AI-Based Chest CT Analysis for Rapid COVID-19 Diagnosis and Prognosis: A Practical Tool to Flag High-Risk Patients and Lower Healthcare Costs
Abstract
:1. Introduction
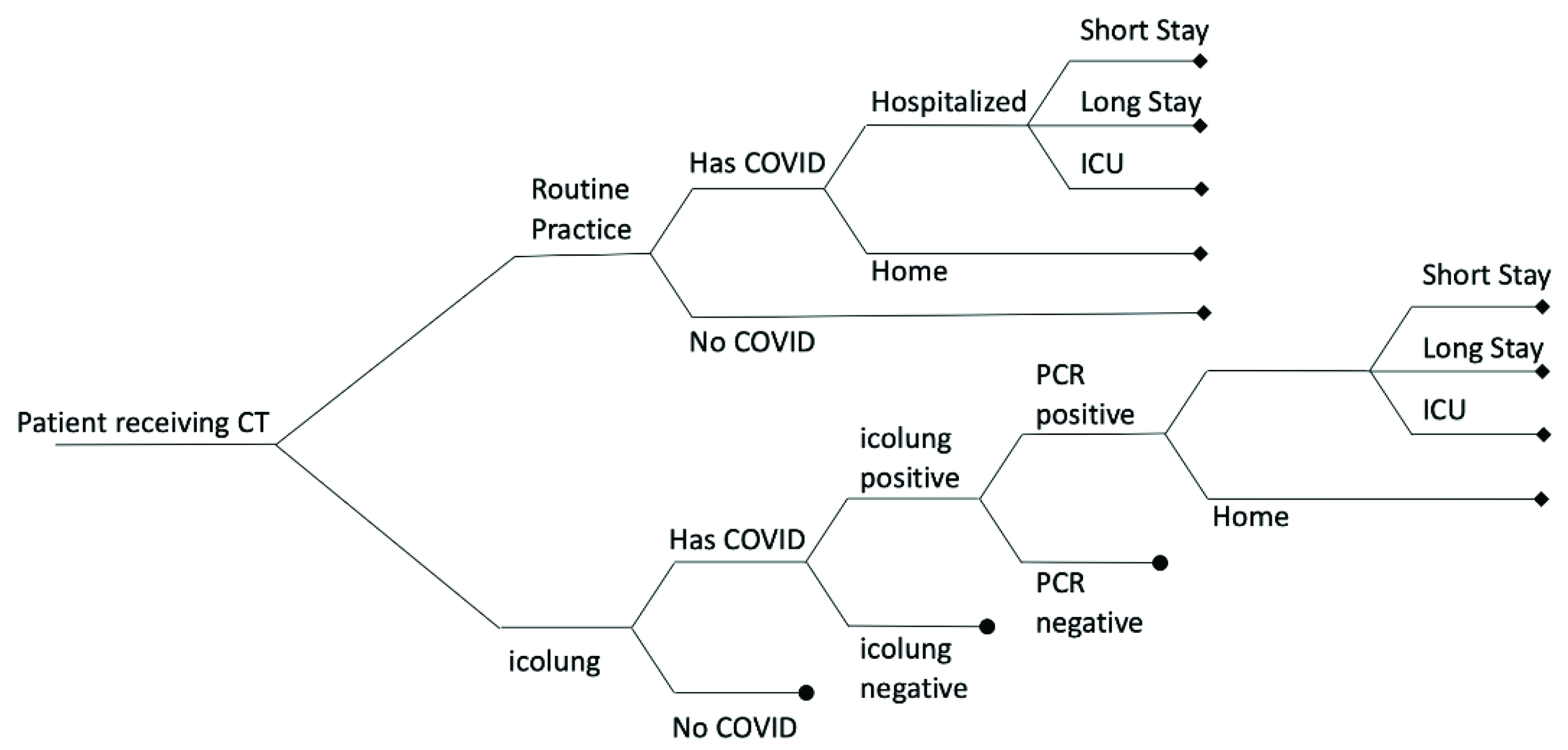
2. Materials and Methods
2.1. Icolung Software
- -
- The 3D segmentation masks of the abnormalities are visualized in 2D axial and coronal views on a report;
- -
- A table with the lung involvement percentages and the corresponding severity scores of both abnormality types. These values are shown for each lung lobe, as well as for the total lungs.
