An Update on the General Features of Breast Cancer in Male Patients—A Literature Review
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Risk, Biology, Diagnosis
3.1.1. General Facts and Specificities of the Geographical Distribution of Male Breast Cancer
3.1.2. Gynecomastia and Pediatric Cases
3.1.3. Metastases
3.1.4. Image-Based Diagnostic Methods
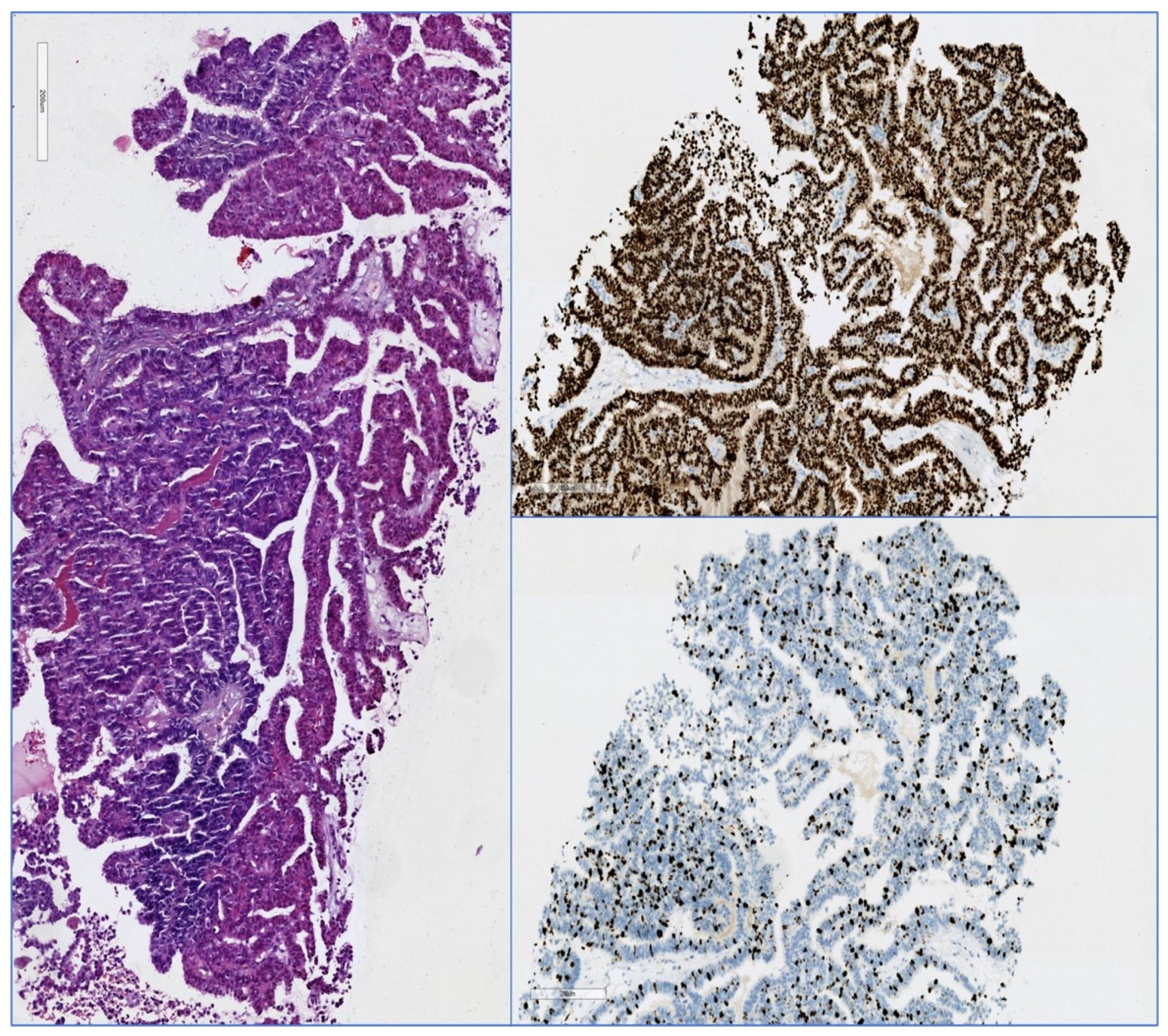
3.1.5. Pathology
3.2. Treatment
3.2.1. Breast Conserving Surgery versus Mastectomy
3.2.2. Adjuvant Treatment
3.3. Breast Cancer in Transgender Patients
3.3.1. Male to Female
3.3.2. Female to Male
3.4. Second Cancers Associated with Breast Cancer in Men
3.5. Prognosis
3.6. Future Trends and Potential Therapeutic Targets in Breast Cancer
3.6.1. Aquaporins
3.6.2. The Androgen Receptor
3.6.3. Breast Pre-Cancer Atlas
3.6.4. Alternative Splicing
3.6.5. Squalene Epoxidase
3.6.6. The Unfolded Protein Response
3.6.7. Proteolytic Neoepitopes for RAS-Driven Cancers
3.6.8. Coumarinyl Thiazolotriazoles
3.6.9. IL-25
3.6.10. ILK/YAP Axis
3.6.11. Antibody-Drug Conjugates and VEGF Receptor Inhibitors
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garreffa, E.; Arora, D. Breast cancer in the elderly, in men and during pregnancy. Surgery 2022, 40, 139–146. [Google Scholar] [CrossRef]
- Abdelwahab Yousef, A.J. Male Breast Cancer: Epidemiology and Risk Factors. Semin. Oncol. 2017, 44, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Kenemans, P.; Verstraeten, R.A.; Verheijen, R.H.M. Oncogenic pathways in hereditary and sporadic breast cancer. Maturitas 2008, 61, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Ruddy, K.J.; Winer, E.P. Male breast cancer: Risk factors, biology, diagnosis, treatment, and survivorship. Ann. Oncol. 2013, 24, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.; Speirs, V.; Shaaban, A.M. Male breast cancer: An update. Virchows Arch. 2022, 480, 85–93. [Google Scholar] [CrossRef]
- Peshkin, B.N.; Ladd, M.K.; Isaacs, C.; Segal, H.; Jacobs, A.; Taylor, K.L.; Graves, K.D.; O’Neill, S.C.; Schwartz, M.D. The Genetic Education for Men (GEM) Trial: Development of Web-Based Education for Untested Men in BRCA1/2-Positive Families. J. Cancer Educ. 2021, 36, 72–84. [Google Scholar] [CrossRef]
- Nguyen, J.V.; Thomas, M.H. Beyond BRCA: Review of Hereditary Syndromes Predisposing to Breast Cancer. J. Breast Imaging 2019, 1, 84–91. [Google Scholar] [CrossRef]
