Association between Nuclear Morphometry Parameters and Gleason Grade in Patients with Prostatic Cancer
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients and Tissue Samples
2.2. Data Acquisition
2.3. Statistical Analysis
3. Results
3.1. General Characteristics of the Study Population
3.2. Difference between Groups Using Univariant Analysis
3.3. Indicators of Nuclear Size
3.4. Variables Characterizing the Optical Density (Gray Levels)
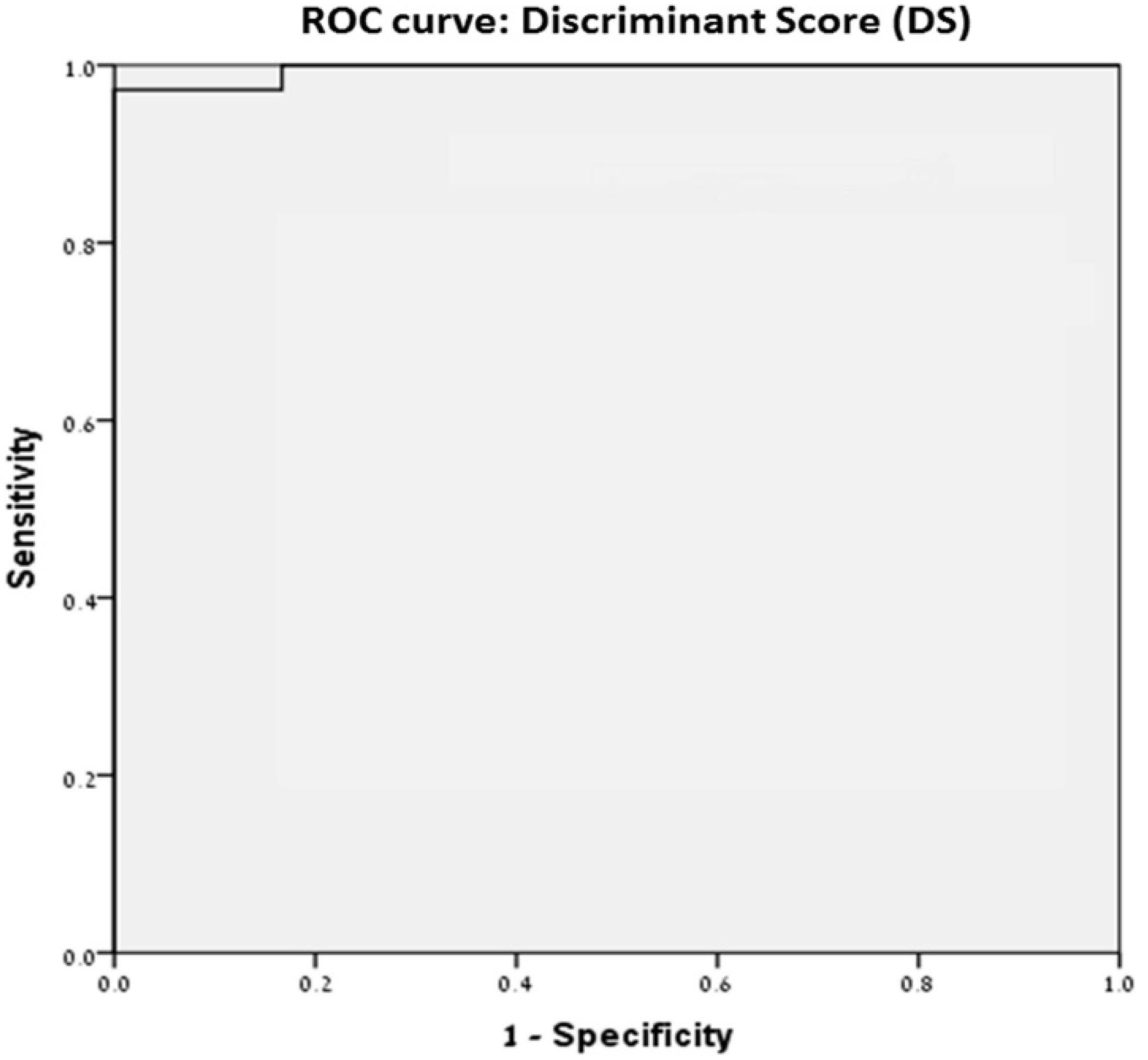
3.5. Discriminate Score
1479.27930311731) + (Gray Level (Min) × 0.40274897061) + (Gray Level (Max) × 0.53105523026) +
(Margination × 275.38324653827) − (Gray Level (Green) × 0.89343166330) + (Gray Level (Blue) ×
0.51289341737)
4. Discussion
4.1. The Relationship between the Increase in the Nucleus Size and Tumor Progression
4.2. The Relationship between Chromatin Density and Overall Quantity and Gleason Score
4.3. Discriminant Score
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA A Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Veltri, R.W.; Christudass, C.S.; Isharwal, S. Nuclear morphometry, nucleomics and prostate cancer progression. Asian J. 2012, 14, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Van Leenders, G.; Van der Kwast, T.H.; Grignon, D. ISUP Grading Workshop Panel Members. The 2019 International Society of Urological Pathology (ISUP) Consensus Conference on Grading of Prostatic Carcinoma. Am. J. Surg. Pathol. 2020, 44, e87–e99. [Google Scholar] [CrossRef] [PubMed]
- Epstain, J.I.; Amin, M.B.; Fine, S.W.; Algaba, F.; Aron, M.; Baydar, D.E.; Lopez Beltran, A.; Brimo, F.; Cheville, J.C.; Colecchia, M.; et al. The 2019 Genitourinary Pathology Society (GUPS) White Paper on Contemporary Grading of Prostate Cancer. Arch. Pathol. Lab. Med. 2021, 145, 461–493. [Google Scholar] [CrossRef] [PubMed]
- Renshaw, A.A.; Schultz, D.; Cote, K.; Loffredo, M.; Ziemba, D.E.; D’amico, A.V. Accurate Gleason grading of prostatic adenocarcinoma in prostate needle biopsies by general pathologists. Arch. Pathol. Lab. Med. 2003, 127, 1007–1008. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Johnson, C.; Gaed, M.; Gómez, J.A.; Moussa, M.; Chin, J.L.; Pautler, S.; Bauman, G.S.; Ward, A.D. Histologic tissue components provide major cues for machine learning-based prostate cancer detection and grading on prostatectomy specimens. Sci. Rep. 2020, 10, 9911. [Google Scholar] [CrossRef] [PubMed]
- Egevad, L. Reproducibility of Gleason grading of prostate cancer can be improved by the use of reference images. Urology 2001, 57, 291–295. [Google Scholar] [CrossRef]