2.2. Model Structure and Parameters
3. Results
3.1. Evaluation of Costs Avoided Using Icolung
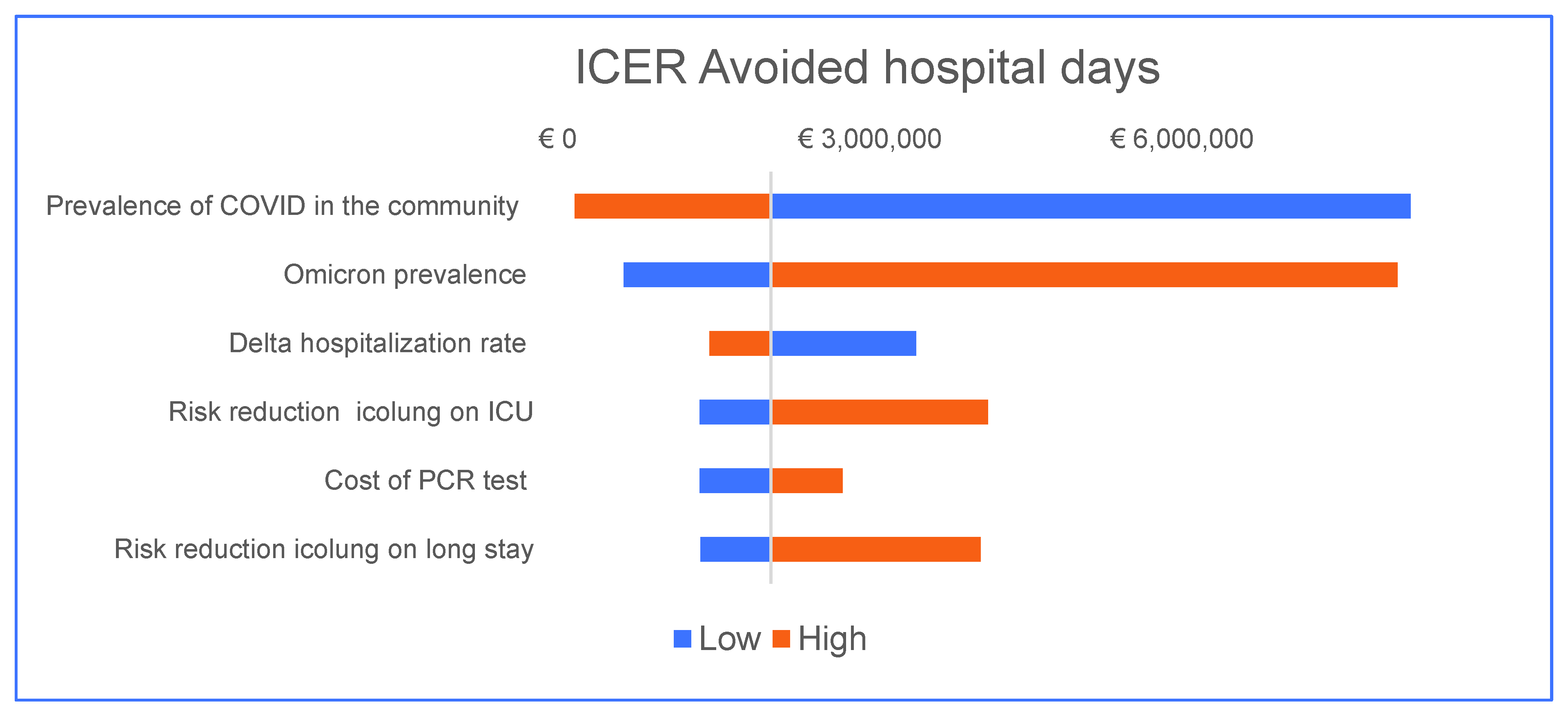
3.2. Sensitivity Analysis
4. Discussion
5. Strengths and Limitations of This Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deprez, L.; Boulanger, Y.-G.; Guiot, J. Added Value of Dual-Energy CT in COVID-19 Pneumopathy. J. Belg. Soc. Radiol. 2021, 105, 62–63. [Google Scholar] [CrossRef] [PubMed]
- Uysal, E.; Kilinçer, A.; Cebeci, H.; Özer, H.; Demir, N.A.; Öztürk, M.; Koplay, M. Chest CT findings in RT-PCR positive asymptomatic COVID-19 patients. Clin. Imaging 2021, 77, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Bernheim, A.; Mei, X.; Huang, M.; Yang, Y.; Fayad, Z.A.; Zhang, N.; Diao, K.; Lin, B.; Zhu, X.; Li, K.; et al. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology 2020, 295, 685–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, M.; Bernheim, A.; Mei, X.; Zhang, N.; Huang, M.; Zeng, X.; Cui, J.; Xu, W.; Yang, Y.; Fayad, Z.A.; et al. CT imaging features of 2019 novel coronavirus (2019–nCoV). Radiology 2020, 295, 202–207. [Google Scholar] [CrossRef] [Green Version]
- Prokop, M.; Van Everdingen, W.; van Rees Vellinga, T.; Quarles van Ufford, H.; Stöger, L.; Beenen, L.; Geurts, B.; Gietema, H.; Krdzalic, J.; Schaefer-Prokop, C.; et al. CO-RADS: A Categorical CT Assessment Scheme for Patients Suspected of Having COVID-19—Definition and Evaluation. Radiology 2020, 296, E97–E104. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.; Kay, F.U.; Abbara, S.; Bhalla, S.; Chung, J.H.; Chung, M.; Henry, T.S.; Kanne, J.P.; Kligerman, S.; Ko, J.P.; et al. Radiological society of North America expert consensus document on reporting chest CT findings related to COVID-19: Endorsed by the society of thoracic radiology, the American college of radiology, and RSNA. Radiol. Cardiothorac. Imaging 2020, 2, e200152. [Google Scholar] [CrossRef] [Green Version]
- Salehi, S.; Abedi, A.; Balakrishnan, S.; Gholamrezanezhad, A. Coronavirus disease 2019 (COVID-19) imaging reporting and data system (COVID-RADS) and common lexicon: A proposal based on the imaging data of 37 studies. Eur. Radiol. 2020, 30, 4930–4942. [Google Scholar] [CrossRef]
