Feasibility of Differential Dose—Volume Histogram Features in Multivariate Prediction Model for Radiation Pneumonitis Occurrence
Abstract: Purpose
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
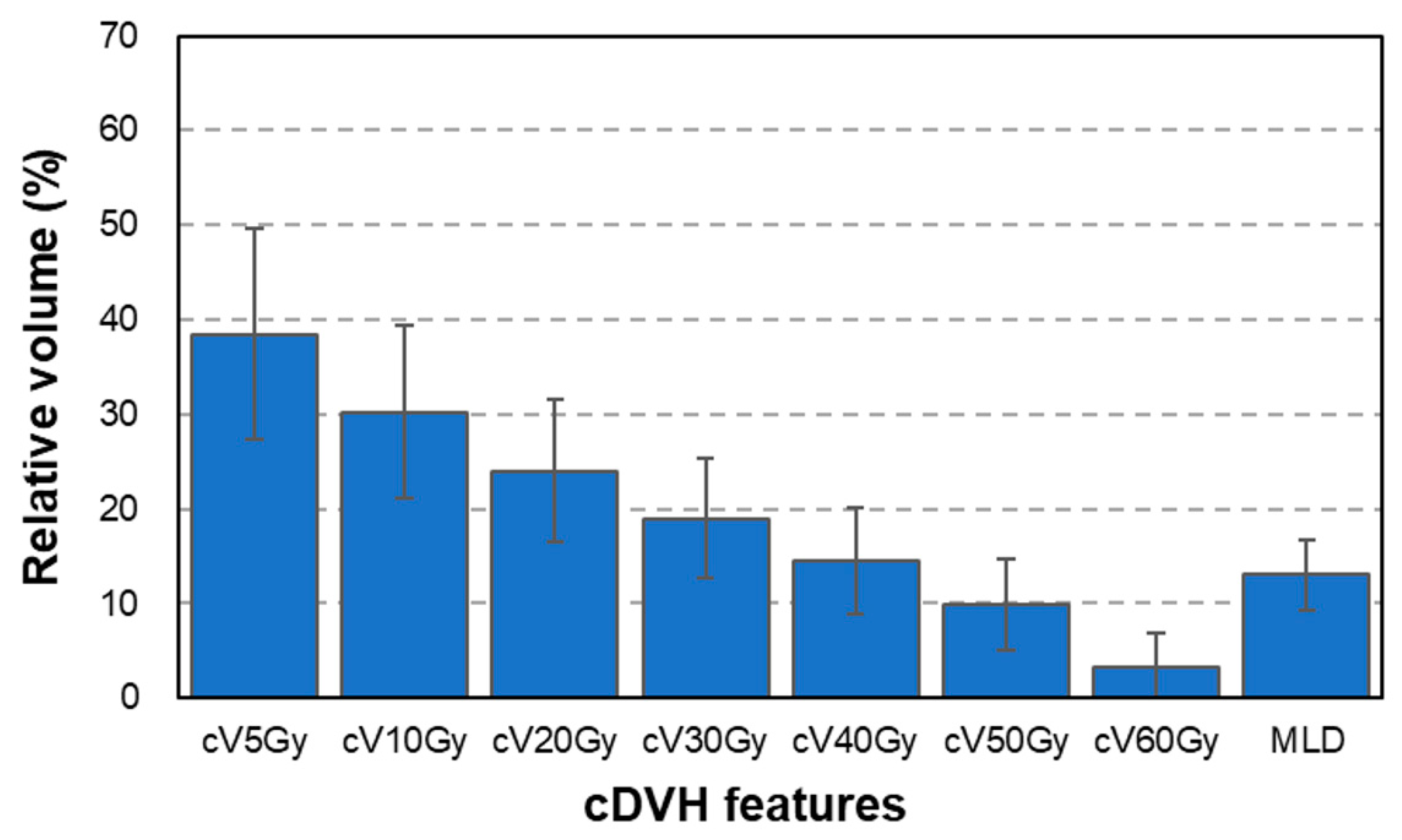
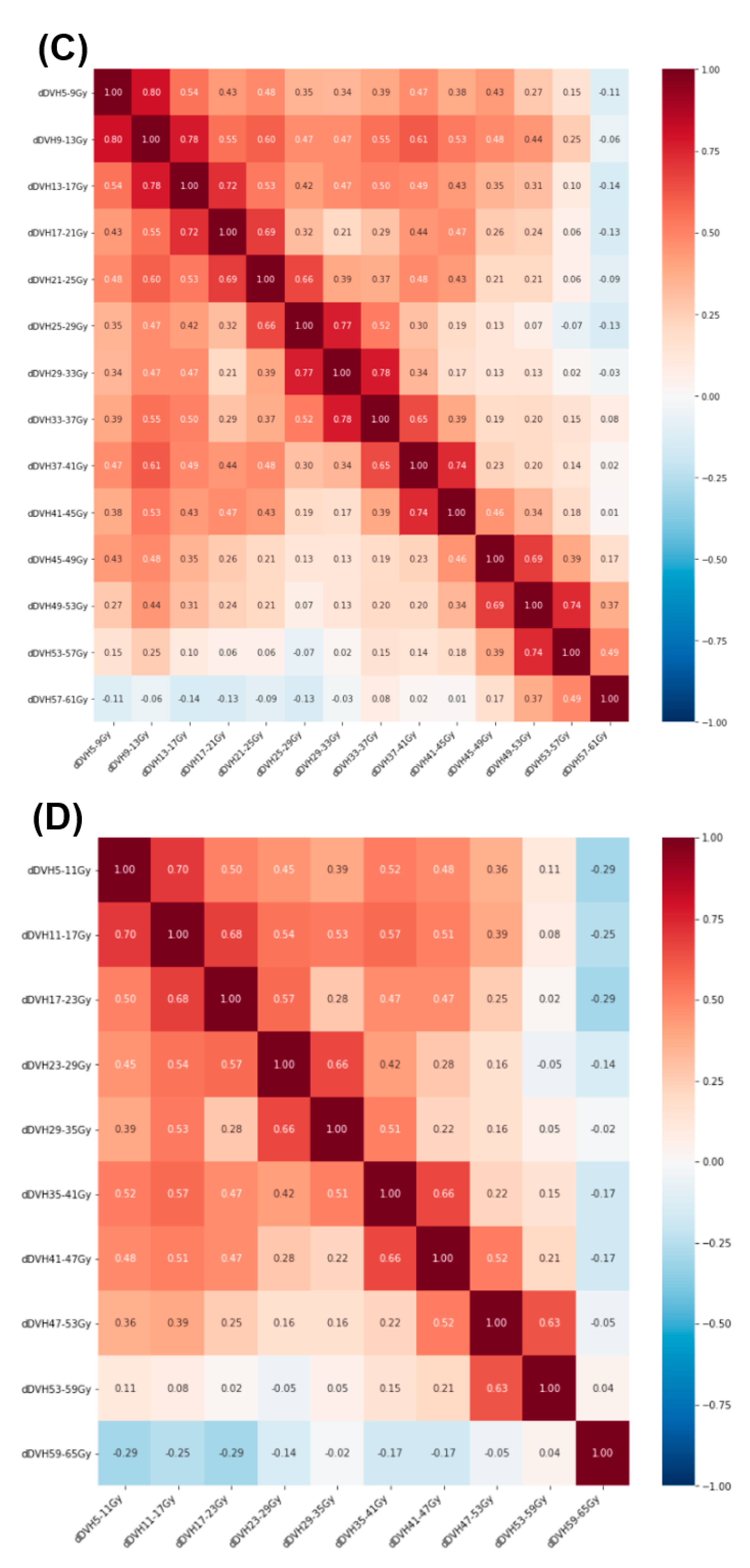
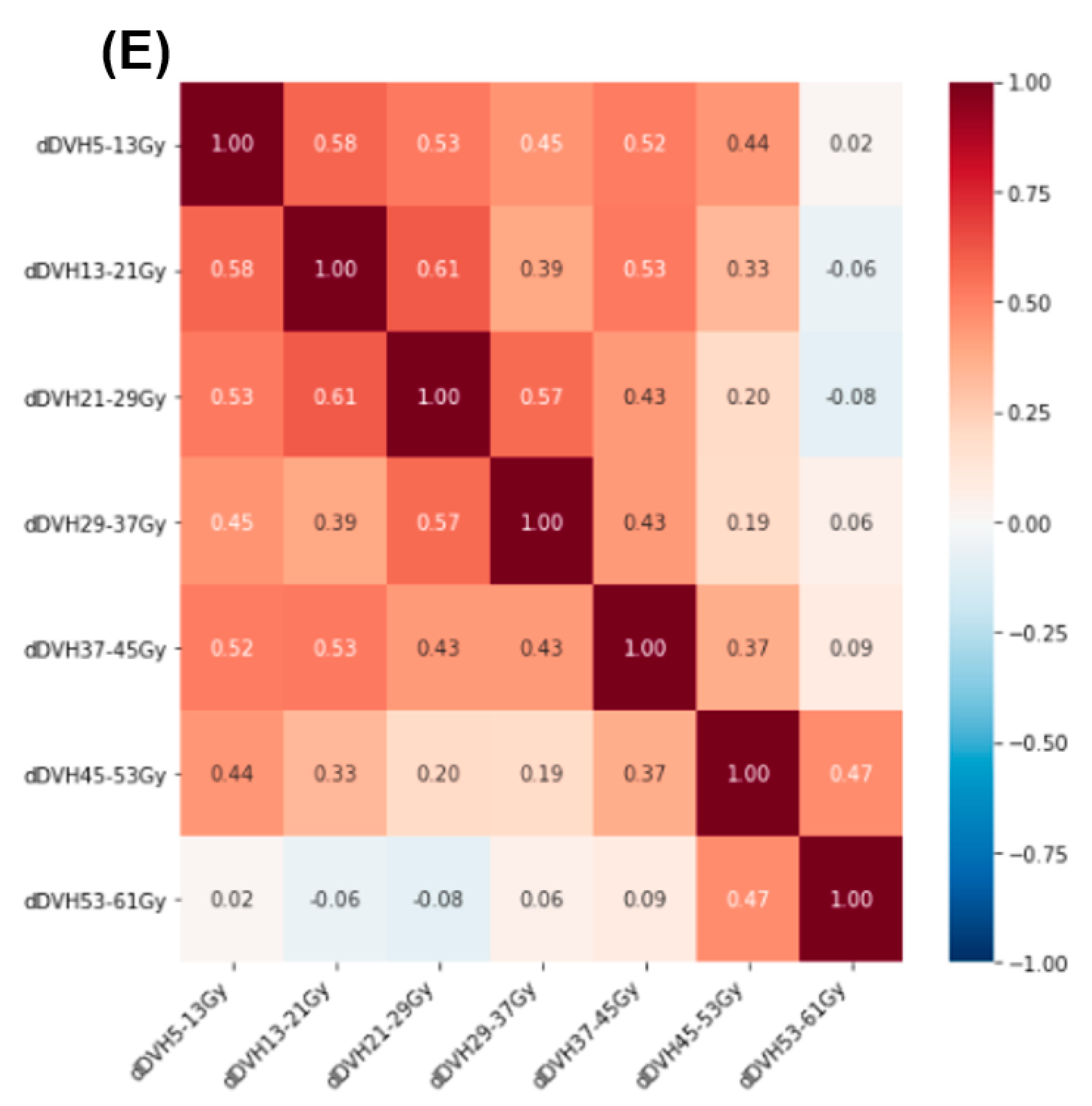
2.2. Radiation Treatment Planning and Dose–Volume Histogram Features
2.3. Prediction Model Development
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Graham, M.V.; Purdy, J.A.; Emami, B.; Harms, W.; Bosch, W.; Lockett, M.A.; Perez, C.A. Clinical dose–volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int. J. Radiat. Oncol. 1999, 45, 323–329. [Google Scholar] [CrossRef]
- Hernando, M.L.; Marks, L.B.; Bentel, G.C.; Zhou, S.-M.; Hollis, D.; Das, S.K.; Fan, M.; Munley, M.T.; Shafman, T.D.; Anscher, M.; et al. Radiation-induced pulmonary toxicity: A dose-volume histogram analysis in 201 patients with lung cancer. Int. J. Radiat. Oncol. 2001, 51, 650–659. [Google Scholar] [CrossRef]
- Marks, L.B.; Bentzen, S.M.; Deasy, J.O.; Kong, F.-M.; Bradley, J.D.; Vogelius, I.S.; El Naqa, I.; Hubbs, J.L.; Lebesque, J.V.; Timmerman, R.D.; et al. Radiation Dose–Volume Effects in the Lung. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S70–S76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yom, S.; Liao, Z.; Liu, H.H.; Tucker, S.L.; Hu, C.; Wei, X.; Wang, X.; Wang, S.; Mohan, R.; Cox, J.D.; et al. Initial Evaluation of Treatment-Related Pneumonitis in Advanced-Stage Non–Small-Cell Lung Cancer Patients Treated With Concurrent Chemotherapy and Intensity-Modulated Radiotherapy. Int. J. Radiat. Oncol. 2007, 68, 94–102. [Google Scholar] [CrossRef]
