Super-Resolution Ultrasound Imaging Provides Quantification of the Renal Cortical and Medullary Vasculature in Obese Zucker Rats: A Pilot Study
Abstract
1. Introduction
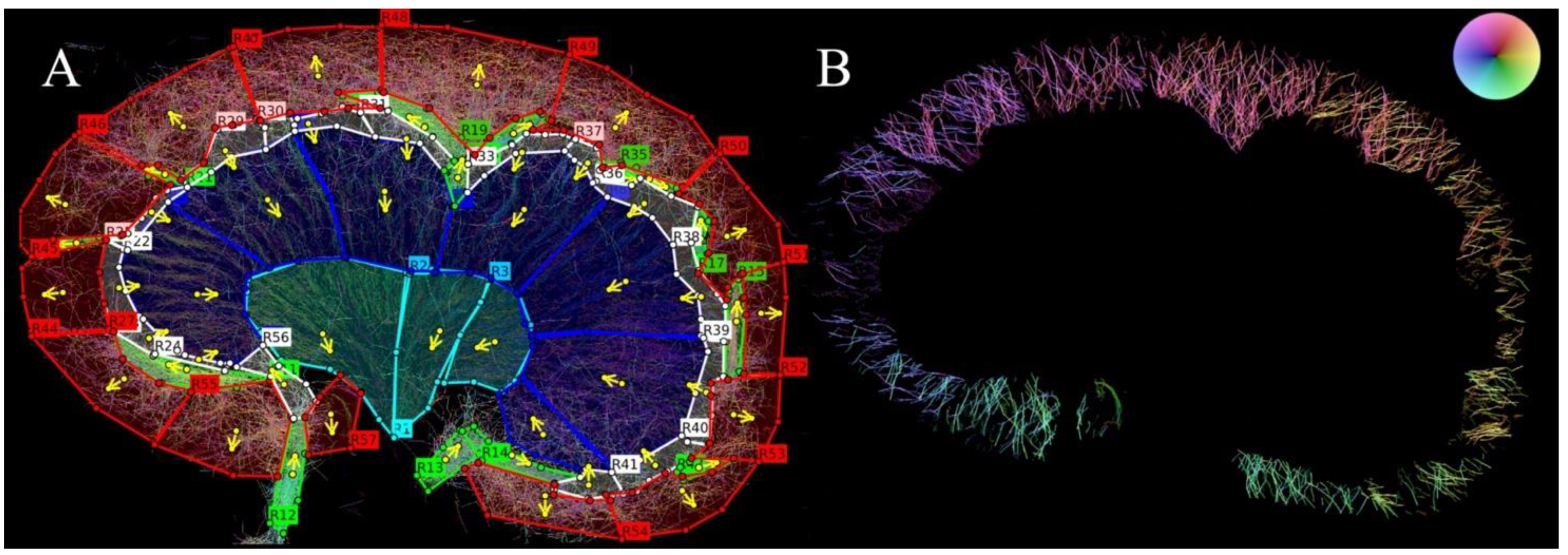
2. Materials and Methods
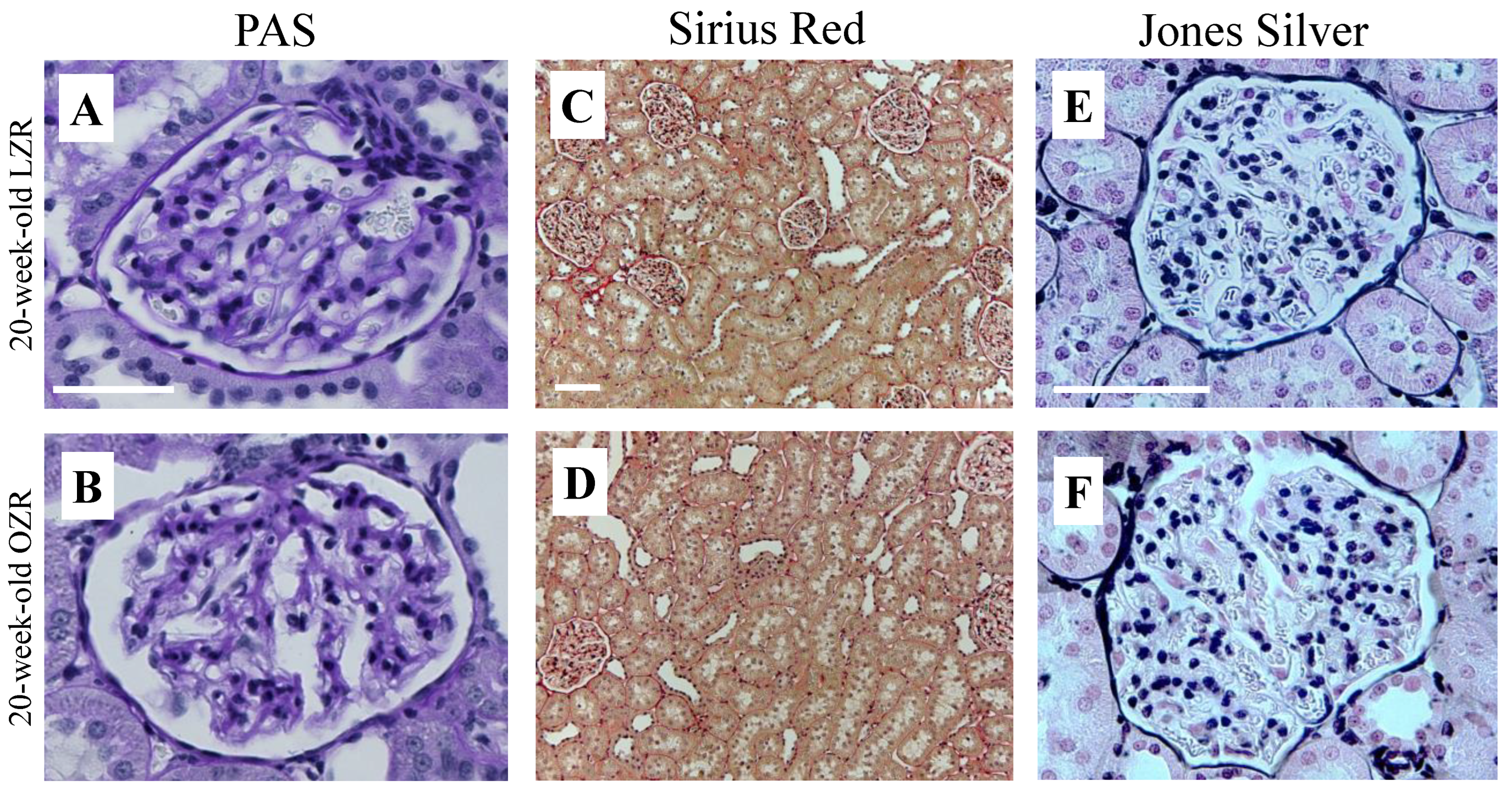
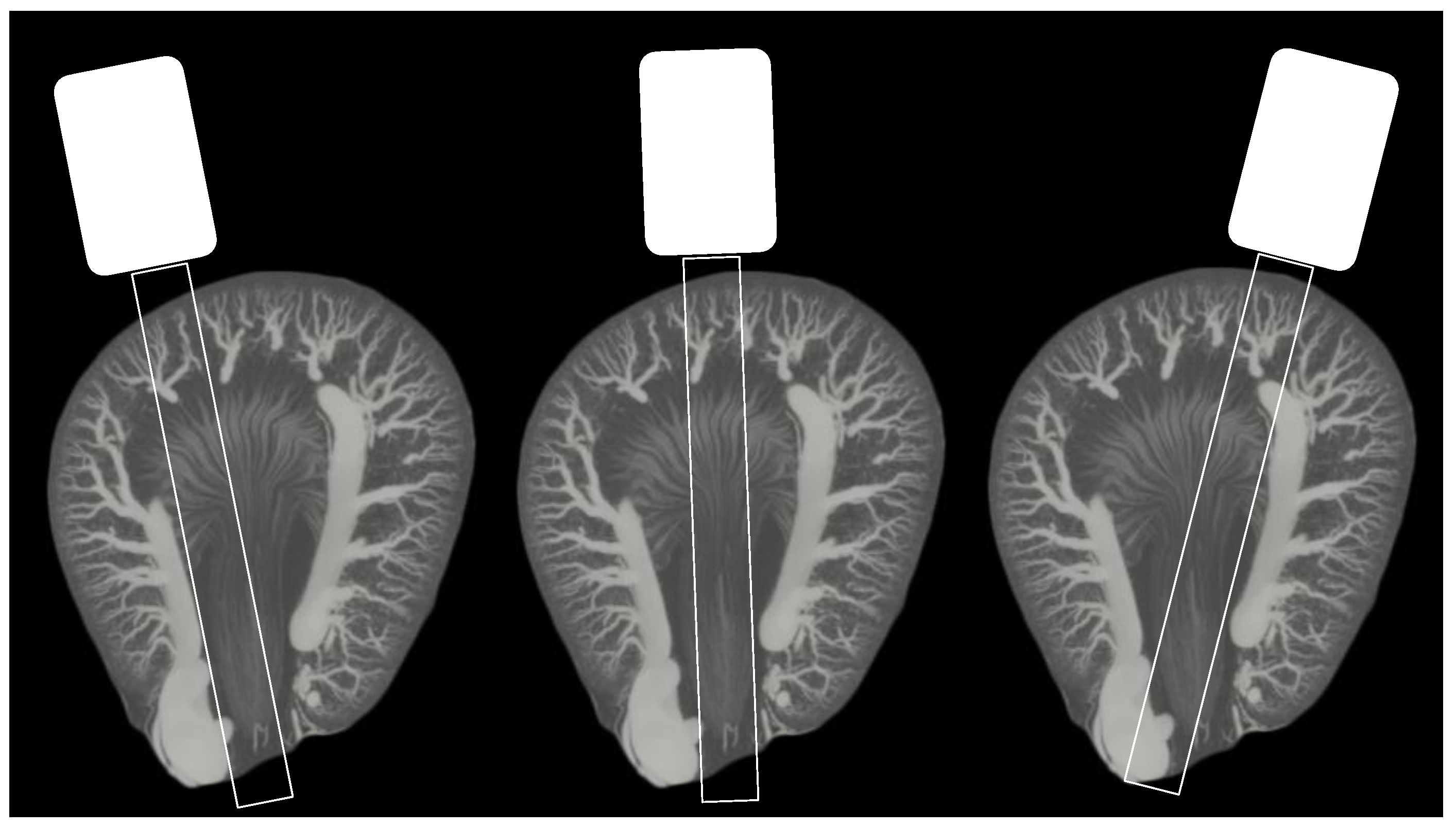
3. Results
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 18 May 2022).
- Wang, Y.; Chen, X.; Song, Y.; Caballero, B.; Cheskin, L.J. Association between Obesity and Kidney Disease: A Systematic Review and Meta-Analysis. Kidney Int. 2008, 73, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Zhou, J.; Yuan, H.; Wu, L.; Chen, Z. Chronic Kidney Disease among Overweight and Obesity with and without Metabolic Syndrome in an Urban Chinese Cohort Epidemiology and Health Outcomes. BMC Nephrol. 2015, 16, 85. [Google Scholar] [CrossRef][Green Version]
- Hall, M.E.; do Carmo, J.M.; da Silva, A.A.; Juncos, L.A.; Wang, Z.; Hall, J.E. Obesity, Hypertension, and Chronic Kidney Disease. Int. J. Nephrol. Renovasc. Dis. 2014, 7, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Chade, A.R.; Hall, J.E. Role of the Renal Microcirculation in Progression of Chronic Kidney Injury in Obesity. Am. J. Nephrol. 2016, 44, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Incio, J.; Soares, R. Angiogenesis and Chronic Inflammation: Cause or Consequence? Angiogenesis 2007, 10, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Han, H.C. Twisted Blood Vessels: Symptoms, Etiology and Biomechanical Mechanisms. J. Vasc. Res. 2012, 49, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Ehling, J.; Bábícková, J.; Gremse, F.; Klinkhammer, B.M.; Baetke, S.; Knuechel, R.; Kiessling, F.; Floege, J.; Lammers, T.; Boor, P. Quantitative Micro-Computed Tomography Imaging of Vascular Dysfunction in Progressive Kidney Diseases. J. Am. Soc. Nephrol. 2016, 27, 520–532. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, J.; Rush, B.M.; Stocker, S.D.; Tan, R.J.; Kim, K. Ultrasound Super-Resolution Imaging Provides a Noninvasive Assessment of Renal Microvasculature Changes during Mouse Acute Kidney Injury. Kidney Int. 2020, 98, 355–365. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, J.; Yang, Y.; Zhang, H.; Lee, F.; He, Q.; Huang, C.; Huang, L.; Qian, L.; Luo, J. In Vivo Assessment of Hypertensive Nephrosclerosis Using Ultrasound Localization Microscopy. Med. Phys. 2022, 49, 2295–2308. [Google Scholar] [CrossRef]
- Andersen, S.B.; Taghavi, I.; Hoyos, C.A.V.; Søgaard, S.B.; Gran, F.; Lönn, L.; Hansen, K.L.; Jensen, J.A.; Nielsen, M.B.; Sørensen, C.M. Super-Resolution Imaging with Ultrasound for Visualization of the Renal Microvasculature in Rats Before and After Renal Ischemia: A Pilot Study. Diagnostics 2020, 10, 862. [Google Scholar] [CrossRef]
- Christensen-Jeffries, K.; Couture, O.; Dayton, P.A.; Eldar, Y.C.; Hynynen, K.; Kiessling, F.; O’Reilly, M.; Pinton, G.F.; Schmitz, G.; Tang, M.X.; et al. Super-Resolution Ultrasound Imaging. Ultrasound Med. Biol. 2020, 46, 865–891. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, I.; Andersen, S.B.; Hoyos, C.A.V.; Nielsen, M.B.; Sorensen, C.M.; Jensen, J.A. In Vivo Motion Correction in Super Resolution Imaging of Rat Kidneys. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 3082–3093. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, I.; Andersen, S.B.; Hoyos, C.A.V.; Schou, M.; Gran, F.; Hansen, K.L.; Nielsen, M.B.; Sørensen, C.M.; Stuart, M.B.; Jensen, J.A. Ultrasound Super-Resolution Imaging with a Hierarchical Kalman Tracker. Ultrasonics 2022, 122, 106695. [Google Scholar] [CrossRef] [PubMed]
- Hingot, V.; Errico, C.; Heiles, B.; Rahal, L.; Tanter, M.; Couture, O. Microvascular Flow Dictates the Compromise between Spatial Resolution and Acquisition Time in Ultrasound Localization Microscopy. Sci. Rep. 2019, 9, 2456. [Google Scholar] [CrossRef] [PubMed]
- Christensen-Jeffries, K.; Brown, J.; Harput, S.; Zhang, G.; Zhu, J.; Tang, M.X.; Dunsby, C.; Eckersley, R.J. Poisson Statistical Model of Ultrasound Super-Resolution Imaging Acquisition Time. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2019, 66, 1246–1254. [Google Scholar] [CrossRef]