- Rahman, S.M.; Itakura, H. Morphometry in histopathology: An image analysis workstation for the pathology laboratory. Anal. Quant. Cytol. Histol. 1996, 18, 471–480. [Google Scholar] [PubMed]
- Ozer, E.; Yörükoğlu, K.; Sagol, O.; Mungan, U.; Demirel, D.; Tüzel, E.; Kirkali, Z. Prognostic significance of nuclear morphometry in renal cell carcinoma. BJU Int. 2002, 90, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Kronqvist, P.; Kuopio, T.; Jalava, P.; Collan, Y. Morphometrical malignancy grading is a valuable prognostic factor in invasive ductal breast cancer. Br. J. Cancer 2002, 87, 1275–1280. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ikeguchi, M.; Sakatani, T.; Endo, K.; Makino, M.; Kaibara, N. Computerized nuclear morphometry is a useful technique for evaluating the high metastatic potential of colorectal adenocarcinoma. Cancer 1999, 86, 1944–1951. [Google Scholar] [CrossRef]
- Epstein, J.I.; Zelefsky, M.J.; Sjoberg, D.D.; Nelson, J.B.; Egevad, L.; Magi-Galluzzi, C.; Vickers, A.; Parwani, A.V.; Reuter, V.E.; Fine, S.W.; et al. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur. Urol. 2015, 69, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Bostwick, D.G. Grading prostate cancer. Am. J. Clin. Pathol. 1994, 102, S38–S56. [Google Scholar] [PubMed]
- Humphrey, P.A. Gleason grading and prognostic factors in carcinoma of the prostate. Mod. Pathol. 2004, 17, 292–306. [Google Scholar] [CrossRef]
- Swanton, C. Intratumor heterogeneity: Evolution through space and time. Cancer Res. 2012, 72, 4875–4882. [Google Scholar] [CrossRef]
- Mohler, J.L.; Partin, A.W.; Lohr, W.D.; Coffey, D.S. Nuclear roundness factor measurement for assessment of prognosis of patients with prostatic carcinoma. Testing of a digitization system. J. Urol. 1988, 139, 1080–1084. [Google Scholar] [CrossRef]
- Mohler, J.L.; Partin, A.W.; Epstein, J.I.; Lohr, W.D.; Coffey, D.S. Nuclear roundness factor measurement for assessment of prognosis of patients with prostatic carcinoma. Standardization of methodology for histologic sections. J. Urol. 1988, 139, 1085–1090. [Google Scholar] [CrossRef]
- Bektas, S.; Bahadir, B.; Dogan Gun, B.; Kertis, G.; Oguz Ozdamar, S. The relation between Gleason score, and nuclear size and shape factors in prostatic adenocarcinoma. Turk. J. Med. Sci. 2009, 39, 381–387. [Google Scholar]