- Krafft, S.P.; Rao, A.; Stingo, F.; Briere, T.M.; Court, L.E.; Liao, Z.; Martel, M.K. The utility of quantitative CT radiomics features for improved prediction of radiation pneumonitis. Med. Phys. 2018, 45, 5317–5324. [Google Scholar] [CrossRef] [PubMed]
- Luna, J.M.; Chao, H.-H.; Diffenderfer, E.S.; Valdes, G.; Chinniah, C.; Ma, G.; Cengel, K.A.; Solberg, T.D.; Berman, A.T.; Simone, C. Predicting radiation pneumonitis in locally advanced stage II–III non-small cell lung cancer using machine learning. Radiother. Oncol. 2019, 133, 106–112. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, S.; Yin, F.F.; Marks, L.B.; Das, S.K. Investigation of the support vector machine algorithm to predict lung radiation-induced pneumonitis. Med. Phys. 2007, 34, 3808–3814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benadjaoud, M.A.; Blanchard, P.; Schwartz, B.; Champoudry, J.; Bouaita, R.; Lefkopoulos, D.; Deutsch, E.; Diallo, I.; Cardot, H.; de Vathaire, F. Functional Data Analysis in NTCP Modeling: A New Method to Explore the Radiation Dose-Volume Effects. Int. J. Radiat. Oncol. 2014, 90, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Chalkidou, A.; O’Doherty, M.J.; Marsden, P.K. False Discovery Rates in PET and CT Studies with Texture Features: A Systematic Review. PLoS ONE 2015, 10, e0124165. [Google Scholar] [CrossRef] [Green Version]
- Matzner-Lober, E.; Suehs, C.; Dohan, A.; Molinari, N. Thoughts on entering correlated imaging variables into a multivariable model: Application to radiomics and texture analysis. Diagn. Interv. Imaging 2018, 99, 269–270. [Google Scholar] [CrossRef]