- Schmitz, P.G.; O’Donnell, M.P.; Kasiske, B.L.; Katz, S.A.; Keane, W.F. Renal Injury in Obese Zucker Rats: Glomerular Hemodynamic Alterations and Effects of Enalapril. Am. J. Physiol. Ren. Fluid Electrolyte Physiol. 1992, 263, F496–F502. [Google Scholar] [CrossRef]
- Zucker, L.M. Hereditary Obesity in the Rat Associated with Hyperlipidemia. Ann. N. Y. Acad. Sci. 1965, 131, 447–458. [Google Scholar] [CrossRef]
- Gades, M.D.; Van Goor, H.; Kaysen, G.A.; Johnson, P.R.; Horwitz, B.A.; Stern, J.S. Brief Periods of Hyperphagia Cause Renal Injury in the Obese Zucker Rat. Kidney Int. 1999, 56, 1779–1787. [Google Scholar] [CrossRef]
- Kasiske, B.L.; O’Donnell, M.P.; Cleary, M.P.; Keane, W.F. Treatment of Hyperlipidemia Reduces Glomerular Injury in Obese Zucker Rats. Kidney Int. 1988, 33, 667–672. [Google Scholar] [CrossRef]
- Lohmaier, S.; Ghanem, A.; Veltmann, C.; Sommer, T.; Bruce, M.; Tiemann, K. In Vitro and in Vivo Studies on Continuous Echo-Contrast Application Strategies Using SonoVue in a Newly Developed Rotating Pump Setup. Ultrasound Med. Biol. 2004, 30, 1145–1151. [Google Scholar] [CrossRef]
- Taghavi, I.; Andersen, S.B.; Hoyos, C.A.V.; Schou, M.; Øygard, S.H.; Gran, F.; Hansen, K.L.; Sørensen, C.M.; Bachmann Nielsen, M.; Stuart, M.B.; et al. Tracking Performance in Ultrasound Super-Resolution Imaging. In Proceedings of the 2020 IEEE International Ultrasonics Symposium (IUS), Las Vegas, NV, USA, 7–11 September 2020. [Google Scholar] [CrossRef]
- Farris, A.B.; Alpers, C.E. What Is the Best Way to Measure Renal Fibrosis¿: A Pathologist’s Perspective. Kidney Int. Suppl. 2014, 4, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Amann, K.; Haas, C.S. What You Should Know about the Work-up of a Renal Biopsy. Nephrol. Dial. Transplant. 2006, 21, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Zimmerhackl, B.; Robertson, C.R.; Jamison, R.L. The Microcirculation of the Renal Medulla. An Off. J. Am. Heart. Assoc. Br. Rev. 1985, 57, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Moffat, D.B.; Fourman, J. The Vascular Pattern of the Rat Kidney. J. Anat. 1963, 97, 543. [Google Scholar]