- Gucalp, A.; Traina, T.A.; Eisner, J.R.; Parker, J.S.; Selitsky, S.R.; Park, B.H.; Elias, A.D.; Baskin-Bey, E.S.; Cardoso, F. Male breast cancer: A disease distinct from female breast cancer. Breast Cancer Res. Treat. 2019, 173, 37–48. [Google Scholar] [CrossRef]
- Ansari, N.; Shahrabi, S.; Khosravi, A.; Shirzad, R.; Rezaeean, H. Prognostic Significance of CHEK2 Mutation in Progression of Breast Cancer. Lab. Med. 2019, 50, e36–e41. [Google Scholar] [CrossRef]
- Liang, M.; Zhang, Y.; Sun, C.; Rizeq, F.K.; Min, M.; Shi, T.; Sun, Y. Association Between CHEK2*1100delC and Breast Cancer: A Systematic Review and Meta-Analysis. Mol. Diagn. Ther. 2018, 22, 397–407. [Google Scholar] [CrossRef]
- Bahassi, E.M.; Robbins, S.B.; Yin, M.; Boivin, G.P.; Kuiper, R.; van Steeg, H.; Stambrook, P.J. Mice with the CHEK2*1100delC SNP are predisposed to cancer with a strong gender bias. Proc. Natl. Acad. Sci. USA 2009, 106, 17111–17116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamseddine, R.S.; Wang, C.; Yin, K.; Wang, J.; Singh, P.; Zhou, J.; Robson, M.E.; Braun, D.; Hughes, K.S. Penetrance of male breast cancer susceptibility genes: A systematic review. Breast Cancer Res. Treat. 2022, 191, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Friebel, T.M.; Domchek, S.M.; Rebbeck, T.R. Modifiers of Cancer Risk in BRCA1 and BRCA2 Mutation Carriers: A Systematic Review and Meta-Analysis. JNCI J. Natl. Cancer Inst. 2014, 106, dju091. [Google Scholar] [CrossRef] [Green Version]
- Davey, M.G.; Davey, C.M.; Bouz, L.; Kerin, E.; McFeetors, C.; Lowery, A.J.; Kerin, M.J. Relevance of the 21-gene expression assay in male breast cancer: A systematic review and meta-analysis. Breast 2022, 64, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Fentiman, I.S. The endocrinology of male breast cancer. Endocr.-Relat. Cancer 2018, 25, R365–R373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woods, R.W.; Salkowski, L.R.; Elezaby, M.; Burnside, E.S.; Strigel, R.M.; Fowler, A.M. Image-based screening for men at high risk for breast cancer: Benefits and drawbacks. Clin. Imaging 2020, 60, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Nofal, M.N.; Yousef, A.J. The diagnosis of male breast cancer. Neth. J. Med. 2019, 77, 356–359. Available online: https://pubmed.ncbi.nlm.nih.gov/31880271/ (accessed on 11 May 2022).