- Tucker, S.L.; Dong, L.; Cheung, R.; Johnson, J.; Mohan, R.; Huang, E.H.; Liu, H.H.; Thames, H.D.; Kuban, D. Comparison of rectal dose–wall histogram versus dose–volume histogram for modeling the incidence of late rectal bleeding after radiotherapy. Int. J. Radiat. Oncol. 2004, 60, 1589–1601. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.G.; Hu, C.; Choy, H.; Komaki, R.U.; Timmerman, R.D.; Schild, S.E.; Bogart, J.A.; Dobelbower, M.C.; Bosch, W.; Galvin, J.M.; et al. Impact of Intensity-Modulated Radiation Therapy Technique for Locally Advanced Non–Small-Cell Lung Cancer: A Secondary Analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J. Clin. Oncol. 2017, 35, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Schwartz, R.; Flickinger, J.; Beriwal, S. Machine Learning Approaches for Predicting Radiation Therapy Outcomes: A Clinician’s Perspective. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Shi, W.; Peng, X.; Zhan, Y.; Zhou, L.; Wang, Y.; Feng, M.; Zhao, J.; Shan, F.; Liu, L. Development and Validation a Nomogram Incorporating CT Radiomics Signatures and Radiological Features for Differentiating Invasive Adenocarcinoma From Adenocarcinoma In Situ and Minimally Invasive Adenocarcinoma Presenting as Ground-Glass Nodules Measuring 5–10 mm in Diameter. Front. Oncol. 2021, 11, 618677. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Lin, P.; Wu, L.; Wan, D.; Zhao, Y.; Liang, L.; Ma, X.; Qin, H.; Liu, Y.; Li, X.; et al. Ultrasound-Based Radiomics Analysis for Preoperatively Predicting Different Histopathological Subtypes of Primary Liver Cancer. Front. Oncol. 