- Dalal, R.; Bruss, Z.S.; Sehdev, J.S. Physiology, Renal Blood Flow and Filtration. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Taghavi, I.; Andersen, S.B.; Sogaard, S.B.; Nielsen, M.B.; Sorensen, C.M.; Stuart, M.B.; Jensen, J.A. Automatic Classification of Arterial and Venous Flow in Super-Resolution Ultrasound Images of Rat Kidneys. In Proceedings of the 2021 IEEE International Ultrasonics Symposium (IUS), Xi’an, China, 11–16 September 2021. [Google Scholar] [CrossRef]
- Lowerison, M.R.; Huang, C.; Lucien, F.; Chen, S.; Song, P. Ultrasound Localization Microscopy of Renal Tumor Xenografts in Chicken Embryo Is Correlated to Hypoxia. Sci. Rep. 2020, 10, 2478. [Google Scholar] [CrossRef]
- Xie, L.; Cianciolo, R.E.; Hulette, B.; Lee, H.W.; Qi, Y.; Cofer, G.; Johnson, G.A. Magnetic Resonance Histology of Age-Related Nephropathy in the Sprague Dawley Rat. Toxicol. Pathol. 2012, 40, 764–778. [Google Scholar] [CrossRef]
- Andersen, S.B.; Taghavi, I.; Kjer, H.M.; Søgaard, S.B.; Gundlach, C.; Dahl, V.A.; Nielsen, M.B.; Dahl, A.B.; Jensen, J.A.; Sørensen, C.M. Evaluation of 2D Super-Resolution Ultrasound Imaging of the Rat Renal Vasculature Using Ex Vivo Micro-Computed Tomography. Sci. Rep. 2021, 11, 24335. [Google Scholar] [CrossRef]
- Maric-Bilkan, C.; Flynn, E.R.; Chade, A.R. Microvascular Disease Precedes the Decline in Renal Function in the Streptozotocin-Induced Diabetic Rat. Am. J. Physiol. Physiol. 2012, 302, F308–F315. [Google Scholar] [CrossRef]
- Bentley, M.D.; Rodriguez-Porcel, M.; Lerman, A.; Hershman Sarafov, M.; Romero, J.C.; Pelaez, L.I.; Grande, J.P.; Ritman, E.L.; Lerman, L.O. Enhanced Renal Cortical Vascularization in Experimental Hypercholesterolemia. Kidney Int. 2002, 61, 1056–1063. [Google Scholar] [CrossRef]
