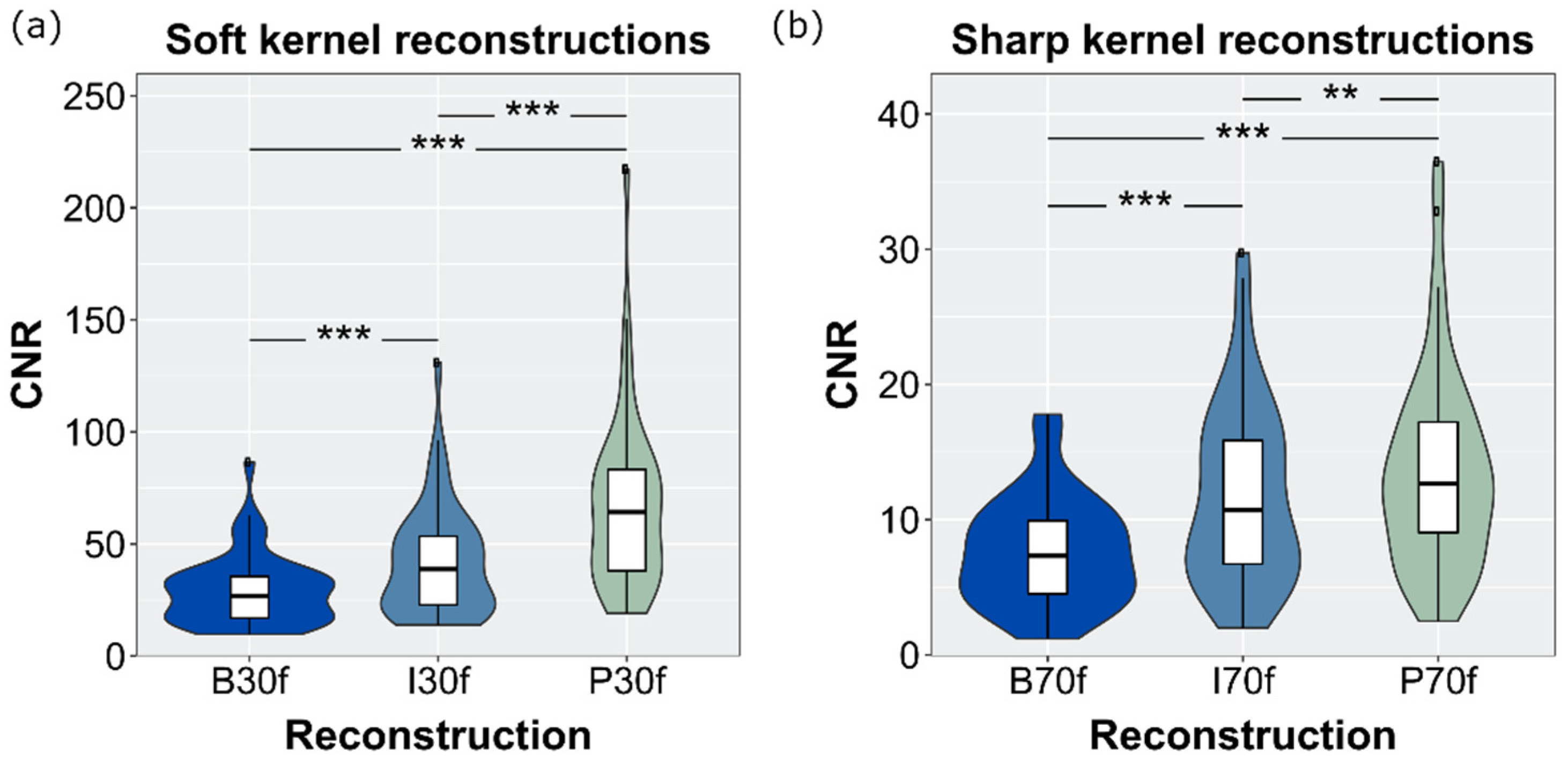
3.6. SNR and CNR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nambiar, A.K.; Bosch, R.; Cruz, F.; Lemack, G.E.; Thiruchelvam, N.; Tubaro, A.; Bedretdinova, D.A.; Ambühl, D.; Farag, F.; Lombardo, R.; et al. EAU Guidelines on Assessment and Nonsurgical Management of Urinary Incontinence. Eur. Urol. 2018, 73, 596–609. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.; Miyamoto, H.; Ito, H.; Terao, H.; Kakizoe, M.; Kato, Y.; Ishiguro, H.; Uemura, H.; Yao, M.; Matsuzaki, J. Predicting the Mineral Composition of Ureteral Stone Using Non-Contrast Computed Tomography. Urolithiasis 2016, 44, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Shahnani, P.S.; Karami, M.; Astane, B.; Janghorbani, M. The comparative survey of Hounsfield units of stone composition in urolithiasis patients. J. Res. Med. Sci. 2014, 19, 650–653. [Google Scholar] [PubMed]
- Andrabi, Y.; Patino, M.; Das, C.J.; Eisner, B.; Sahani, D.V.; Kambadakone, A. Advances in CT Imaging for Urolithiasis. Indian J. Urol. 2015, 31, 185–193. [Google Scholar] [CrossRef]
- Coll, D.M.; Varanelli, M.J.; Smith, R.C. Relationship of Spontaneous Passage of Ureteral Calculi to Stone Size and Location as Revealed by Unenhanced Helical CT. AJR Am. J. Roentgenol. 2002, 178, 101–103. [Google Scholar] [CrossRef]
- Argüelles Salido, E.; Aguilar García, J.; Lozano-Blasco, J.M.; Subirá Rios, J.; Beardo Villar, P.; Campoy-Martínez, P.; Medina-López, R.A. Lithiasis Size Estimation Variability Depending on Image Technical Methodology. Urolithiasis 2013, 41, 517–522. [Google Scholar] [CrossRef]
- Brenner, D.J.; Hall, E.J. Computed Tomography—An Increasing Source of Radiation Exposure. N. Eng. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef] [Green Version]
- Brendlin, A.S.; Plajer, D.; Chaika, M.; Wrazidlo, R.; Estler, A.; Tsiflikas, I.; Artzner, C.P.; Afat, S.; Bongers, M.N. AI Denoising Significantly Improves Image Quality in Whole-Body Low-Dose Computed Tomography Staging. Diagnostics 2022, 12, 225. [Google Scholar] [CrossRef]
- Yeoh, H.; Hong, S.H.; Ahn, C.; Choi, J.-Y.; Chae, H.-D.; Yoo, H.J.; Kim, J.H. Deep Learning Algorithm for Simultaneous Noise Reduction and Edge Sharpening in Low-Dose CT Images: A Pilot Study Using Lumbar Spine CT. Korean J. Radiol. 2021, 22, 1850. [Google Scholar] [CrossRef]
- Steuwe, A.; Weber, M.; Bethge, O.T.; Rademacher, C.; Boschheidgen, M.; Sawicki, L.M.; Antoch, G.; Aissa, J. Influence of a novel deep-learning based reconstruction software on the objective and subjective image quality in low-dose abdominal computed tomography. Br. J. Radiol. 2021, 94, 20200677. [Google Scholar] [CrossRef]
- Deak, P.D.; Smal, Y.; Kalender, W.A. Multisection CT Protocols: Sex- and Age-Specific Conversion Factors Used to Determine Effective Dose from Dose-Length Product. Radiology 2010, 257, 158–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franck, P.; Nabet, P.; Dousset, B. Applications of Infrared Spectroscopy to Medical Biology. Cell. Mol. Biol. 1998, 44, 273–275. [Google Scholar] [PubMed]