- Guiot, J.; Vaidyanathan, A.; Deprez, L.; Zerka, F.; Danthine, D.; Frix, A.-N.; Thys, M.; Henket, M.; Canivet, G.; Mathieu, S.; et al. Development and Validation of an Automated Radiomic CT Signature for Detecting COVID-19. Diagnostics 2020, 11, 41. [Google Scholar] [CrossRef]
- Naidoo, J.; Wang, X.; Woo, K.M.; Iyriboz, T.; Halpenny, D.; Cunningham, J.; Chaft, J.E.; Segal, N.H.; Callahan, M.K.; Lesokhin, A.M.; et al. Pneumonitis in Patients Treated With Anti–Programmed Death-1/Programmed Death Ligand 1 Therapy. J. Clin. Oncol. 2017, 35, 709–717. [Google Scholar] [CrossRef] [Green Version]
- Picard, S.; Goh, D.; Tan, A.; Sikotra, N.; Gabbay, E.; Clay, T. Patterns of immunotherapy-induced pneumonitis in patients with non-small-cell lung cancer: A case series. J. Med. Case Rep. 2021, 15, 332. [Google Scholar] [CrossRef]
- Jin, C.; Chen, W.; Cao, Y.; Xu, Z.; Tan, Z.; Zhang, X.; Deng, L.; Zheng, C.; Zhou, J.; Shi, H.; et al. Development and evaluation of an artificial intelligence system for COVID-19 diagnosis. Nat. Commun. 2020, 11, 5088. [Google Scholar] [CrossRef] [PubMed]
- Ardakani, A.A.; Kanafi, A.R.; Acharya, U.R.; Khadem, N.; Mohammadi, A. Application of deep learning technique to manage COVID-19 in routine clinical practice using CT images: Results of 10 convolutional neural networks. Comput. Biol. Med. 2020, 121, 103795. [Google Scholar] [CrossRef] [PubMed]
- Ozsahin, I.; Sekeroglu, B.; Musa, M.S.; Mustapha, M.T.; Ozsahin, D.U. Review on Diagnosis of COVID-19 from Chest CT Images Using Artificial Intelligence. Comput. Math. Methods Med. 2020, 2020, 9756518. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Dong, D.; Li, L.; Niu, M.; Bai, Y.; Wang, M.; Qiu, X.; Zha, Y.; Tian, J. A Deep Learning Prognosis Model Help Alert for COVID-19 Patients at High-Risk of Death: A Multi-Center Study. IEEE J. Biomed. Health Inform. 2020, 24, 9195. [Google Scholar] [CrossRef]
- Wang, S.; Zha, Y.; Li, W.; Wu, Q.; Li, X.; Niu, M.; Wang, M.; Qiu, X.; Li, H.; Yu, H.; et al. A fully automatic deep learning system for COVID-19 diagnostic and prognostic analysis. Eur. Respir. J. 2020, 56, 2000775. [Google Scholar] [CrossRef]