- Dencks, S.; Piepenbrock, M.; Opacic, T.; Krauspe, B.; Stickeler, E.; Kiessling, F.; Schmitz, G. Clinical Pilot Application of Super-Resolution US Imaging in Breast Cancer. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2019, 66, 517–526. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, W.; Gong, P.; Lok, U.W.; Tang, S.; Yin, T.; Zhang, X.; Zhu, L.; Sang, M.; Song, P.; et al. Super-Resolution Ultrasound Localization Microscopy Based on a High Frame-Rate Clinical Ultrasound Scanner: An in-Human Feasibility Study. Phys. Med. Biol. 2021, 66, 08NT01. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Peng, J.; Brown, K.; Sirsi, S.; Mineo, C.; Shaul, P.W.; Hoyt, K. Super-Resolution Ultrasound Imaging of Skeletal Muscle Microvascular Dysfunction in an Animal Model of Type 2 Diabetes. J. Ultrasound Med. 2019, 38, 2589. [Google Scholar] [CrossRef] [PubMed]
- Foiret, J.; Zhang, H.; Ilovitsh, T.; Mahakian, L.; Tam, S.; Ferrara, K.W. Ultrasound Localization Microscopy to Image and Assess Microvasculature in a Rat Kidney. Sci. Rep. 2017, 7, 13662. [Google Scholar] [CrossRef] [PubMed]
- Duke, J. Renal Function and Anesthesia. In Anesthesia Secrets; Elsevier: Amsterdam, The Netherlands, 2011; pp. 308–316. [Google Scholar] [CrossRef]
- Damkjær, M.; Vafaee, M.; Møller, M.L.; Braad, P.E.; Petersen, H.; Høilund-Carlsen, P.F.; Bie, P. Renal Cortical and Medullary Blood Flow Responses to Altered NO Availability in Humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, 1449–1455. [Google Scholar] [CrossRef]
- Iliescu, R.; Chade, A.R. Progressive Renal Vascular Proliferation and Injury in Obese Zucker Rats. Microcirculation 2010, 17, 250–258. [Google Scholar] [CrossRef]
- Ebenezer, P.J.; Mariappan, N.; Elks, C.M.; Haque, M.; Soltani, Z.; Reisin, E.; Francis, J. Effects of Pyrrolidine Dithiocarbamate on High-Fat Diet-Induced Metabolic and Renal Alterations in Rats. Life Sci. 2009, 85, 357–364. [Google Scholar] [CrossRef][Green Version]

| Young | Old | |||
|---|---|---|---|---|
| Basic characteristics | LZR | OZR | LZR | OZR |
| Body weight (g) | 329.5 ± 30.4 | 413 ± 24.0 | 458 ± 33.9 | 591.5 ± 6.4 |
| Kidney weight (g) | 1.15 * | 1.20 * | 1.47 ± 0.08 | 1.51 ± 0.13 |
| KW/BW (%) | 0.33 | 0.28 | 0.32 | 0.26 |
| Biochemical parameters | ||||