- Patil, I. Visualizations with Statistical Details: The “ggstatsplot” Approach. J. Open Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 6 May 2021).
- Tian, S.; Liu, A.; Liu, J.; Liu, Y.; Pan, J. Potential Value of the PixelShine Deep Learning Algorithm for Increasing Quality of 70 KVp+ASiR-V Reconstruction Pelvic Arterial Phase CT Images. Jpn. J. Radiol. 2019, 37, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Rozema, R.; Kruitbosch, H.T.; van Minnen, B.; Dorgelo, B.; Kraeima, J.; van Ooijen, P.M.A. Iterative Reconstruction and Deep Learning Algorithms for Enabling Low-Dose Computed Tomography in Midfacial Trauma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 132, 247–254. [Google Scholar] [CrossRef]
- Rozema, R.; Kruitbosch, H.T.; van Minnen, B.; Dorgelo, B.; Kraeima, J.; van Ooijen, P.M.A. Structural Similarity Analysis of Midfacial Fractures—a Feasibility Study. Quant. Imaging Med. Surg. 2022, 12, 1558–1565. [Google Scholar] [CrossRef]
- Hasegawa, A.; Ishihara, T.; Thomas, M.A.; Pan, T. Noise Reduction Profile: A New Method for Evaluation of Noise Reduction Techniques in CT. Med. Phys. 2022, 49, 186–200. [Google Scholar] [CrossRef]
- Pan, T.; Hasegawa, A.; Luo, D.; Wu, C.C.; Vikram, R. Technical Note: Impact on Central Frequency and Noise Magnitude Ratios by Advanced CT Image Reconstruction Techniques. Med. Phys. 2020, 47, 480–487. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, X.; Xu, L.; Bai, X.; Jin, R.; Xu, M.; Yan, J.; Jin, Z.; Sun, H. Value of Deep Learning Reconstruction at Ultra-Low-Dose CT for Evaluation of Urolithiasis. Eur. Radiol. 2022. [Google Scholar] [CrossRef]
- Thapaliya, S.; Brady, S.L.; Somasundaram, E.; Anton, C.G.; Coley, B.D.; Towbin, A.J.; Zhang, B.; Dillman, J.R.; Trout, A.T. Detection of Urinary Tract Calculi on CT Images Reconstructed with Deep Learning Algorithms. Abdom. Radiol. 2022, 47, 265–271. [Google Scholar] [CrossRef]
- Delabie, A.; Bouzerar, R.; Pichois, R.; Desdoit, X.; Vial, J.; Renard, C. Diagnostic Performance and Image Quality of Deep Learning Image Reconstruction (DLIR) on Unenhanced Low-Dose Abdominal CT for Urolithiasis. Acta Radiol. 2021, 028418512110358. [Google Scholar] [CrossRef] [PubMed]
- Lidén, M.; Andersson, T.; Geijer, H. Making Renal Stones Change Size—Impact of CT Image Post Processing and Reader Variability. Eur. Radiol. 2011, 21, 2218–2225. [Google Scholar] [CrossRef] [PubMed]
- Appel, E.; Thomas, C.; Steuwe, A.; Schaarschmidt, B.M.; Brook, O.R.; Aissa, J.; Hennenlotter, J.; Antoch, G.; Boos, J. Evaluation of Split-Filter Dual-Energy CT for Characterization of Urinary Stones. Br. J. Radiol. 2021, 94, 20210084. [Google Scholar] [CrossRef] [PubMed]
