Correlation of Optical Coherence Tomography Angiography Characteristics with Visual Function to Define Vision-Threatening Diabetic Macular Ischemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Visual Function Assessment
2.4. OCTA Acquisition
2.5. Statistical Analysis
3. Results
3.1. Demographics and Clinical Characteristics
3.2. The Correlation between Visual Performance and OCTA Parameters
3.3. Cutoff Points for Identifying Visual Impairment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EDTRS. Classification of Diabetic Retinopathy from Fluorescein Angiograms: ETDRS Report Number 11. Ophthalmology 1991, 98, 807–822. [Google Scholar] [CrossRef]
- Sim, D.A.; Keane, P.A.; Zarranz-Ventura, J.; Fung, S.; Powner, M.B.; Platteau, E.; Bunce, C.V.; Fruttiger, M.; Patel, P.J.; Tufail, A.; et al. The Effects of Macular Ischemia on Visual Acuity in Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2353–2360. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F. Retinal Vascular Cystoid Macular Edema: Review and New Theory. Retina 2016, 36, 1823–1842. [Google Scholar] [CrossRef]
- Cennamo, G.; Romano, M.R.; Nicoletti, G.; Velotti, N.; de Crecchio, G. Optical coherence tomography angiography versus fluorescein angiography in the diagnosis of ischaemic diabetic maculopathy. Acta Ophthalmol. 2017, 95, e36–e42. [Google Scholar] [CrossRef]
- Rocholz, R.; Corvi, F.; Weichsel, J.; Schmidt, S.; Staurenghi, G. OCT Angiography (OCTA) in retinal diagnostics. In High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics; Bille, J.F., Ed.; Springer: Cham, Switzlerland, 2019; pp. 135–160. [Google Scholar]
- Elnahry, A.G.; Ramsey, D.J. Optical coherence tomography angiography imaging of the retinal microvasculature is unimpeded by macular xanthophyll pigment. Clin. Exp. Ophthalmol. 2020, 48, 1012–1014. [Google Scholar] [CrossRef] [PubMed]
- Sabaner, M.C.; Dogan, M.; Akdogan, M.; Simsek, M. Panretinal laser photocoagulation decreases large foveal avascular zone area in non-proliferative diabetic retinopathy: A prospective OCTA study. Photodiagnosis Photodyn.Ther. 2021, 34, 102298. [Google Scholar] [CrossRef]
- Gross, J.G.; Glassman, A.R.; Liu, D.; Sun, J.K.; Antoszyk, A.N.; Baker, C.W.; Bressler, N.M.; Elman, M.J.; Ferris, F.L., III; Gardner, T.W.; et al. Five-Year Outcomes of Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA Ophthalmol. 2018, 136, 1138–1148. [Google Scholar] [CrossRef] [Green Version]
- Foote, K.G.; Loumou, P.; Griffin, S.; Qin, J.; Ratnam, K.; Porco, T.C.; Roorda, A.; Duncan, J.L. Relationship Between Foveal Cone Structure and Visual Acuity Measured With Adaptive Optics Scanning Laser Ophthalmoscopy in Retinal Degeneration. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3385–3393. [Google Scholar] [CrossRef] [Green Version]
- Abdelshafy, M.; Abdelshafy, A. Correlations Between Optical Coherence Tomography Angiography Parameters and the Visual Acuity in Patients with Diabetic Retinopathy. Clin. Ophthalmol. 2020, 14, 1107–1115. [Google Scholar] [CrossRef] [Green Version]
- Stockman, A.; Sharpe, L.T. Into the twilight zone: The complexities of mesopic vision and luminous efficiency. Ophthalmic. Physiol. Opt. 2006, 26, 225–239. [Google Scholar] [CrossRef]
- Karatsai, E.; Sen, P.; Gurudas, S.; Sivaprasad, S. Low Luminance Visual Acuity and Low Luminance Deficit in Proliferative Diabetic Retinopathy. J. Clin. Med. 2021, 10, 358. [Google Scholar] [CrossRef] [PubMed]
- Optovue. The New Gold Standard in OCTA Imaging. Available online: https://www.optovue.com/products/angiovue (accessed on 2 November 2021).