- Epstein, J.I.; Pizov, G.; Steinberg, G.D.; Carter, H.B.; Pitcock, R.; Armas, O.A.; Partin, A.; Walsh, P.C. Correlation of prostate cancer nucleus deoxyribinucleic acid, size, shape and Gleason grade with pathological stage at radical prostatectomy. J. Urol. 1992, 148, 87–91. [Google Scholar] [CrossRef]
- Pretorius, M.E.; Waehre, H.; Abeler, V.M.; Davidson, B.; Vlatkovic, L.; Lothe, R.A.; Giercksky, K.E.; Danielson, H.E. Large scale genomic instability as an additive prognostic marker in early prostate cancer. Cell Oncol. 2009, 31, 251–259. [Google Scholar] [CrossRef] [PubMed]
| Morphometric Variant | Description | Illustration | GleasonGrade 1–3 | GleasonGrade 4 | Gleason Grade 5 | p-Value (ANOVA) | Bonferroni Test |
|---|---|---|---|---|---|---|---|
| Nuclear Area | The area contained in the polygon encloses the object |  | 54.01 ± 7.48 | 61.16 ± 17.069 | 71.14 ± 148 | 0.006 | 3 vs. 5 |
| Nuclear Aspect | The relation between axial and ellipse axes to the object |  | 1.28 ± 0.072 | 1.22 ± 0.048 | 1.32 ± 0.10 | 0.000 | 3 vs. 4 4 vs. 5 |
| Area/Box | The ratio of the area to the area of an enclosing box |  | 0.745 ± 0.011 | 0.75 ± 0.006 | 0.741 ± 0.013 | 0.022 | 4 vs. 5 |
| Gray Level-Mean | The average density or intensity of an object |  | 126.37 ± 17.64 | 150.84 ± 17.87 | 122.10 ± 22.09 | 0.000 | 3 vs. 4/4 vs. 5 |
| Axis Major | The length of the main axis of an ellipse |  | 9.28 ± 0.61 | 9.60 ± 1.32 | 10.75 ± 1.77 | 0.002 | 3 vs. 5/4 vs. 5 |
| Axis Minor | The length of the secondary axis of an ellipse equals torque |  | 7.34 ± 0.59 | 7.91 ± 1.078 | 8.199 ± 1.126 | 0.016 | 3 vs. 5 |
| Maximal Diameter | The length of the longest line passing through the center |  | 9.26 ± 0.618 | 9.60 ± 1.34 | 10.733 ± 1.79 | 0.002 | 3 vs. 5/4 vs. 5 |
| Minimal Diameter | The length of theshortest line passing through the center |  | 7.02 ± 0.589 | 7.58 ± 1.043 | 7.83 ± 1.079 | 0.019 | 3 vs. 5 |
| Mean Diameter | Average of locomotives passing through the center |  | 8.06 ± 0.568 | 8.53 ± 1.16 | 9.14 ± 1.34 | 0.008 | 3 vs. 5 |
| Maximal Radius | Maximum distance from center to outline |  | 4.809 ± 0.319 | 4.97 ± 0.69 | 5.59 ± 0.945 | 0.002 | 3 vs. 5/4 vs. 5 |
| Minimal Radius | Minimum distance from center to outline |  | 3.36 ± 0.29 | 3.63 ± 0.5 | 3.72 ± 0.518 | 0.027 | 3 vs. 5 |
| Holes | Number of holes in the object |  | 26.36 ± 1.811 | 27.72 ± 3.83 | 30.199 ± 4.66 | 0.005 | 3 vs. 5 |
| Radius Ratio | The ratio between the maximum radius and the minimum |  | 1.466 ± 0.11 | 1.37 ± 0.0578 | 1.529 ± 0.139 | 0.000 | 3 vs. 4/4 vs. 5 |