- Pizzato, M.; Carioli, G.; Bertuccio, P.; Malvezzi, M.; Levi, F.; Boffetta, P.; Negri, E.; La Vecchia, C. Trends in male breast cancer mortality: A global overview. Eur. J. Cancer Prev. 2021, 30, 472–479. [Google Scholar] [CrossRef]
- Ndom, P.; Um, G.; Bell, E.M.D.; Eloundou, A.; Hossain, N.M.; Huo, D. A meta-analysis of male breast cancer in Africa. Breast 2012, 21, 237–241. [Google Scholar] [CrossRef]
- Methamem, M.; Ghadhab, I.; Hidar, S.; Briki, R. Breast cancer in men: A serie of 45 cases and literature review. Pan Afr. Med. J. 2020, 36, 1–10. [Google Scholar] [CrossRef]
- Ssentongo, P.; Lewcun, J.A.; Candela, X.; Ssentongo, A.E.; Kwon, E.G.; Ba, D.M.; Oh, J.S.; Amponsah-Manu, F.; McDonald, A.C.; Chinchilli, V.M.; et al. Regional, racial, gender, and tumor biology disparities in breast cancer survival rates in Africa: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0225039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaaban, A.M. Pathology of the male breast. Diagn. Histopathol. 2019, 25, 138–142. [Google Scholar] [CrossRef]
- Billa, E.; Kanakis, G.A.; Goulis, D.G. Imaging in gynecomastia. Andrology 2021, 9, 1444–1456. [Google Scholar] [CrossRef] [PubMed]
- Hoda, R.S.; Arpin, R.N.; Gottumukkala, R.V.; Hughes, K.S.; Ly, A.; Brachtel, E.F. Diagnostic Value of Fine-Needle Aspiration in Male Breast Lesions. Acta Cytol. 2019, 63, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Ghilli, M.; Mariniello, M.D.; Scatena, C.; Dosa, L.; Traficante, G.; Tamburini, A.; Caporalini, C.; Buccoliero, A.M.; Facchini, F.; Colizzi, L.; et al. Male secretory breast cancer: Case in a 6-year-old boy with a peculiar gene duplication and review of the literature. Breast Cancer Res. Treat. 2018, 170, 445–454. [Google Scholar] [CrossRef]
- Shahriari, M.; Ghasemi, K.; Bordbar, M.; Shakibazad, N. Gynecomastia as a late complication of childhood cancer and its treatment that can affect the quality of life of male survivors. Semin. Oncol. 2019, 46, 155–159. [Google Scholar] [CrossRef]
- Wang, Y.; Reulen, R.C.; Kremer, L.C.M.; de Vathaire, F.; Haupt, R.; Zadravec Zaletel, L.; Bagnasco, F.; Demoor-Goldschmidt, C.; van Dorp, W.J.; Haddy, N.; et al. Male breast cancer after childhood cancer: Systematic review and analyses in the PanCareSurFup cohort. Eur. J. Cancer 2022, 165, 27–47. [Google Scholar] [CrossRef]
- de Almeida Freire, N.; de Andrade, B.A.B.; Silva Canedo, N.H.; Agostini, M.; Romañach, M.J. Oral and maxillofacial metastasis of male breast cancer: Report of a rare case and literature review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, e18–e22. [Google Scholar] [CrossRef]
- Kesting, M.R.; Loeffelbein, D.J.; Hölzle, F.; Wolff, K.D.; Ebsen, M. Male breast cancer metastasis presenting as submandibular swelling. Auris Nasus Larynx 2006, 33, 483–485. [Google Scholar] [CrossRef]
- González-Pérez, L.M.; Infante-Cossio, P.; Crespo-Torres, S.; Sanchez-Gallego, F. Mandibular metastases as first clinical sign of an occult male breast cancer. Int. J. Oral Maxillofac. Surg. 2012, 41, 1211–1214. [Google Scholar] [CrossRef]
- Mor, A.G.; Das, S.; Joshi, S.P.; Chaudhari, V.A.; Desai, S. Metastatic Lobular Carcinoma of the Male Breast Masquerading as a Pancreatic Head Mass, a Diagnostic Dilemma-Rare Case and Literature Review. Indian J. Med. Paediatr. Oncol. 2022, 43, 124–128. [Google Scholar] [CrossRef]
- Che, W.; Wang, Y.; Wang, X.; Lyu, J. Midlife brain metastases in the United States: Is male at risk? Cancer Med. 2022, 11, 1202–1216. [Google Scholar] [CrossRef]
- Leone, J.P.; Leone, B.A.; Zwenger, A.O.; Vallejo, C.T.; Romero, A.O.; Machiavelli, M.R.; Pérez, J.E., Leone. The prognostic significance of metastatic pattern in stage IV male breast cancer at initial diagnosis: A population-based study. Breast Cancer Res. Treat. 2021, 187, 237–244. [Google Scholar] [CrossRef]
- Singh, R.; Stoltzfus, K.C.; Chen, H.; Louie, A.V.; Lehrer, E.J.; Horn, S.R.; Palmer, J.D.; Trifiletti, D.M.; Brown, P.D.; Zaorsky, N.G. Epidemiology of synchronous brain metastases. Neuro-Oncol. Adv. 2020, 2, vdaa041. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.H.; Woo, C.G.; Lee, Y.J.; Park, Y.S. Brain metastasis with subtype conversion in a patient with male breast cancer: A case report. Medicine 2021, 100, e24373. [Google Scholar] [CrossRef] [PubMed]
- Tahrir, Y.; Bertal, A.; Mawhoub, S.; Makhchoune, M.; Ibahiouin, K.; Lakhdar, A. A cerebellopontine angle metastatis of a male breast cancer: Case report. Ann. Med. Surg. 2022, 75, 103421. [Google Scholar] [CrossRef] [PubMed]
- Fuchinoue, Y.; Node, Y.; Masuda, H.; Kondo, K.; Harada, N.; Nemoto, M.; Sugo, N. A Case of Male Breast Cancer with Brain Metastasis 24 Years after a Mastectomy. No Shinkei Geka Neurol. Surg. 2018, 46, 683–689. [Google Scholar] [CrossRef]
- Yang, S.; Leng, Y.; Chau, C.M.; Ma, K.F.J.; Fung, W.Y.; Chan, R.L.S.; Yung, W.T.A.; Leong, P.W.; Li, O.C.A.; Wong, T. The ins and outs of male breast and anterior chest wall lesions from childhood to adulthood. Clin. Radiol. 2022, 77, 503–513. [Google Scholar] [CrossRef]
- Mango, V.L.; Goodman, S.; Clarkin, K.; Wynn, R.T.; Friedlander, L.; Hibshoosh, H.; Ha, R. The unusual suspects: A review of unusual benign and malignant male breast imaging cases. Clin. Imaging 2018, 50, 78–85. [Google Scholar] [CrossRef]