| Blood pressure (mmHg) | 86.69 ± 10.6 | 92.60 ± 2.7 | 89.53 ± 6.9 | 112.76 ± 5.5 |
| Blood glucose before laparotomy (mmol/L) | - | 10.1 ± 1.5 | 9.05 ± 0.9 | 11.8 ± 4.9 |
| Plasma creatinine (mg/dL) | 0.46 ± 0.4 | 0.40 ± 0.9 | 0.53 ± 0.4 | 0.54 ± 0.5 |
| Plasma urea (mg/dL) | 15.86 ± 0.64 | 20.44 ± 3.25 | 20.13 ± 1.15 | 22.42 ± 3.10 |
| Urinary creatinine (mg/dL) | 86.00 ± 52.61 | 19.50 ± 9.97 | 40.71 ± 12.47 | 10.39 ** |
| Urinary albumin (mg/dL) | 6.67 ± 6.11 | 6.83 ± 0.86 | 20.97 ± 22.04 | 102.62 ± 36.70 |
| Creatinine excretion rate (mg/min) | 0.007 | 0.004 | 0.006 | 0.002 ** |
| Albumin excretion rate (mg/min) | 0.0005 | 0.001 | 0.003 | 0.02 |
| Albumin/creatinine ratio (mg/g) | 65.15 ± 35.78 | 413.35 ± 171.87 | 447.29 ± 350.72 | 10,466.52 ** |
| GFR/Creatinine clearance (ml/min) | 0.63 ± 0.06 | 0.41 ± 0.01 | 0.57 ± 0.31 | 0.43 ** |
| Diuresis (µl/min) | 7.57 ± 2.71 | 19.53 ± 5.33 | 15.43 ± 5.73 | 22.63 ± 15.58 |
| Rat age | Young | |||||||||||
| Rat type | LZR | OZR | ||||||||||
| Area | IM | OM | CO | IM | OM | CO | ||||||
| Vesselkind | A | V | A | V | A | V | A | V | A | V | A | V |
| No. of tracks | 1036 | 661 | 2173 | 1565 | 1225 | 1632 | 890 | 627 | 1623 | 1265 | 637 | 869 |
| No. of MBs | 32,341 | 29,091 | 59,117 | 60,303 | 18,43 | 22,904 | 34,282 | 25,838 | 49,267 | 42,671 | 9524 | 12,612 |
| Rat age | Old | |||||||||||
| Rat type | LZR | OZR | ||||||||||
| Area | IM | OM | CO | IM | OM | CO | ||||||
| Vesselkind | A | V | A | V | A | V | A | V | A | V | A | V |
| No. of tracks | 1705 | 865 | 3021 | 2024 | 1400 | 2128 | 1192 | 658 | 4201 | 2222 | 1592 | 1974 |
| No. of MBs | 45,215 | 32,365 | 76,814 | 64,762 | 22,565 | 31,639 | 42,857 | 28,929 | 140,094 | 86,223 | 29,232 | 32,476 |
| Vascular Density | Young | Old |
|---|---|---|
| OZR vs. LZR | OZR vs. LZR | |
| CO arteries/arterioles | −0.68 [−0.81;−0.55] | −0.04 [−0.17;0.09] |
| CO veins/venules | −0.57 [−0.69;−0.45] | 0.23 [0.12;0.34] |
| OM arterioles | −0.16 [−0.25;−0.06] | 0.02 [−0.07;0.12] |
| OM venules | −0.06 [−0.21;0.09] | 0.42 [0.29;0.55] |