- Jia, Y.; Tan, O.; Tokayer, J.; Potsaid, B.; Wang, Y.; Liu, J.J.; Kraus, M.F.; Subhash, H.; Fujimoto, J.G.; Hornegger, J.; et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt. Express 2012, 20, 4710–4725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarinci, F.; Picconi, F.; Virgili, G.; Varano, M.; Giorno, P.; Frontoni, S.; Parravano, M. Microvascular impairment as a biomarker of diabetic retinopathy progression in the long-term follow up in type 1 diabetes. Sci. Rep. 2020, 10, 18266. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi Falavarjani, K.; Habibi, A.; Anvari, P.; Ghasemizadeh, S.; Ashraf Khorasani, M.; Shenazandi, H.; Sarraf, D. Effect of segmentation error correction on optical coherence tomography angiography measurements in healthy subjects and diabetic macular oedema. Br. J. Ophthalmol. 2020, 104, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Garrity, S.T.; Iafe, N.A.; Phasukkijwatana, N.; Chen, X.; Sarraf, D. Quantitative Analysis of Three Distinct Retinal Capillary Plexuses in Healthy Eyes Using Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5548–5555. [Google Scholar] [CrossRef] [Green Version]
- Camino, A.; Zhang, M.; Gao, S.S.; Hwang, T.S.; Sharma, U.; Wilson, D.J.; Huang, D.; Jia, Y. Evaluation of artifact reduction in optical coherence tomography angiography with real-time tracking and motion correction technology. Biomed. Opt. Express 2016, 7, 3905–3915. [Google Scholar] [CrossRef]
- Kashani, A.H.; Green, K.M.; Kwon, J.; Chu, Z.; Zhang, Q.; Wang, R.K.; Garrity, S.; Sarraf, D.; Rebhun, C.B.; Waheed, N.K.; et al. Suspended Scattering Particles in Motion: A Novel Feature of OCT Angiography in Exudative Maculopathies. Ophthalmol. Retin. 2018, 2, 694–702. [Google Scholar] [CrossRef]
- Yao, X.; Alam, M.N.; Le, D.; Toslak, D. Quantitative optical coherence tomography angiography: A review. Exp. Biol. Med. 2020, 245, 301–312. [Google Scholar] [CrossRef]
- Munk, M.R.; Kashani, A.H.; Tadayoni, R.; Korobelnik, J.F.; Wolf, S.; Pichi, F.; Tian, M. Standardization of OCT Angiography Nomenclature in Retinal Vascular Diseases: First Survey Results. Ophthalmol. Retin. 2021, 5, 981–990. [Google Scholar] [CrossRef]
- Ying, G.-S.; Maguire, M.G.; Glynn, R.J.; Rosner, B. Tutorial on Biostatistics: Receiver-Operating Characteristic (ROC) Analysis for Correlated Eye Data. Ophthalmic. Epidemiol. 2021, 28, 1–11. [Google Scholar] [CrossRef]
- Huang, F.L. Using Cluster Bootstrapping to Analyze Nested Data With a Few Clusters. Educ. Psychol. Meas. 2018, 78, 297–318. [Google Scholar] [CrossRef] [PubMed]
- Efron, B. Better Bootstrap Confidence Intervals. J. Am. Stat. Assoc. 1987, 82, 171–185. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC. 2017. Available online: https://www.stata.com/support/faqs/resources/citing-software-documentation-faqs/ (accessed on 2 November 2021).