- Chesebro, A.L.; Rives, A.F.; Shaffer, K. Male Breast Disease: What the Radiologist Needs to Know. Curr. Probl. Diagn. Radiol. 2019, 48, 482–493. [Google Scholar] [CrossRef]
- Dondi, F.; Albano, D.; Giubbini, R.; Bertagna, F. 18F-FDG PET/CT for the evaluation of male breast cancer: A systematic review. Nucl. Med. Commun. 2022, 43, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Gherghe, M.; Mutuleanu, M.D.; Stanciu, A.E.; Irimescu, I.; Lazar, A.; Bacinschi, X.; Anghel, R.M. Quantitative Analysis of SPECT-CT Data in Metastatic Breast Cancer Patients-The Clinical Significance. Cancers 2022, 14, 273. [Google Scholar] [CrossRef] [PubMed]
- Caldarone, A.; Piccotti, F.; Morasso, C.; Truffi, M.; Sottotetti, F.; Guerra, C.; Albasini, S.; Agozzino, M.; Villani, L.; Corsi, F. Raman analysis of microcalcifications in male breast cancer. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 263, 120185. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Martaindale, S.; Whitman, G.J. Male Breast Magnetic Resonance Imaging: When is it Helpful? Our Experience Over the Last Decade. Curr. Probl. Diagn. Radiol. 2019, 48, 196–203. [Google Scholar] [CrossRef]
- Önder, Ö.; Azizova, A.; Durhan, G.; Elibol, F.D.; Akpınar, M.G.; Demirkazık, F. Imaging findings and classification of the common and uncommon male breast diseases. Insights Imaging 2020, 11, 27. [Google Scholar] [CrossRef] [Green Version]
- Fentiman, I.S. The biology of male breast cancer. Breast 2018, 38, 132–135. [Google Scholar] [CrossRef]
- Cho, S.Y.; Lee, J.H.; Ryu, J.M.; Lee, J.E.; Cho, E.Y.; Ahn, C.H.; Paeng, K.; Yoo, I.; Ock, C.Y.; Song, S.Y. Deep learning from HE slides predicts the clinical benefit from adjuvant chemotherapy in hormone receptor-positive breast cancer patients. Sci. Rep. 2021, 11, 17363. [Google Scholar] [CrossRef]
- Zhong, E.; Cheng, E.; Goldfischer, M.; Hoda, S.A. Papillary Lesions of the Male Breast: A Study of 117 Cases and Brief Review of the Literature Demonstrate a Broad Clinicopathologic Spectrum. Am. J. Surg. Pathol. 2020, 44, 68–76. [Google Scholar] [CrossRef]
- Avau, F.; Chintinne, M.; Baudry, S.; Buxant, F. Literature review and case report of bilateral intracystic papillary carcinoma associated with an invasive ductal carcinoma in a male breast. Breast Dis. 2022, 41, 5–13. [Google Scholar] [CrossRef]
- Akinseye, O.; Hayes, J.C. Male with Metastases to the Breasts. J. Breast Imaging 2020, 2, 515–516. [Google Scholar] [CrossRef]
- Anagnostopoulou, V.; Mantha, N.; Sapalidis, K.; Tolparidou, E.; Georgiou, E.; Koletsa, T. Male breast involvement in chronic lymphocytic leukemia. A case report and review of the literature. Rom. J. Morphol. Embryol. 2020, 61, 241–245. [Google Scholar] [CrossRef]
- Coopey, S.B.; Kartal, K.; Li, C.; Yala, A.; Barzilay, R.; Faulkner, H.R.; King, T.A.; Acevedo, F.; Garber, J.E.; Guidi, A.J.; et al. Atypical ductal hyperplasia in men with gynecomastia: What is their breast cancer risk? Breast Cancer Res. Treat. 2019, 175, 1–4. [Google Scholar] [CrossRef]
- Wu, J.; Chen, H.; Dong, J.; Cao, Y.; Li, W.; Zhang, F.; Zeng, X. Axillary masses as clinical manifestations of male sweat gland carcinoma associated with extramammary Paget’s disease and accessory breast carcinoma: Two cases report and literature review. World J. Surg. Oncol. 2022, 20, 109. [Google Scholar] [CrossRef]
- Carder, P.J.; Shaaban, A. Mesenchymal lesions of the breast. Diagn. Histopathol. 2019, 25, 123–131. [Google Scholar] [CrossRef]
- Raj, S.D.; Sweetwood, K.; Kapoor, M.M.; Raj, K.M.; Nagi, C.; Sepulveda, K.A.; Sedgwick, E.L. Spindle cell lesions of the breast: Multimodality imaging and clinical differentiation of pathologically similar neoplasms. Eur. J. Radiol. 2017, 90, 60–72. [Google Scholar] [CrossRef]
- Panigrahi, C.; Jha, S.; Kumar, P.; Mishra, T.S.; Sasmal, P.K.; Adhya, A.K. Squamous Metaplasia in a Borderline Phyllodes Tumor-an Undocumented Histological Finding in Male Breast: Report of a Case and Review of Literature. Int. J. Surg. Pathol. 2022, 30, 106–113. [Google Scholar] [CrossRef]
- Lerwill, M.F.; Lee, A.H.S.; Tan, P.H. Fibroepithelial tumours of the breast—A review. Virchows Arch. 2022, 480, 45–63. [Google Scholar] [CrossRef]
- Ma, X.; Shen, L.; Hu, F.; Tang, W.; Gu, Y.; Peng, W. Predicting the pathological grade of breast phyllodes tumors: A nomogram based on clinical and magnetic resonance imaging features. Br. J. Radiol. 2021, 94, 1124. [Google Scholar] [CrossRef]
- Bouhani, M.; Fertani, Y.; Zemni, I.; Adouni, O.; Bouida, A.; Chargui, R.; Khaled, R. Dermatofibrosarcoma Protuberans of the Breast in Man: An Extremely Rare Entity With a Review of the Literature. J. Investig. Med. High Impact. Case Rep. 2019, 7, 2324709619875634. [Google Scholar] [CrossRef] [Green Version]
- Sung, T.H.T.; Tam, A.C.W.; Khoo, J.L.S. Dermatofibrosarcoma Protuberans: A comprehensive review on the spectrum of clinico-radiological presentations. J. Med. Imaging Radiat. Oncol. 2017, 61, 9–17. [Google Scholar] [CrossRef]
- Vergine, M.; Musella, A.; Gulotta, E.; Frusone, F.; De Luca, A.; Maceli, F.; Libia, A.; Benedetti Panici, P.; Monti, M. Paget’s disease of the male breast: Case report and a point of view from actual literature. Il G. Di Chir.-J. Ital. Surg. Assoc. 2018, 39, 114–117. Available online: https://pubmed.ncbi.nlm.nih.gov/29694313/ (accessed on 11 May 2022).