- Shiri, I.; Sorouri, M.; Geramifar, P.; Nazari, M.; Abdollahi, M.; Salimi, Y.; Khosravi, B.; Askari, D.; Aghaghazvini, L.; Hajianfar, G.; et al. Machine learning-based prognostic modeling using clinical data and quantitative radiomic features from chest CT images in COVID-19 patients. Comput. Biol. Med. 2021, 132, 104304. [Google Scholar] [CrossRef]
- Shi, F.; Wang, J.; Shi, J.; Wu, Z.; Wang, Q.; Tang, Z.; He, K.; Shi, Y.; Shen, D. Review of Artificial Intelligence Techniques in Imaging Data Acquisition, Segmentation, and Diagnosis for COVID-19. IEEE Rev. Biomed. Eng. 2020, 14, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Fusco, R.; Grassi, R.; Granata, V.; Setola, S.V.; Grassi, F.; Cozzi, D.; Pecori, B.; Izzo, F.; Petrillo, A. Artificial Intelligence and COVID-19 Using Chest CT Scan and Chest X-ray Images: Machine Learning and Deep Learning Approaches for Diagnosis and Treatment. J. Pers. Med. 2021, 11, 993. [Google Scholar] [CrossRef]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional networks for biomedical image segmentation. In Medical Image Computing and Computer-Assisted Intervention 2015; Navab, N., Hornegger, J., Wells, W.M., Frangi, A.F., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 234–241. [Google Scholar] [CrossRef] [Green Version]
- Çiçek, Ö.; Abdulkadir, A.; Lienkamp, S.S.; Brox, T.; Ronneberger, O. 3D U-Net: Learning Dense Volumetric Segmentation from Sparse Annotation. In International Conference on Medical Image Computing and Computer-Assisted Intervention; Springer: Cham, Switzerland, 2016; pp. 424–432. [Google Scholar]
- Pan, F.; Ye, T.; Sun, P.; Gui, S.; Liang, B.; Li, L.; Zheng, D.; Wang, J.; Hesketh, R.L.; Yang, L.; et al. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology 2020, 295, 715–721. [Google Scholar] [CrossRef] [Green Version]
- Temime, L.; Gustin, M.-P.; Duval, A.; Buetti, N.; Crépey, P.; Guillemot, D.; ThiéBaut, R.; Vanhems, P.; Zahar, J.-R.; Smith, D.R.M.; et al. Estimating R0 of Sars-Cov-2 in Healthcare Settings. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Savitsky, L.M.; Albright, C.M. Preventing COVID-19 Transmission on Labor and Delivery: A Decision Analysis. Am. J. Perinatol. 2020, 37, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Belgium: Coronavirus Pandemic Country Profile—Our World in Data. Available online: https://ourworldindata.org/coronavirus/country/belgium?country (accessed on 16 February 2022).