- McGee, S. Simplifying likelihood ratios. J. Gen. Intern. Med. 2002, 17, 646–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samara, W.A.; Shahlaee, A.; Adam, M.K.; Khan, M.A.; Chiang, A.; Maguire, J.I.; Hsu, J.; Ho, A.C. Quantification of Diabetic Macular Ischemia Using Optical Coherence Tomography Angiography and Its Relationship with Visual Acuity. Ophthalmology 2017, 124, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, D.J.; Arden, G.B. Hypoxia and Dark Adaptation in Diabetic Retinopathy: Interactions, Consequences, and Therapy. Curr. Diab. Rep. 2015, 15, 118. [Google Scholar] [CrossRef] [PubMed]
- Sivaprasad, S.; Arden, G. Spare the rods and spoil the retina: Revisited. Eye 2016, 30, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Shen, M.; Pan, D.; Xu, S.-Z.; Shen, R.-J.; Shao, Y.; Shi, C.; Lu, F.; Jin, Z.-B. Relationship Between Cone Loss and Microvasculature Change in Retinitis Pigmentosa. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4520–4531. [Google Scholar] [CrossRef] [Green Version]
- Ingram, N.T.; Fain, G.L.; Sampath, A.P. Elevated energy requirement of cone photoreceptors. Proc. Natl. Acad. Sci. USA 2020, 117, 19599–19603. [Google Scholar] [CrossRef]
- Frenkel, R.E.; Shapiro, H.; Stoilov, I. Predicting vision gains with anti-VEGF therapy in neovascular age-related macular degeneration patients by using low-luminance vision. Br. J. Ophthalmol. 2016, 100, 1052–1057. [Google Scholar] [CrossRef]