- Adams, S.J.; Kanthan, R. Paget’s disease of the male breast in the 21st century: A systematic review. Breast 2016, 29, 14–23. [Google Scholar] [CrossRef]
- Eggemann, H.; Ignatov, A.; Smith, B.J.; Altmann, U.; von Minckwitz, G.; Röhl, F.W.; Jahn, M.; Costa, S.D. Adjuvant therapy with tamoxifen compared to aromatase inhibitors for 257 male breast cancer patients. Breast Cancer Res. Treat. 2012, 137, 465–470. [Google Scholar] [CrossRef]
- Hassett, M.J.; Somerfield, M.R.; Baker, E.R.; Cardoso, F.; Kansal, K.J.; Kwait, D.C.; Plichta, J.K.; Ricker, C.; Roshal, A.; Ruddy, K.J.; et al. Management of Male Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2020, 38, 1849–1863. [Google Scholar] [CrossRef]
- Trapani, D.; Douillard, J.Y.; Winer, E.P.; Burstein, H.; Carey, L.A.; Cortes, J.; Lopes, G.; Gralow, J.R.; Gradishar, W.J.; Magrini, N.; et al. The Global Landscape of Treatment Standards for Breast Cancer. JNCI J. Natl. Cancer Inst. 2021, 113, 1143–1155. [Google Scholar] [CrossRef]
- Reinisch, M.; Seiler, S.; Hauzenberger, T.; Kamischke, A.; Schmatloch, S.; Strittmatter, H.J.; Zahm, D.M.; Thode, C.; Furlanetto, J.; Strik, D.; et al. Efficacy of Endocrine Therapy for the Treatment of Breast Cancer in Men: Results from the MALE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 565–572. [Google Scholar] [CrossRef]
- Duso, B.A.; Trapani, D.; Marra, A.; D’Amico, P.; Guerini Rocco, E.; Fusco, N.; Mazzarella, L.; Criscitiello, C.; Esposito, A.; Curigliano, G. Pharmacological management of male breast cancer. Expert Opin. Pharmacother. 2020, 21, 1493–1504. [Google Scholar] [CrossRef]
- Khan, N.A.J.; Tirona, M. An updated review of epidemiology, risk factors, and management of male breast cancer. Med. Oncol. 2021, 38, 39. [Google Scholar] [CrossRef]
- Lin, A.P.; Huang, T.W.; Tam, K.W. Treatment of male breast cancer: Meta-analysis of real-world evidence. Br. J. Surg. 2021, 108, 1034–1042. [Google Scholar] [CrossRef]
- Carter, M.; Reyna, C.; Shaughnessy, E.; Hanseman, D.; Meier, T.; Barrord, M.; Lewis, J.D. Trends and Outcomes Associated with Axillary Management of Males with Clinical N0 Breast Cancer—An NCDB Analysis. J. Surg. Res. 2021, 268, 97–104. [Google Scholar] [CrossRef]
- Gherghe, M.; Bordea, C.; Blidaru, A. Clinical significance of the lymphoscintigraphy in the evaluation of non-axillary sentinel lymph node localization in breast cancer. Chirurgia 2015, 110, 26–32. [Google Scholar]
- Bordea, C.; Pleæca, M.; Condrea, I.; Gherghe, M.; Gociman, A.; Blidaru, A. Occult breast lesion localization and concomitant sentinel lymph node biopsy in early breast cancer (SNOLL). Chirurgia 2012, 109, 722–729. [Google Scholar] [CrossRef]
- Fentiman, I.S. Surgical options for male breast cancer. Breast Cancer Res Treat. 2018, 172, 539–544. [Google Scholar] [CrossRef] [Green Version]
- Williams, A.D.; Ciocca, R.; Sabol, J.L.; Carp, N.Z. The use of neoadjuvant therapy increases the rate of breast conservation in men with locally advanced breast cancer. Clin. Breast Cancer 2022, 22, 343–358. [Google Scholar] [CrossRef]
- Bakalov, V.; Jayakrishnan, T.T.; Abel, S.; Hilton, C.; Rusia, B.; Wegner, R.E. The use of adjuvant radiation therapy in male breast cancer and its impact on outcomes. Cancer Treat. Res. Commun. 2021, 27, 100359. [Google Scholar] [CrossRef]
- Sauder, C.A.M.; Bateni, S.B.; Davidson, A.J.; Nishijima, D.K. Breast Conserving Surgery Compared with Mastectomy in Male Breast Cancer: A Brief Systematic Review. Clin. Breast Cancer 2020, 20, e309–e314. [Google Scholar] [CrossRef]
- de La Cruz, L.M.; Thiruchelvam, P.T.R.; Shivani, J.; Trina, J.; Blankenship, S.A.; Fisher, C.S. Saving the Male Breast: A Systematic Literature Review of Breast-Conservation Surgery for Male Breast Cancer. Ann. Surg. Oncol. 2019, 26, 3939–3944. [Google Scholar] [CrossRef]
- Deldar, R.; Sayyed, A.A.; Towfighi, P.; Aminpour, N.; Sogunro, O.; Son, J.D.; Fan, K.L.; Song, D.H. Postmastectomy Reconstruction in Male Breast Cancer. Breast J. 2022, 2022, 5482261. [Google Scholar] [CrossRef]
- Dreger, N.M.; Degener, S.; Roth, S.; Ahmad-Nejad, P.; Kamper, L.; Müller, E.; von Rundstedt, F.C.; Brandt, A.S. Impact of CYP2D6 Polymorphisms on Tamoxifen Treatment in Patients with Retroperitoneal Fibrosis: A First Step Towards Tailored Therapy? Urology 2020, 137, 84–90. [Google Scholar] [CrossRef]