2020, 10, 1646. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Chen, Y.; Tan, J.; Wang, L.; Zhang, J.; Yang, C.; Ma, Q.; Ge, Y.; Xu, Z.; et al. The Performance of a Dual-Energy CT Derived Radiomics Model in Differentiating Serosal Invasion for Advanced Gastric Cancer Patients After Neoadjuvant Chemotherapy: Iodine Map Combined With 120-kV Equivalent Mixed Images. Front. Oncol. 2021, 10, 562945. [Google Scholar] [CrossRef] [PubMed]
- Seppenwoolde, Y.; Shirato, H.; Kitamura, K.; Shimizu, S.; van Herk, M.; Lebesque, J.V.; Miyasaka, K. Precise and real-time measurement of 3D tumor motion in lung due to breathing and heartbeat, measured during radiotherapy. Int. J. Radiat. Oncol. 2002, 53, 822–834. [Google Scholar] [CrossRef]
- Hope, A.J.; Lindsay, P.E.; El Naqa, I.; Alaly, J.R.; Vicic, M.; Bradley, J.D.; Deasy, J. Modeling radiation pneumonitis risk with clinical, dosimetric, and spatial parameters. Int. J. Radiat. Oncol. 2006, 65, 112–124. [Google Scholar] [CrossRef] [PubMed]







| Characteristic | n | % | |
|---|---|---|---|
| Gender | Male | 130 | 85.0 |
| Female | 23 | 15.0 | |
| RP classification | <Grade 2 | 112 | 73.2 |
| ≥Grade 2 | 41 | 26.8 | |
| Smoking history | Yes | 97 | 63.4 |
| No | 56 | 36.6 | |
| Total dose | 60 Gy | 120 | 78.4 |
| 66 Gy | 33 | 21.6 | |
| Dose per fraction | 2.0 Gy | 152 | 99.3 |
| 1.8 Gy | 1 | 0.7 |
| Developed Model | AUC Mean (95% CI) | |
|---|---|---|
| Training Partition | Testing Partition | |