- Sunness, J.S.; Rubin, G.S.; Broman, A.; Applegate, C.A.; Bressler, N.M.; Hawkins, B.S. Low luminance visual dysfunction as a predictor of subsequent visual acuity loss from geographic atrophy in age-related macular degeneration. Ophthalmology 2008, 115, 1480–1488. [Google Scholar] [CrossRef] [Green Version]
- Ragkousis, A.; Kozobolis, V.; Kabanarou, S.; Bontzos, G.; Mangouritsas, G.; Heliopoulos, I.; Chatziralli, I. Vessel Density around Foveal Avascular Zone as a Potential Imaging Biomarker for Detecting Preclinical Diabetic Retinopathy: An Optical Coherence Tomography Angiography Study. Semin. Ophthalmol. 2020, 35, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, M.; Chung, H.; Kim, H.C. Quantification Of Retinal Vessel Tortuosity In Diabetic Retinopathy Using Optical Coherence Tomography Angiography. Retina-J. Ret. Vit. Dis. 2017, 38, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Le, D.; Lim, J.I.; Yao, X. Vascular Complexity Analysis In Optical Coherence Tomography Angiography Of Diabetic Retinopathy. Retina 2021, 41, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, G.; Montorio, D.; Fossataro, F.; Fossataro, C.; Tranfa, F. Evaluation of vessel density in disorganization of retinal inner layers after resolved diabetic macular edema using optical coherence tomography angiography. PLoS ONE 2021, 16, e0244789. [Google Scholar] [CrossRef] [PubMed]