- Sanchez-Spitman, A.B.; Swen, J.J.; Dezentje, V.O.; Moes, D.J.A.R.; Gelderblom, H.; Guchelaar, H.J. Clinical pharmacokinetics and pharmacogenetics of tamoxifen and endoxifen. Expert Rev. Clin. Pharmacol. 2019, 12, 523–536. [Google Scholar] [CrossRef]
- Ahmed, N.S.; Elghazawy, N.H.; ElHady, A.K.; Engel, M.; Hartmann, R.W.; Abadi, A.H. Design and synthesis of novel tamoxifen analogues that avoid CYP2D6 metabolism. Eur. J. Med. Chem. 2016, 112, 171–179. [Google Scholar] [CrossRef]
- Jayaraman, S.; Reid, J.M.; Hawse, J.R.; Goetz, M.P. Endoxifen, an Estrogen Receptor Targeted Therapy: From Bench to Bedside. Endocrinology 2021, 162, bqab191. [Google Scholar] [CrossRef]
- Kraus, A.L.; Yu-Kite, M.; Mardekian, J.; Cotter, M.J.; Kim, S.; Decembrino, J.; Snow, T.; Carson, K.R.; Motyl Rockland, J.; Gossai, A.; et al. Real-World Data of Palbociclib in Combination with Endocrine Therapy for the Treatment of Metastatic Breast Cancer in Men. Clin. Pharmacol. Ther. 2022, 111, 302–309. [Google Scholar] [CrossRef]
- Campone, M.; De Laurentiis, M.; Zamagni, C.; Kudryavcev, I.; Agterof, M.; Brown-Glaberman, U.; Palácová, M.; Chatterjee, S.; Menon-Singh, L.; Wu, J.; et al. Ribociclib plus letrozole in male patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: Subgroup analysis of the phase IIIb CompLEEment-1 trial. Breast Cancer Res. Treat. 2022, 193, 95–103. [Google Scholar] [CrossRef]
- T’Sjoen, G.; Arcelus, J.; Gooren, L.; Klink, D.T.; Tangpricha, V. Endocrinology of Transgender Medicine. Endocr. Rev. 2019, 40, 97–117. [Google Scholar] [CrossRef] [Green Version]
- Parikh, U.; Mausner, E.; Chhor, C.M.; Gao, Y.; Karrington, I.; Heller, S.L. Breast Imaging in Transgender Patients: What the Radiologist Should Know. Radiographics 2020, 40, 13–27. [Google Scholar] [CrossRef]
- Ramos, D.M.; Monterde, L.S.; García, R.M.; Vidagany, N.E.; Piqueres, C.S.; Marti, R.Q.; Sastre, M.L.; Sos, J.E. Cáncer de mama en pacientes transgénero. Revisión de la literatura. Rev. Senología Patología Mamar. 2019, 32, 140–144. [Google Scholar] [CrossRef]
- Hartley, R.L.; Stone, J.P.; Temple-Oberle, C. Breast cancer in transgender patients: A systematic review. Part 1: Male to female. Eur. J. Surg. Oncol. 2018, 44, 1455–1462. [Google Scholar] [CrossRef]
- Ali, N.; Sindhu, K.; Bakst, R.L. A Rare Case of a Transgender Female with Breast Implant-Associated Anaplastic Large Cell Lymphoma Treated with Radiotherapy and a Review of the Literature. J. Investig. Med. High Impact Case Rep. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Stone, J.P.; Hartley, R.L.; Temple-Oberle, C. Breast cancer in transgender patients: A systematic review. Part 2: Female to Male. Eur. J. Surg. Oncol. 2018, 44, 1463–1468. [Google Scholar] [CrossRef]
- Fledderus, A.C.; Gout, H.A.; Ogilvie, A.C.; van Loenen, D.K.G. Breast malignancy in female-to-male transsexuals: Systematic review, case report, and recommendations for screening. Breast 2020, 53, 92–100. [Google Scholar] [CrossRef]
- Patel, H.; Arruarana, V.; Yao, L.; Cui, X.; Ray, E. Effects of hormones and hormone therapy on breast tissue in transgender patients: A concise review. Endocrine 2020, 68, 6–15. [Google Scholar] [CrossRef]
- Grenader, T.; Goldberg, A.; Shavit, L. Second cancers in patients with male breast cancer: A literature review. J. Cancer Surviv. 2008, 2, 73–78. [Google Scholar] [CrossRef]
- O’Leary, T.R.; Shriver, C.D.; Wind, G. Metachronous Contralateral Male Breast Cancer: Case Report and Literature Review. Mil. Med. 2019, 184, E578–E583. [Google Scholar] [CrossRef] [Green Version]
- Charalambous, A.; Giannakopoulou, M.; Bozas, E.; Paikousis, L. Parallel and serial mediation analysis between pain, anxiety, depression, fatigue and nausea, vomiting and retching within a randomised controlled trial in patients with breast and prostate cancer. BMJ Open 2019, 9, e026809. [Google Scholar] [CrossRef] [Green Version]
- Benassai, G.; Miletti, A.; Calemma, F.; Furino, E.; de Palma, G.D.; Quarto, G. Male breast cancer: An update. Ann. Ital. Chir. 2020, 91, 359–365. Available online: https://pubmed.ncbi.nlm.nih.gov/33055389/ (accessed on 11 May 2022).