- Lazar, M.; Ringl, H.; Baltzer, P.; Toth, D.; Seitz, C.; Krauss, B.; Unger, E.; Polanec, S.; Tamandl, D.; Herold, C.J.; et al. Protocol Analysis of Dual-Energy CT for Optimization of Kidney Stone Detection in Virtual Non-Contrast Reconstructions. Eur. Radiol. 2020, 30, 4295–4305. [Google Scholar] [CrossRef]
- Bonatti, M.; Lombardo, F.; Zamboni, G.A.; Pernter, P.; Pycha, A.; Mucelli, R.P.; Bonatti, G. Renal Stones Composition in Vivo Determination: Comparison between 100/Sn140 KV Dual-Energy CT and 120 KV Single-Energy CT. Urolithiasis 2017, 45, 255–261. [Google Scholar] [CrossRef]
- Sheir, K.Z.; Mansour, O.; Madbouly, K.; Elsobky, E.; Abdel-Khalek, M. Determination of the Chemical Composition of Urinary Calculi by Noncontrast Spiral Computerized Tomography. Urol. Res. 2005, 33, 99–104. [Google Scholar] [CrossRef]
- Gallioli, A.; de Lorenzis, E.; Boeri, L.; Delor, M.; Zanetti, S.P.; Longo, F.; Trinchieri, A.; Montanari, E. Clinical Utility of Computed Tomography Hounsfield Characterization for Percutaneous Nephrolithotomy: A Cross-Sectional Study. BMC Urol. 2017, 17, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Torricelli, F.C.M.; Marchini, G.S.; De, S.; Yamaçake, K.G.R.; Mazzucchi, E.; Monga, M. Predicting Urinary Stone Composition Based on Single-Energy Noncontrast Computed Tomography: The Challenge of Cystine. Urology 2014, 83, 1258–1264. [Google Scholar] [CrossRef]
- Thomas, C.; Heuschmid, M.; Schilling, D.; Ketelsen, D.; Tsiflikas, I.; Stenzl, A.; Claussen, C.D.; Schlemmer, H.-P. Urinary Calculi Composed of Uric Acid, Cystine, and Mineral Salts: Differentiation with Dual-Energy CT at a Radiation Dose Comparable to That of Intravenous Pyelography. Radiology 2010, 257, 402–409. [Google Scholar] [CrossRef] [Green Version]
- Mackin, D.; Ger, R.; Gay, S.; Dodge, C.; Zhang, L.; Yang, J.; Jones, A.K.; Court, L. Matching and Homogenizing Convolution Kernels for Quantitative Studies in Computed Tomography. Investig. Radiol. 2019, 54, 288–295. [Google Scholar] [CrossRef]
- Danilovic, A.; Rocha, B.A.; Marchini, G.S.; Traxer, O.; Batagello, C.; Vicentini, F.C.; Torricelli, F.C.M.; Srougi, M.; Nahas, W.C.; Mazzucchi, E. Computed Tomography Window Affects Kidney Stones Measurements. Int. Braz. J. Urol. 2019, 45, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Umbach, R.; Müller, J.-K.; Wendt-Nordahl, G.; Knoll, T.; Jessen, J.P. In-Vitro Comparison of Different Slice Thicknesses and Kernel Settings for Measurement of Urinary Stone Size by Computed Tomography. Urolithiasis 2019, 47, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Eisner, B.H.; Kambadakone, A.; Monga, M.; Anderson, J.K.; Thoreson, A.A.; Lee, H.; Dretler, S.P.; Sahani, D.v. Computerized Tomography Magnified Bone Windows Are Superior to Standard Soft Tissue Windows for Accurate Measurement of Stone Size: An In Vitro and Clinical Study. J. Urol. 2009, 181, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Apfaltrer, G.; Dutschke, A.; Baltzer, P.A.T.; Schestak, C.; Özsoy, M.; Seitz, C.; Veser, J.; Petter, E.; Helbich, T.H.; Ringl, H.; et al. Substantial Radiation Dose Reduction with Consistent Image Quality Using a Novel Low-Dose Stone Composition Protocol. World J. Urol. 2020, 38, 2971–2979. [Google Scholar] [CrossRef]