- Pauleikhoff, D.; Bonelli, R.; Dubis, A.M.; Gunnemann, F.; Rothaus, K.; Charbel Issa, P.; Heeren, T.F.; Peto, T.; Clemons, T.E.; Chew, E.Y.; et al. Progression characteristics of ellipsoid zone loss in macular telangiectasia type 2. Acta Ophthalmol. 2019, 97, e998–e1005. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.K.; Lin, M.M.; Lammer, J.; Prager, S.; Sarangi, R.; Silva, P.S.; Aiello, L.P. Disorganization of the Retinal Inner Layers as a Predictor of Visual Acuity in Eyes With Center-Involved Diabetic Macular Edema. JAMA Ophthalmol. 2014, 132, 1309–1316. [Google Scholar] [CrossRef] [Green Version]
- Moein, H.R.; Novais, E.A.; Rebhun, C.B.; Cole, E.D.; Louzada, R.N.; Witkin, A.J.; Baumal, C.R.; Duker, J.S.; Waheed, N.K. Optical Coherence Tomography Angiography to Detect Macular Capillary Ischemia in Patients with Inner Retinal Changes after Resolved Diabetic Macular Edema. Retina 2018, 38, 2277–2284. [Google Scholar] [CrossRef]
- Nesper, P.L.; Scarinci, F.; Fawzi, A.A. Adaptive Optics Reveals Photoreceptor Abnormalities in Diabetic Macular Ischemia. PLoS ONE 2017, 12, e0169926. [Google Scholar] [CrossRef] [Green Version]
- Cheung, G.; Pearce, E.; Fenner, B.; Sen, P.; Chong, V.; Sivaprasad, S. Looking Ahead: Visual and Anatomical Endpoints in Future Trials of Diabetic Macular Ischemia. Ophthalmologica 2021, 24, 24. [Google Scholar] [CrossRef]

| Demographics | Results | N |
| PATIENTS | 87 | |
| Age (years) (mean ± SD) | 56.1 ± 12.5 | 87 |
| >50 years | 70.1% | 61 |
| Males | 59.8% | 52 |
| Females | 40.2% | 35 |
| T1DM | 37% | 32 |
| T2DM | 63% | 55 |
| Duration of diabetes (years) (mean ± SD) | 27.1 ± 13.2 | 78 |