| Nuclear Roundness | Roundness according to the ratio between the outline and the surface |  | 1.088 ± 0.027 | 1.06 ± 0.011 | 1.096 ± 0.027 | 0.001 | 3 vs. 4/4 vs. 5 |
| Gray Level–Red | Average red value in object |  | 146.34 ± 21.48 | 183.02 ± 29.21 | 140.06 ± 36.48 | 0.000 | 3 vs. 4/4 vs. 5 |
| Gray Level–Green | Average green value in object |  | 97.079 ± 22.5 | 114.22 ± 20.81 | 89.46 ± 21.71 | 0.03 | 3 vs. 4/4 vs. 5 |
| Gray Level–Blue | Average blue value in object |  | 135.68 ± 14.29 | 155.28 ± 13.96 | 136.79 ± 15.39 | 0.000 | 3 vs. 4/4 vs. 5 |
| Length | Evaluation of object length by descriptor |  | 9.28 ± 0.616 | 9.60 ± 1.33 | 10.76 ± 1.80 | 0.002 | 3 vs. 5/4 vs. 5 |
| Width | The width of the object is weighted by the curvature of a structure |  | 7.38 ± 0.587 | 7.94 ± 1.09 | 8.26 ± 1.159 | 0.015 | 3 vs. 5 |
| Perimeter2 | The chain code for the outline includes holes |  | 27.859 ± 1.925 | 29.357 ± 4.119 | 31.97 ± 4.889 | 0.004 | 3 vs. 5 |
| IOD | The optical density of the field |  | 6937.39 ± 1578 | 9426.03 ± 3208 | 8796.673 ± 3221 | 0.009 | 3 vs. 4 |
| Perimeter (convex) | The length of the convex outline of the object |  | 26.074 ± 1.80 | 27.42 ± 3.81 | 29.876 | 0.005 | 3 vs. 5 |
| Perimeter (ellipse) | Equivalent ellipse outline length |  | 26.23 ± 1.81 | 27.60 ± 3.75 | 29.95 ± 4.531 | 0.005 | 3 vs. 5 |
| Perimeter (ratio) | The ratio of the convex outline to the outline of the object |  | 51.69 ± 7.31 | 58.718 ± 16.75 | 68.49 ± 22.10 | 0.006 | 3 vs. 5 |
| Fractal Dimension | Fractal dimension of the object boundary |  | 1.058 ± 0.00159 | 1.057 ± 0.0017 | 1.056 ± 0.0013 | 0.002 | 3 vs. 5 |
| Box Width | Width of enclosing box |  | 8.54 ± 0.656 | 9.01 ± 1.141 | 9.7229 ± 1.50 | 0.006 | 3 vs. 5 |
| Box Height | Height of enclosing box |  | 8.40 ± 0.6106 | 8.82 ± 1.34 | 9.63 ± 1.568 | 0.008 | 3 vs. 5 |
| Feret (min) | The shortest distance in the defined direction |  | 7.277 ± 0.58 | 7.82 ± 1.077 | 8.14 ± 1.135 | 0.016 | 3 vs. 5 |
| Feret (mean) | Average distances in different directions |  | 8.383 ± 0.576 | 8.816 ± 1.21 | 9.596 ± 1.47 | 0.005 | 3 vs. 5 |
| Gray Level (min) | Density or minimal power in the object |  | 85.54 ± 13.98 | 102.94 ± 15.25 | 82.446 ± 20.027 | 0.000 | 3 vs. 4/4 vs. 5 |
| Gray Level (max) | Density or maximum intensity in the object |  | 178.70 ± 15.86 | 205.66 ± 15.05 | 180.61 ± 18.85 | 0.000 | 3 vs. 4/4 vs. 5 |
| Gray Level (std.dv) | Standard Deviation The density or intensity of an object |  | 17.53 ± 2.4255 | 20.12 ± 3.188 | 17.88 ± 2.03 | 0.005 | 3 vs. 4/4 vs. 5 |