- Veneti, L.; Bøås, H.; Kristoffersen, A.B.; Stålcrantz, J.; Bragstad, K.; Hungnes, O.; Storm, M.L.; Aasand, N.; Rø, G.; Starrfelt, J.; et al. Reduced risk of hospitalisation among reported COVID-19 cases infected with the SARS-CoV-2 Omicron BA.1 variant compared with the Delta variant, Norway, December 2021 to January 2022. Eurosurveillance 2022, 27, 2200077. [Google Scholar] [CrossRef] [PubMed]
- Hellou, M.M.; Górska, A.; Mazzaferri, F.; Cremonini, E.; Gentilotti, E.; De Nardo, P.; Poran, I.; Leeflang, M.M.; Tacconelli, E.; Paul, M. Nucleic acid amplification tests on respiratory samples for the diagnosis of coronavirus infections: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2020, 27, 341. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.; Sahli, H.; Joukovsky, B.; Kvasnytsia, M.; Dirks, I.; Deligiannis, N. Explainable-by-design Semi-Supervised Representation Learning for COVID-19 Diagnosis from CT Imaging. arXiv 2020, arXiv:2011.11719. [Google Scholar]
- Kucharski, A.J.; Klepac, P.; Conlan, A.J.; Kissler, S.M.; Tang, M.L.; Fry, H.; Gog, J.R.; Edmunds, W.J.; Emery, J.C.; Medley, G.; et al. Effectiveness of isolation, testing, contact tracing, and physical distancing on reducing transmission of SARS-CoV-2 in different settings: A mathematical modelling study. Lancet Infect. Dis. 2020, 20, 1151–1160. [Google Scholar] [CrossRef]
- Teleman, M.D.; Boudville, I.C.; Heng, B.H.; Zhu, D.; Leo, Y.S. Factors associated with transmission of severe acute respiratory syndrome among health-care workers in Singapore. Epidemiol. Infect. 2004, 132, 797–803. [Google Scholar] [CrossRef] [Green Version]
- Butler, R.; Monsalve, M.; Thomas, G.W.; Herman, T.; Segre, A.M.; Polgreen, P.M.; Suneja, M. Estimating Time Physicians and Other Health Care Workers Spend with Patients in an Intensive Care Unit Using a Sensor Network. Am. J. Med. 2018, 131, 972.e9–972.e15. [Google Scholar] [CrossRef]
- Lessmann, N.; Sánchez, C.I.; Beenen, L.; Boulogne, L.H.; Brink, M.; Calli, E.; Charbonnier, J.-P.; Dofferhoff, T.; van Everdingen, W.M.; Gerke, P.K.; et al. Automated Assessment of COVID-19 Reporting and Data System and Chest CT Severity Scores in Patients Suspected of Having COVID-19 Using Artificial Intelligence. Radiology 2021, 298, E18–E28. [Google Scholar] [CrossRef]
- Bai, H.X.; Hsieh, B.; Xiong, Z.; Halsey, K.; Choi, J.W.; Tran, T.M.L.; Pan, I.; Shi, L.-B.; Wang, D.-C.; Mei, J.; et al. Performance of Radiologists in Differentiating COVID-19 from Non-COVID-19 Viral Pneumonia at Chest CT. Radiology 2020, 296, E46–E54. [Google Scholar] [CrossRef]
- Ning, W.; Lei, S.; Yang, J.; Cao, Y.; Jiang, P.; Yang, Q.; Zhang, J.; Wang, X.; Chen, F.; Geng, Z.; et al. Open resource of clinical data from patients with pneumonia for the prediction of COVID-19 outcomes via deep learning. Nat. Biomed. Eng. 2020, 4, 1197–1207. [Google Scholar] [CrossRef]