| IM arterioles | 0.31 [0.13;0.50] | −0.20 [−0.31;−0.08] |
| IM venules | 0.20 [0.03;0.38] | 0.01 [−0.12;0.15] |
| Vascular Tortuosity | Young | Old | ||
|---|---|---|---|---|
| LZR | OZR | LZR | OZR | |
| CO arteries/arterioles | 1.12 | 1.12 | 1.13 | 1.13 |
| CO veins/venules | 1.12 | 1.12 | 1.13 | 1.13 |
| OM arterioles | 1.12 | 1.11 | 1.12 | 1.11 |
| OM venules | 1.13 | 1.13 | 1.13 | 1.14 |
| IM arterioles | 1.12 | 1.11 | 1.12 | 1.12 |
| IM venules | 1.13 | 1.13 | 1.13 | 1.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Søgaard, S.B.; Andersen, S.B.; Taghavi, I.; Hoyos, C.A.V.; Christoffersen, C.; Hansen, K.L.; Jensen, J.A.; Nielsen, M.B.; Sørensen, C.M. Super-Resolution Ultrasound Imaging Provides Quantification of the Renal Cortical and Medullary Vasculature in Obese Zucker Rats: A Pilot Study. Diagnostics 2022, 12, 1626. https://doi.org/10.3390/diagnostics12071626
Søgaard SB, Andersen SB, Taghavi I, Hoyos CAV, Christoffersen C, Hansen KL, Jensen JA, Nielsen MB, Sørensen CM. Super-Resolution Ultrasound Imaging Provides Quantification of the Renal Cortical and Medullary Vasculature in Obese Zucker Rats: A Pilot Study. Diagnostics. 2022; 12(7):1626. https://doi.org/10.3390/diagnostics12071626
Chicago/Turabian StyleSøgaard, Stinne Byrholdt, Sofie Bech Andersen, Iman Taghavi, Carlos Armando Villagómez Hoyos, Christina Christoffersen, Kristoffer Lindskov Hansen, Jørgen Arendt Jensen, Michael Bachmann Nielsen, and Charlotte Mehlin Sørensen. 2022. "Super-Resolution Ultrasound Imaging Provides Quantification of the Renal Cortical and Medullary Vasculature in Obese Zucker Rats: A Pilot Study" Diagnostics 12, no. 7: 1626. https://doi.org/10.3390/diagnostics12071626
APA StyleSøgaard, S. B., Andersen, S. B., Taghavi, I., Hoyos, C. A. V., Christoffersen, C., Hansen, K. L., Jensen, J. A., Nielsen, M. B., & Sørensen, C. M. (2022). Super-Resolution Ultrasound Imaging Provides Quantification of the Renal Cortical and Medullary Vasculature in Obese Zucker Rats: A Pilot Study. Diagnostics, 12(7), 1626. https://doi.org/10.3390/diagnostics12071626