- Malinda, S.; Vithana, P.; Chathuranga, L.S.; Jayasinghe, S.; Arachchige, E.; Udayakumara, D. Male breast cancer: A Sri Lankan case report and review of literature. 2022, 11, BMT61. Breast Cancer Manag. [CrossRef]
- Yao, N.; Shi, W.; Liu, T.; Siyin, S.T.; Wang, W.; Duan, N.; Xu, G.; Qu, J. Clinicopathologic characteristics and prognosis for male breast cancer compared to female breast cancer. Sci. Rep. 2022, 12, 220. [Google Scholar] [CrossRef]
- Ali, A.; Xie, Z.; Stanko, L.; De Leo, E.; Hong, Y.R.; Bian, J.; Daily, K.C. Endocrine adherence in male versus female breast cancer: A seer-medicare review. Breast Cancer Res. Treat. 2022, 192, 491–499. [Google Scholar] [CrossRef]
- Pensabene, M.; Von Arx, C.; De Laurentiis, M. Male Breast Cancer: From Molecular Genetics to Clinical Management. Cancers 2022, 14, 2006. [Google Scholar] [CrossRef]
- Stahl, K.; Dodge, D.; Wong, W.; Shen, C. ASO Author Reflection: Trimodality Therapy Offers Survival Advantage in Metastatic Male Breast Cancer. Ann. Surg. Oncol. 2021, 29, 1018. [Google Scholar] [CrossRef] [PubMed]
- Zografos, E.; Proikakis, S.C.; Anagnostopoulos, A.K.; Korakiti, A.M.; Zagouri, F.; Gazouli, M.; Tsangaris, G.T. High-throughput Proteomic Profiling of Male Breast Cancer Tissue. Cancer Genom. Proteom. 2022, 19, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ricciardelli, C.; Yool, A.J. Targeting Aquaporins in Novel Therapies for Male and Female Breast and Reproductive Cancers. Cells 2021, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Magouliotis, D.E.; Tasiopoulou, V.S.; Svokos, A.A.; Svokos, K.A. Aquaporins in health and disease. Adv. Clin. Chem. 2020, 98, 149–171. [Google Scholar] [CrossRef]
- Jung, H.J.; Jang, H.J.; Kwon, T.H. Aquaporins implicated in the cell proliferation and the signaling pathways of cell stemness. Biochimie 2021, 188, 52–60. [Google Scholar] [CrossRef]
- Ala, M.; Mohammad Jafari, R.; Hajiabbasi, A.; Dehpour, A.R. Aquaporins and diseases pathogenesis: From trivial to undeniable involvements, a disease-based point of view. J. Cell. Physiol. 2021, 236, 6115–6135. [Google Scholar] [CrossRef]
- Bystrup, M.; Login, F.H.; Edamana, S.; Borgquist, S.; Tramm, T.; Kwon, T.H.; Nejsum, L.N. Aquaporin-5 in breast cancer. APMIS 2022, 130, 253–260. [Google Scholar] [CrossRef]
- Traberg-Nyborg, L.; Login, F.H.; Edamana, S.; Tramm, T.; Borgquist, S.; Nejsum, L.N. Aquaporin-1 in breast cancer. APMIS 2022, 130, 3–10. [Google Scholar] [CrossRef]
- Milković, L.; Gašparović, A.Č. AQP3 and AQP5-Potential Regulators of Redox Status in Breast Cancer. Molecules 2021, 26, 2613. [Google Scholar] [CrossRef]
- Moosavi, M.S.; Elham, Y. Aquaporins 1, 3 and 5 in Different Tumors, their Expression, Prognosis Value and Role as New Therapeutic Targets. Pathol. Oncol. Res. 2020, 26, 615–625. [Google Scholar] [CrossRef]
- Zhu, L.; Ma, N.; Wang, B.; Wang, L.; Zhou, C.; Yan, Y.; He, J.; Ren, Y. Significant prognostic values of aquaporin mRNA expression in breast cancer. Cancer Manag. Res. 2019, 11, 1503–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forooshani, M.K.; Scarpitta, R.; Fanelli, G.N.; Miccoli, M.; Naccarato, A.G.; Scatena, C. Is It Time to Consider the Androgen Receptor as a Therapeutic Target in Breast Cancer? Anti-Cancer Agents Med. Chem. 2021, 22, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Yardley, D.A.; Young, R.R.; Adelson, K.B.; Silber, A.L.; Najera, J.E.; Daniel, D.B.; Peacock, N.; Finney, L.; Hoekstra, S.J.; Shastry, M.; et al. A Phase II Study Evaluating Orteronel, an Inhibitor of Androgen Biosynthesis, in Patients with Androgen Receptor (AR)-Expressing Metastatic Breast Cancer (MBC). Clin. Breast Cancer 2022, 22, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Nachmanson, D.; Officer, A.; Mori, H.; Gordon, J.; Evans, M.F.; Steward, J.; Yao, H.; O’Keefe, T.; Hasteh, F.; Stein, G.S.; et al. The breast pre-cancer atlas illustrates the molecular and micro-environmental diversity of ductal carcinoma in situ. NPJ Breast Cancer 2022, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.; Decock, J. Targeting of lactate dehydrogenase C dysregulates the cell cycle and sensitizes breast cancer cells to DNA damage response targeted therapy. Mol. Oncol. 2022, 16, 885–903. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.J.; Li, A.H.; Li, P.; Sun, H. Therapeutic Targeting of Alternative Splicing: A New Frontier in Cancer Treatment. Front. Oncol. 2022, 12, 1349. [Google Scholar] [CrossRef]