| Margination | Dissemination of the intensity ratios between center and perimeter |  | 0.3400 ± 0.015 | 0.334 ± 016 | 0.352 ± 0.0158 | 0.004 | 4 vs. 5 |
| Heterogeneity | Percentage of pixels that deviate above 10% |  | 0.148 ± 0.0154 | 0.208 ± 0.0176 | 0.152 ± 0.0447 | 0.004 | 3 vs. 4/4 vs. 5 |
| Clumpiness | Reflects texture variation |  | 0.122 ± 0.0163 | 0.178 ± 0.107 | 0.110 ± 0.061 | 0.025 | 4 vs. 5 |
| Gray Level (sum) | Total density or optical intensity |  | 270125 ± 61457 | 367027 ± 12494 | 342521 ± 12542 | 0.009 | 3 vs. 4 |
| Perimeter3 | Corrected chain code for outline, without holes |  | 26.270 ± 1.811 | 27.611 ± 3.82 | 30.09 ± 4.66 | 0.005 | 3 vs. 5 |
| Perimeter Length | Evaluation of object length by layout |  | 13.135 ± 0.90 | 13.80 ± 1.91 | 15.14 ± 2.333 | 0.005 | 3 vs. 5 |
| Independent Variable | Beta, Slope (Incline) | p Value |
|---|---|---|
| Minimal Radius | 5.9556 | <0.0001 |
| Gray Level (Green) | −0.8934 | <0.0001 |
| Gray Level (Blue) | 0.512 | <0.0001 |
| Fractal Dimension | −1479.2 | <0.0001 |
| Gray Level (Min) | 0.4027 | <0.0001 |
| Gray Level (Max) | 0.531 | <0.0001 |
| Margination | 275.38 | <0.0001 |
| Point of intersection with the Y-axis (Constant) | 1331.5 |
| Gleason Grade | Gray Level Green | Optic Density | Nuclear Area | Total Chromatin Quantity |
|---|---|---|---|---|
| 3 | 97 | 256 − 97 = 159 | 54 | 8586 |
| 4 | 114 | 256 − 114 = 142 | 61 | 8662 * |
| 5 | 89 | 256 − 89 = 167 | 71 | 11,857 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malshy, K.; Amiel, G.E.; Hershkovitz, D.; Sabo, E.; Hoffman, A. Association between Nuclear Morphometry Parameters and Gleason Grade in Patients with Prostatic Cancer. Diagnostics 2022, 12, 1356. https://doi.org/10.3390/diagnostics12061356
Malshy K, Amiel GE, Hershkovitz D, Sabo E, Hoffman A. Association between Nuclear Morphometry Parameters and Gleason Grade in Patients with Prostatic Cancer. Diagnostics. 2022; 12(6):1356. https://doi.org/10.3390/diagnostics12061356
Chicago/Turabian StyleMalshy, Kamil, Gilad E. Amiel, Dov Hershkovitz, Edmond Sabo, and Azik Hoffman. 2022. "Association between Nuclear Morphometry Parameters and Gleason Grade in Patients with Prostatic Cancer" Diagnostics 12, no. 6: 1356. https://doi.org/10.3390/diagnostics12061356
APA StyleMalshy, K., Amiel, G. E., Hershkovitz, D., Sabo, E., & Hoffman, A. (2022). Association between Nuclear Morphometry Parameters and Gleason Grade in Patients with Prostatic Cancer. Diagnostics, 12(6), 1356. https://doi.org/10.3390/diagnostics12061356