- Bai, H.X.; Wang, R.; Xiong, Z.; Hsieh, B.; Chang, K.; Halsey, K.; Tran, T.M.L.; Choi, J.W.; Wang, D.-C.; Shi, L.-B.; et al. Artificial Intelligence Augmentation of Radiologist Performance in Distinguishing COVID-19 from Pneumonia of other Origin at Chest CT. Radiology 2020, 296, E156–E165. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, L.; D’Angelo, E.C.; Paolisso, P.; Toniolo, S.; Fabrizio, M.; Angeli, F.; Donati, F.; Magnani, I.; Rinaldi, A.; Bartoli, L.; et al. The value of ECG changes in risk stratification of COVID-19 patients. Ann. Noninvasive Electrocardiol. 2021, 26, e12815. [Google Scholar] [CrossRef] [PubMed]
- Zaher, E.A.; Keller, D.M.; Suntharampillai, N.; Ujkani, E.; Lesiak, M. Cardiovascular risk factors of poor prognosis in COVID-19—A review. J. Med. Sci. 2021, 90, e571. [Google Scholar] [CrossRef]
- Paolisso, P.; Bergamaschi, L.; D’Angelo, E.C.; Donati, F.; Giannella, M.; Tedeschi, S.; Pascale, R.; Bartoletti, M.; Tesini, G.; Biffi, M.; et al. Preliminary Experience With Low Molecular Weight Heparin Strategy in COVID-19 Patients. Front. Pharmacol. 2020, 11, 1124. [Google Scholar] [CrossRef]

| Variable | Base Case Value | Range Considered in the Sensitivity Analysis | Reference |
|---|---|---|---|
| Prevalence of COVID in the community | 4.00% | 1.00–50.00% | [24] |
| Omicron prevalence | 75.00% | 0.00–100.00% | Unpublished data |
| Delta prevalence | 25.00% | - | Unpublished data |
| Omicron hospitalization rate | 0.20% | 0.10–0.30% | [25] |
| Delta hospitalization rate | 1.10% | 0.55–1.65% | |
| Probability of hospitalization | 7.70% | - | Estimated * |
| Omicron ICU rate (among hospitalized) | 24.00% | 3.85–11.50% | [25] |
| Delta ICU rate (among hospitalized) | 0.43% | 12.50–36.00% | |
| Probability of ICU admission (among hospitalized) | 17.65% | - | Estimated ** |
| Probability of short stay (1.5 days) | 18.25% | - | Estimated *** |
| Probability of long stay (5 days) | 64.11% | - | Estimated **** |
| Sensitivity PCR test | 96.20% | 91.00–98.40% | [26] |
| Specificity PCR test | 98.70% | 95.00–99.00% | |
| Sensitivity icolung | 96.00% | 94.00–99.00% | [27] |
| Specificity icolung | 60.00% | 59.00–61.00% | |
| Cost of hospitalization per patient per day | EUR 1000.00 | EUR 500.00–1500.00 | Assumption |
| Cost of ICU per patient per day | EUR 3000.00 | EUR 1500.00–4500.00 | Assumption |
| Cost of PCR test | EUR 100.00 | EUR 50.00–150.00 | Assumption |
| Cost of CT chest scan | EUR 300.00 | EUR 150.00–450.00 | Assumption |
| Cost of icolung per patient | EUR 50.00 | EUR 25.00–75.00 | Assumption |
| Average hospital short stay duration (days) | 1.50 | - | Expert opinion |
| Average hospital long stay duration (days) | 5.00 | - | Expert opinion |
| Average ICU stay duration (days) | 14.00 | - | Expert opinion |
| Risk reduction icolung on long stay | 0.90 | 0.80–1.00 | Expert opinion |
| Risk reduction icolung on ICU | 0.90 | 0.80–1.00 | Expert opinion |
| Reproduction number (community) | 1.25 | 0.25–2.25 | |