| Bilateral eyes | 52.9% | 46 |
| Ocular Characteristics | Results | N |
| EYES | 123 | |
| BCVA (ETDRS Letters) (mean ± SD) | 76 ± 10 | 123 |
| ≥70 letters | 82.1% | 101 |
| <70 letters | 17.9% | 22 |
| LLVA (ETDRS Letters) (mean ± SD) | 66 ± 12 | 121 |
| ≥70 letters | 47.1% | 57 |
| <70 letters | 52.9% | 64 |
| LLD (ETDRS Letters) (median [IQR]) | 10 [6,7,8,9,10,11,12,13] | 121 |
| ≥10 letters | 48.8% | 62 |
| <10 letters | 51.2% | 59 |
| Pseudophakia | 47.2% | 58 |
| OCTA (3 × 3 mm scan) | Results | N a |
| Image quality | 7 ± 1 | 115 |
| FAZ area (mm2) | 0.57 ± 0.38 | 115 |
| FAZ perimeter (mm) | 3.37 ± 1.30 | 115 |
| FAZ–AI | 1.30 ± 0.18 | 115 |
| Whole image SVC VD (%) | 36.29 ± 5.31 | 115 |
| Whole image DVC VD (%) | 41.81 ± 4.88 | 115 |
| Parafoveal SVC VD (%) | 38.15 ± 5.97 | 115 |
| Parafoveal DVC VD (%) | 43.43 ± 5.01 | 115 |
| FD–300 (%) | 43.61 ± 4.92 | 115 |
| Whole image SVC VD/DVC VD ratio | 0.87 ± 0.12 | 115 |
| SVC VD/DVC VD > 1.0 | 16.5% | 19 |
| SVC VD/DVC VD ≤ 1.0 | 83.5% | 96 |
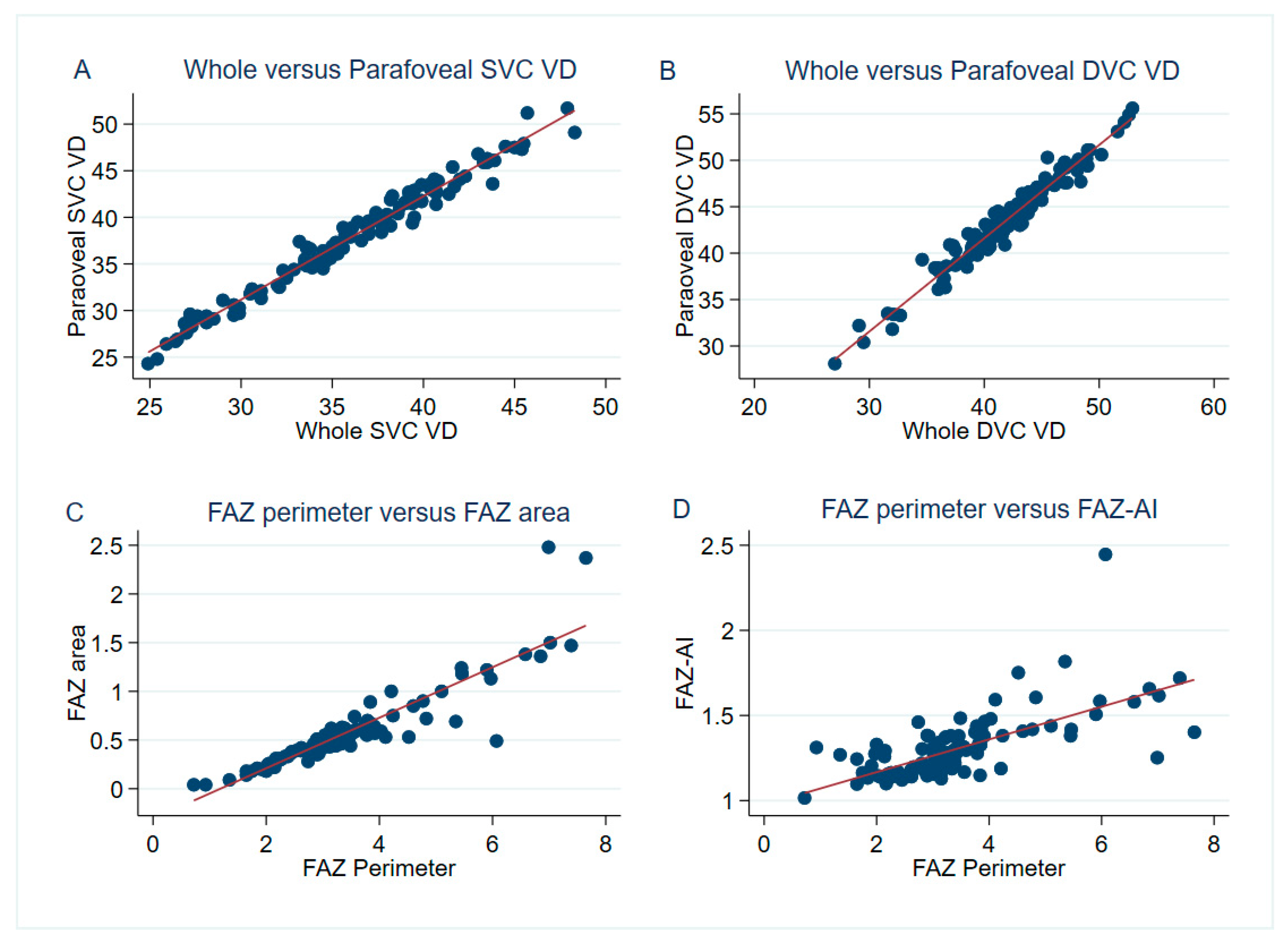
| Pearson Correlation | BCVA | LLVA | FAZ Area | FAZ Perimeter | FAZ–AI | wi SVC VD | wi DVC VD | para SVC VD | para DVC VD | FD–300 |
|---|---|---|---|---|---|---|---|---|---|---|
| BCVA | 1.00 | |||||||||
| LLVA | 0.79 *** | 1.00 | ||||||||
| FAZ area | −0.33 ** | –0.27 ** | 1.00 | |||||||
| FAZ perimeter | –0.33 ** | –0.30 ** | 0.90 ** | |||||||
| FAZ–AI | –0.18 | –0.20 * | 0.35 * | 0.68 *** | 1.00 | |||||