- Lam, J.P.; Alexander, L.F.; William, H.E.; Hodge, D.O.; Kofler, J.M.; Morin, R.L.; Thiel, D.D.; Cernigliaro, J.G. In Vivo Comparison of Radiation Exposure in Third-Generation vs Second-Generation Dual-Source Dual-Energy CT for Imaging Urinary Calculi. J. Endourol. 2021, 35, 1581–1585. [Google Scholar] [CrossRef]

| x (mm) | y (mm) | x (mm) | y (mm) | |
|---|---|---|---|---|
| Soft Kernels | Sharp Kernels | |||
| B | 5.6 ± 3.0 AC | 4.2 ± 2.2 E | 4.4 ± 3.0 | 3.1 ± 2.0 |
| I | 5.1 ± 3.1 AB | 3.8 ± 2.1 DE | 4.4 ± 3.0 | 3.1 ± 2.1 |
| P | 5.4 ± 3.1 BC | 4.1 ± 2.2 D | 4.4 ± 3.0 | 3.2 ± 2.1 |
| Soft Kernels | Sharp Kernels | |
|---|---|---|
| B | 717.6 ± 405.9 A | 1047.3 ± 490.7 C |
| I | 714.4 ± 459.4 A | 986.2 ± 516.5 B |
| P | 704.2 ± 424.5 | 915.9 ± 449.6 BC |
| Soft Kernel Reconstruction | Sharp Kernel Reconstruction | ||||||
|---|---|---|---|---|---|---|---|
| Composition | n | B | I | P | B | I | P |
| CaOx | 15 | 772 (523–1059) | 739 (508–1147) | 829 (531–1044) | 1316 (1045–1583) | 1251 (703–1565) | 1119 (791–1399) |
| Calcium-Oxalate-carbonate apatite | 5 | 703 (663–777) | 672 (668–675) | 632 (598–699) | 1092 (1000–1339) | 1251 (1156–1254) | 850 (764–1124) |
| Uric acid | 3 | 501 (430–664) | 513 (412–645) | 515 (422–562) | 460 (452–967) | 464 (434–1040) | 507 (463–972) |
| Cystine | 1 | 721 | 714 | 725 | 731 | 745 | 714 |
| Carbonate-Apatite-mix | 1 | 1174 | 1257 | 1202 | 1149 | 1358 | 1320 |
| (a) | |||
| CT Value | B | I | P |
| Air (sharp) | −940.0 ± 12.0 A | −933.4 ± 11.2 AB | −939.4 ± 11.9 B |
| Bone (sharp) | 188.8 ± 63.3 D | 186.0 ± 66.7 C | 196.2 ± 63.5 CD |
| Liver (soft) | 44.7 ± 16.3 | 44.7 ± 16.1 | 44.6 ± 16.1 |
| Muscle (soft) | 51.3 ± 6.7 EF | 51.1 ± 6.7 F | 50.8 ± 7.0 E |
| Spleen (soft) | 45.4 ± 3.5 | 45.4 ± 3.4 | 45.4 ± 3.4 |
| Fat (soft) | −116.1 ± 9.7 H | −116. ± 9.6 G | −115.6 ± 9.6 GH |
| Results of the statistical comparisons: A–G: p < 0.001, H: p = 0.001. | |||
| (b) | |||
| Image Noise | B | I | P |
| Air (sharp) | 77.5 ± 15.9 | 49.4 ± 13.5 | 28.5 ± 14.3 |
| Bone (sharp) | 212.3 ± 40.1 | 143.0 ± 31.1 | 124.0 ± 20.7 |
| Liver (soft) | 38.2 ± 7.7 | 26.2 ± 5.0 | 20.3 ± 4.8 |
| Muscle (soft) | 31.6 ± 5.6 | 21.7 ± 3.9 | 14.5 ± 2.6 |
| Spleen (soft) | 34.5 ± 7.0 | 23.4 ± 4.8 | 16.4 ± 4.1 |
| Fat (soft) | 29.4 ± 5.6 | 20.4 ± 4.2 | 12.8 ± 3.1 |
| Statistical differences (p < 0.001) among all reconstructions within one ROI. Abbreviations: B: filtered back-projection, I: iterative reconstruction; P: PixelShine. | |||
| Parameter | This Study | Zhang et al. [20] | Thapaliya et al. [21] | Delabie et al. [22] | ||||
|---|---|---|---|---|---|---|---|---|
| Vendor | Algomedica | Siemens Healthineers | Canon Medical Systems | Canon Medical Systems | GE Healthcare | |||