| cDVH | 0.62 (0.58–0.65) | 0.60 (0.57–0.63) |
| +dDVH (bin = 2 Gy) | 0.71 (0.69–0.74) | 0.72 (0.70–0.74) |
| +dDVH (bin = 4 Gy) | 0.73 (0.71–0.76) | 0.73 (0.72–0.74) |
| +dDVH (bin = 6 Gy) | 0.69 (0.66–0.71) | 0.69 (0.66–0.72) |
| +dDVH (bin = 8 Gy) | 0.68 (0.65–0.72) | 0.69 (0.67–0.72) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsuta, Y.; Kadoya, N.; Sugai, Y.; Katagiri, Y.; Yamamoto, T.; Takeda, K.; Tanaka, S.; Jingu, K. Feasibility of Differential Dose—Volume Histogram Features in Multivariate Prediction Model for Radiation Pneumonitis Occurrence. Diagnostics 2022, 12, 1354. https://doi.org/10.3390/diagnostics12061354
Katsuta Y, Kadoya N, Sugai Y, Katagiri Y, Yamamoto T, Takeda K, Tanaka S, Jingu K. Feasibility of Differential Dose—Volume Histogram Features in Multivariate Prediction Model for Radiation Pneumonitis Occurrence. Diagnostics. 2022; 12(6):1354. https://doi.org/10.3390/diagnostics12061354
Chicago/Turabian StyleKatsuta, Yoshiyuki, Noriyuki Kadoya, Yuto Sugai, Yu Katagiri, Takaya Yamamoto, Kazuya Takeda, Shohei Tanaka, and Keiichi Jingu. 2022. "Feasibility of Differential Dose—Volume Histogram Features in Multivariate Prediction Model for Radiation Pneumonitis Occurrence" Diagnostics 12, no. 6: 1354. https://doi.org/10.3390/diagnostics12061354
APA StyleKatsuta, Y., Kadoya, N., Sugai, Y., Katagiri, Y., Yamamoto, T., Takeda, K., Tanaka, S., & Jingu, K. (2022). Feasibility of Differential Dose—Volume Histogram Features in Multivariate Prediction Model for Radiation Pneumonitis Occurrence. Diagnostics, 12(6), 1354. https://doi.org/10.3390/diagnostics12061354