| Reproduction number short stay | 1.25 | - | Assumed to be as community |
| Reproduction number long stay | 1.87 | - | Estimated ***** |
| Reproduction number ICU | 2.19 | - | |
| Risk reduction self-isolation plus household quarantine | 0.63 | 0.50–0.76 | [28] |
| Risk reduction personal protection equipment | 0.07 | 0.06–0.08 | [29] |
| Strategy | Estimated Costs (EUR) | Incremental Costs (EUR) | Infections | Hospital Days | Infections Avoided | Hospital Days Avoided |
|---|---|---|---|---|---|---|
| Routine practice (RP) | 301,910 | 49.81 | 1.02 | |||
| RP + icolung | 453,129 | 151,220 | 31.41 | 0.95 | 18.4 | 0.07 |
| Outcomes | ICER |
|---|---|
| Infections avoided | EUR 8221 |
| Hospital days avoided | EUR 2,047,902 |
| Prevalence COVID | Rt | ICER |
|---|---|---|
| 1.0% | 0.250 | EUR64,480 |
| 4.0% | 0.520 | EUR 19,761 |
| 11.0% | 0.790 | EUR 4726 |
| 16.0% | 1.060 | EUR 2420 |
| 21.0% | 1.330 | EUR 1468 |
| 26.0% | 1.600 | EUR 985 |
| 31.0% | 1.870 | EUR 707 |
| 36.0% | 2.140 | EUR 531 |
| 41.0% | 2.410 | EUR 414 |
| 46.0% | 2.680 | EUR 332 |
| Prevalence COVID | Hospitalization Risk | ICER |
|---|---|---|
| 1.0% | 0.4% | EUR 8,585,600 |
| 6.0% | 1.3% | EUR 525,664 |
| 11.0% | 2.1% | EUR 182,785 |
| 16.0% | 3.3% | EUR 82,140 |
| 21.0% | 4.3% | EUR 47,747 |
| 26.0% | 5.3% | EUR 30,949 |
| 31.0% | 6.3% | EUR 21,508 |
| 36.0% | 7.3% | EUR 15,680 |
| 41.0% | 8.3% | EUR 11,831 |
| 46.0% | 9.3% | EUR 9157 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, G.; Ernst, B.; Henket, M.; Winandy, M.; Chatterjee, A.; Van Eyndhoven, S.; Praet, J.; Smeets, D.; Meunier, P.; Louis, R.; et al. AI-Based Chest CT Analysis for Rapid COVID-19 Diagnosis and Prognosis: A Practical Tool to Flag High-Risk Patients and Lower Healthcare Costs. Diagnostics 2022, 12, 1608. https://doi.org/10.3390/diagnostics12071608
Esposito G, Ernst B, Henket M, Winandy M, Chatterjee A, Van Eyndhoven S, Praet J, Smeets D, Meunier P, Louis R, et al. AI-Based Chest CT Analysis for Rapid COVID-19 Diagnosis and Prognosis: A Practical Tool to Flag High-Risk Patients and Lower Healthcare Costs. Diagnostics. 2022; 12(7):1608. https://doi.org/10.3390/diagnostics12071608
Chicago/Turabian StyleEsposito, Giovanni, Benoit Ernst, Monique Henket, Marie Winandy, Avishek Chatterjee, Simon Van Eyndhoven, Jelle Praet, Dirk Smeets, Paul Meunier, Renaud Louis, and et al. 2022. "AI-Based Chest CT Analysis for Rapid COVID-19 Diagnosis and Prognosis: A Practical Tool to Flag High-Risk Patients and Lower Healthcare Costs" Diagnostics 12, no. 7: 1608. https://doi.org/10.3390/diagnostics12071608
APA StyleEsposito, G., Ernst, B., Henket, M., Winandy, M., Chatterjee, A., Van Eyndhoven, S., Praet, J., Smeets, D., Meunier, P., Louis, R., Kolh, P., & Guiot, J. (2022). AI-Based Chest CT Analysis for Rapid COVID-19 Diagnosis and Prognosis: A Practical Tool to Flag High-Risk Patients and Lower Healthcare Costs. Diagnostics, 12(7), 1608. https://doi.org/10.3390/diagnostics12071608