| Techniques used | PixelShine | IR (Safire), FBP | DLR (AiCE) | HIR | DLR (AiCE, six options evaluated) | AIDR3D | DLR (TrueFidelityTM) | FBP ASiR-V |
| Preprocessing techniques | FBP | Raw data | None described | Raw data | Raw data | None described | ||
| Type of dataset used | CT of kidney stones in 45 patients, both soft tissue and bone kernel and window settings | CT of kidney stones in 51 patients with intra-individual comparison, soft tissue window settings; LDCT-HIR as gold standard | CT of kidney stones in 7 patients, AIDR3D as gold standard, soft tissue window | CT of kidney stones in 75 patients, soft tissue window (stone detection), bone window (stone count) | ||||
| Evaluation measures | Image noise, CNR, SNR, attenuation, stone size | Radiation exposure, stone characteristics, image noise, SNR, subjective IQ | Stone detection, stone size, inter-rater reliability | Attenuation, noise measurements, SNR, contrast, CNR, detectability, IQ, stone size category; | ||||
| Advantage | Higher objective IQ | Direct reconstruction | Reduced radiation exposure, higher IQ | High level of agreement with AIDR3D | Quantitative and qualitative IQ improved | |||
| Disadvantage | Secondary reconstruction | Image noise | Lower sensibility | Higher sensibility | More image noise than AiCE | Contrast between kidney and spleen different to ASiR-V | Image noise | |
| Recommendation | Usage of PixelShine to reduce image noise; use sharp kernel reconstructions bone window to improve differentiation between stone compositions | DLR with ultra-low dose CT to reduce dose, though it might miss stones <3mm | Usage of DLR to potentially reduce radiation exposure | Usage of DLR to improve IQ, though it still might miss stones <3mm | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steuwe, A.; Valentin, B.; Bethge, O.T.; Ljimani, A.; Niegisch, G.; Antoch, G.; Aissa, J. Influence of a Deep Learning Noise Reduction on the CT Values, Image Noise and Characterization of Kidney and Ureter Stones. Diagnostics 2022, 12, 1627. https://doi.org/10.3390/diagnostics12071627
Steuwe A, Valentin B, Bethge OT, Ljimani A, Niegisch G, Antoch G, Aissa J. Influence of a Deep Learning Noise Reduction on the CT Values, Image Noise and Characterization of Kidney and Ureter Stones. Diagnostics. 2022; 12(7):1627. https://doi.org/10.3390/diagnostics12071627
Chicago/Turabian StyleSteuwe, Andrea, Birte Valentin, Oliver T. Bethge, Alexandra Ljimani, Günter Niegisch, Gerald Antoch, and Joel Aissa. 2022. "Influence of a Deep Learning Noise Reduction on the CT Values, Image Noise and Characterization of Kidney and Ureter Stones" Diagnostics 12, no. 7: 1627. https://doi.org/10.3390/diagnostics12071627
APA StyleSteuwe, A., Valentin, B., Bethge, O. T., Ljimani, A., Niegisch, G., Antoch, G., & Aissa, J. (2022). Influence of a Deep Learning Noise Reduction on the CT Values, Image Noise and Characterization of Kidney and Ureter Stones. Diagnostics, 12(7), 1627. https://doi.org/10.3390/diagnostics12071627