- Hong, Z.; Liu, T.; Wan, L.; Fa, P.; Kumar, P.; Cao, Y.; Prasad, C.B.; Qiu, Z.; Liu, J.; Wang, H.; et al. Targeting Squalene Epoxidase Interrupts Homologous Recombination via the ER Stress Response and Promotes Radiotherapy Efficacy. Cancer Res. 2022, 82, 1298–1312. [Google Scholar] [CrossRef]
- Fan, P.; Jordan, V.C. Estrogen Receptor and the Unfolded Protein Response: Double-Edged Swords in Therapy for Estrogen Receptor-Positive Breast Cancer. Target. Oncol. 2022, 17, 111–124. [Google Scholar] [CrossRef]
- Direito, I.; Fardilha, M.; Helguero, L.A. Contribution of the unfolded protein response to breast and prostate tissue homeostasis and its significance to cancer endocrine response. Carcinogenesis 2019, 40, 203–215. [Google Scholar] [CrossRef]
- Lim, S.A.; Zhou, J.; Martinko, A.J.; Wang, Y.H.; Filippova, E.V.; Steri, V.; Wang, D.; Remesh, S.G.; Liu, J.; Hann, B.; et al. Targeting a proteolytic neoepitope on CUB domain containing protein 1 (CDCP1) for RAS-driven cancers. J. Clin. Investig. 2022, 132, e154604. [Google Scholar] [CrossRef]
- Praveenkumar, E.; Gurrapu, N.; Kolluri, P.K.; Shivaraj; Subhashini, N.J.P.; Dokala, A. Selective CDK4/6 inhibition of novel 1,2,3-triazole tethered acridinedione derivatives induces G1/S cell cycle transition arrest via Rb phosphorylation blockade in breast cancer models. Bioorg. Chem. 2021, 116, 105377. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Zaib, S.; Javed, M.; Rashid, F.; Iqbal, J.; Ibrar, A. Antiproliferative and Pro-Apoptotic Effects of Thiazolo[3,2-b][1,2,4]triazoles in Breast and Cervical Cancer Cells. Anticancer Agents Med. Chem. 2021, 21, 2181–2191. [Google Scholar] [CrossRef] [PubMed]
- Gowhari Shabgah, A.; Amir, A.; Gardanova, Z.R.; Olegovna Zekiy, A.; Thangavelu, L.; Ebrahimi Nik, M.; Ahmadi, M.; Gholizadeh Navashenaq, J. Interleukin-25: New perspective and state-of-the-art in cancer prognosis and treatment approaches. Cancer Med. 2021, 10, 5191–5202. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Li, J.; Gong, S.Y.; Huang, M.; Li, R.; Xiong, G.X.; Wang, F.; Zou, Q.M.; Qi, Q.; Yin, X.X. Targeting the ILK/YAP axis by LFG-500 blocks epithelial-mesenchymal transition and metastasis. Acta Pharmacol. Sin. 2021, 42, 1847–1859. [Google Scholar] [CrossRef]
- Marmé, F. Antibody-Drug Conjugates for Breast Cancer. Oncol. Res. Treat. 2022, 45, 26–36. [Google Scholar] [CrossRef]
- Cha, J.H.; Chan, L.C.; Wang, Y.N.; Chu, Y.Y.; Wang, C.H.; Lee, H.H.; Xia, W.; Shyu, W.C.; Liu, S.P.; Yao, J.; et al. Ephrin receptor A10 monoclonal antibodies and the derived chimeric antigen receptor T cells exert an antitumor response in mouse models of triple-negative breast cancer. J. Biol. Chem. 2022, 298, 101817. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionescu, S.; Nicolescu, A.C.; Marincas, M.; Madge, O.-L.; Simion, L. An Update on the General Features of Breast Cancer in Male Patients—A Literature Review. Diagnostics 2022, 12, 1554. https://doi.org/10.3390/diagnostics12071554
Ionescu S, Nicolescu AC, Marincas M, Madge O-L, Simion L. An Update on the General Features of Breast Cancer in Male Patients—A Literature Review. Diagnostics. 2022; 12(7):1554. https://doi.org/10.3390/diagnostics12071554
Chicago/Turabian StyleIonescu, Sinziana, Alin Codrut Nicolescu, Marian Marincas, Octavia-Luciana Madge, and Laurentiu Simion. 2022. "An Update on the General Features of Breast Cancer in Male Patients—A Literature Review" Diagnostics 12, no. 7: 1554. https://doi.org/10.3390/diagnostics12071554
APA StyleIonescu, S., Nicolescu, A. C., Marincas, M., Madge, O.-L., & Simion, L. (2022). An Update on the General Features of Breast Cancer in Male Patients—A Literature Review. Diagnostics, 12(7), 1554. https://doi.org/10.3390/diagnostics12071554