| wi SVC VD | 0.30 ** | 0.34 ** | –0.32 *** | –0.32 ** | –0.09 | 1.00 | ||||
| wi DVC VD | 0.43 *** | 0.42 *** | –0.48 *** | –0.45 *** | –0.13 | 0.50 *** | 1.00 | |||
| para SVC VD | 0.29 ** | 0.31 *** | –0.29 ** | –0.27 ** | –0.07 | 0.99 *** | 0.47 *** | 1.00 | ||
| para DVC VD | 0.39 *** | 0.38 *** | –0.43 *** | –0.37 *** | –0.07 | 0.46 *** | 0.98 *** | 0.44 *** | 1.00 | |
| FD–300 | 0.27 ** | 0.23 * | 0.12 | 0.15 | 0.18 | 0.54 *** | 0.48 *** | 0.54 *** | 0.50 *** | 1.00 |
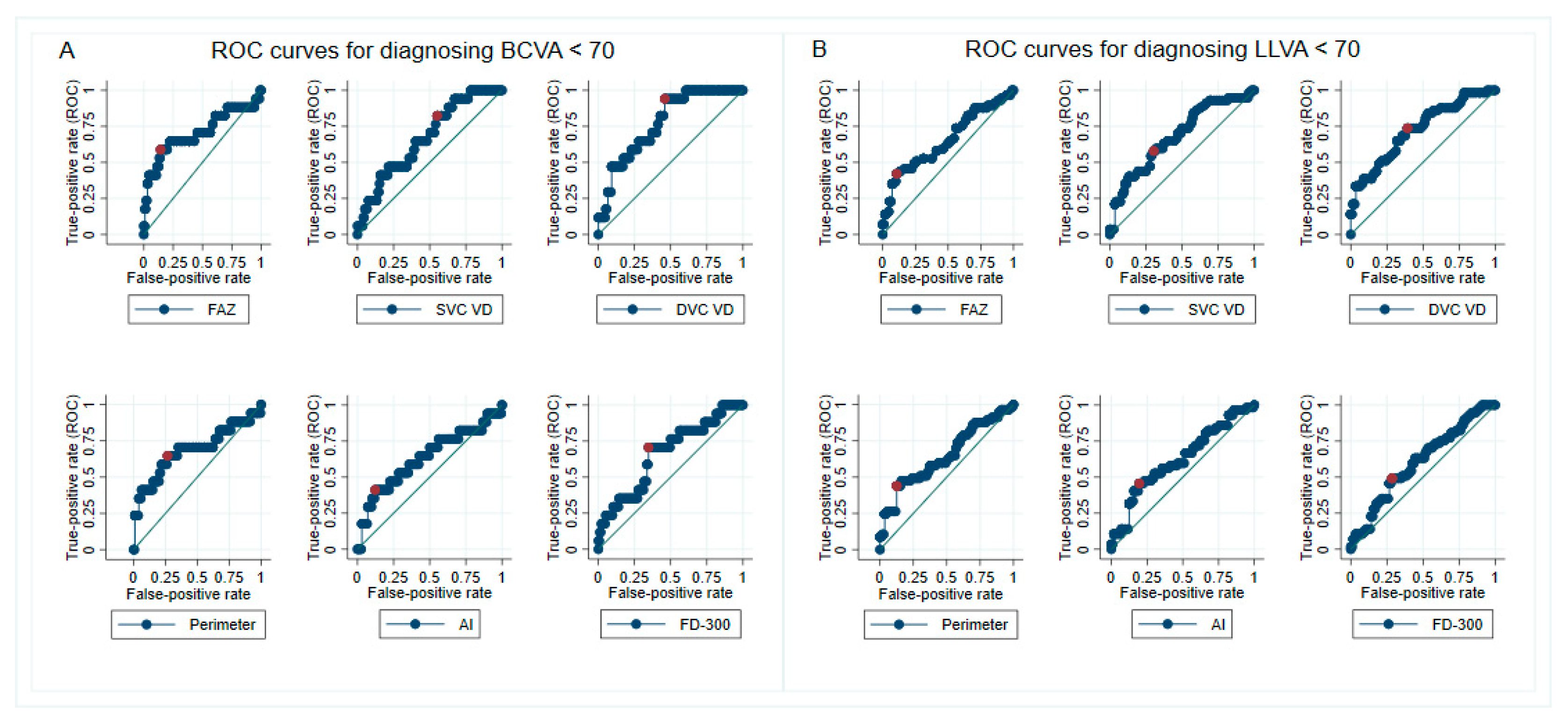
| For Diagnosing BCVA < 70 ETDRS Letters | |||||||
| Parameters | AUC (95% CI) a | Cutoff Point | Sensitivity | Specificity | PPV | NPV | LR+ |
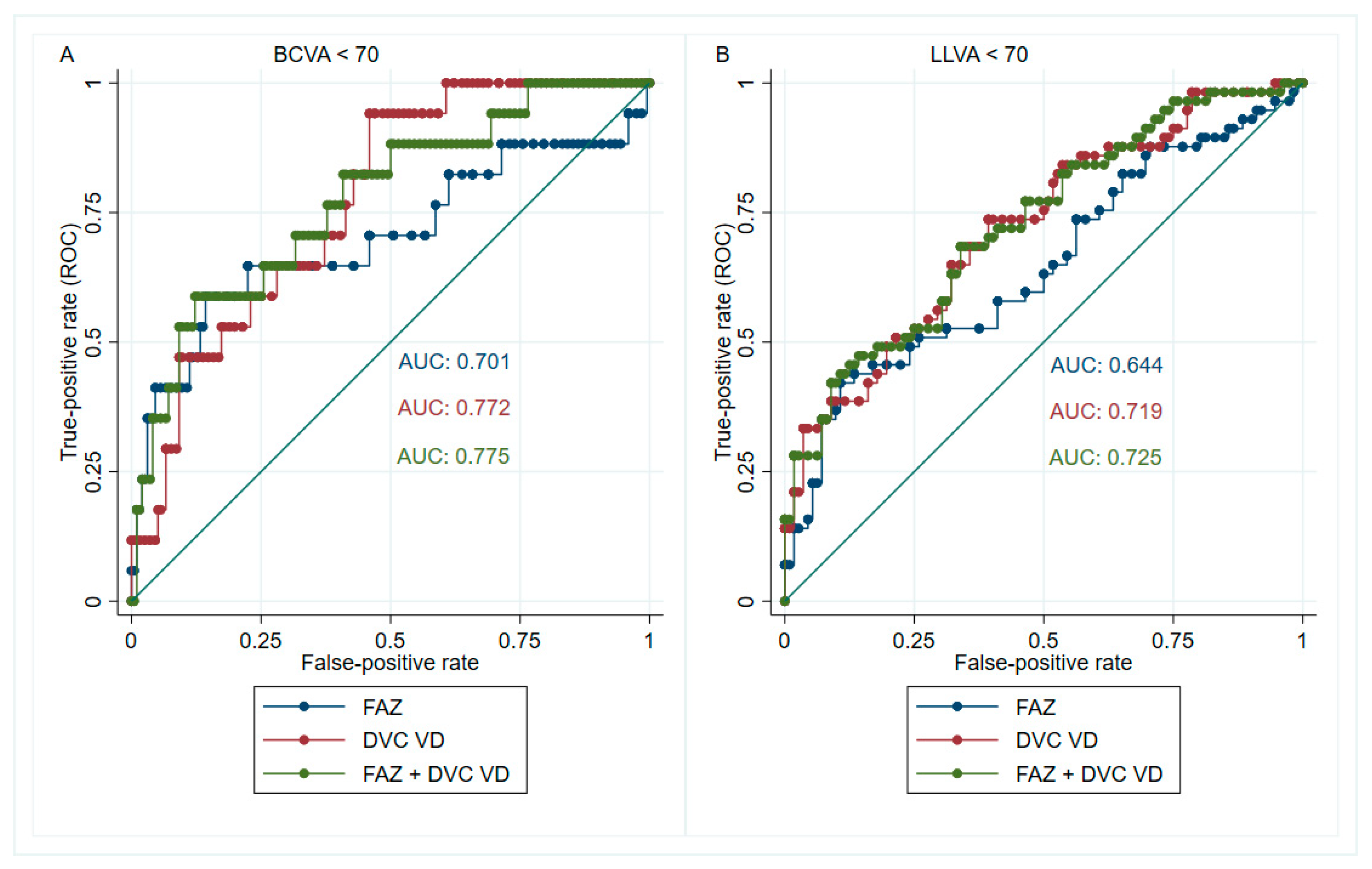
| FAZ area | 0.701 (0.525–0.865) | ≥0.64 mm2 | 0.59 | 0.86 | 0.42 | 0.92 | 4.21 |
| FAZ perimeter | 0.684 (0.516–0.846) | ≥3.49 mm | 0.65 | 0.73 | 0.30 | 0.92 | 2.41 |
| FAZ–AI | 0.630 (0.451–0.785) | ≥1.42 | 0.35 | 0.88 | 0.35 | 0.89 | 2.92 |
| Whole image SVC VD | 0.666 (0.528–0.797) | ≤37.70% | 0.82 | 0.44 | 0.20 | 0.93 | 1.46 |
| Whole image DVC VD | 0.772 (0.657–0.872) | ≤41.9% | 0.94 | 0.54 | 0.26 | 0.98 | 2.04 |
| FD–300 | 0.661 (0.508–0.804) | ≤42.82% | 0.71 | 0.65 | 0.26 | 0.93 | 2.03 |
| For Diagnosing LLVA < 70 ETDRS Letters | |||||||
| Parameters | AUC (95% CI) a | Cutoff Point | Sensitivity | Specificity | PPV | NPV | LR+ |
| FAZ area | 0.644 (0.544–0.744) | ≥0.60 mm2 | 0.42 | 0.89 | 0.80 | 0.60 | 3.82 |
| FAZ perimeter | 0.642 (0.534–0.741) | ≥3.59 mm | 0.44 | 0.88 | 0.78 | 0.60 | 3.67 |
| FAZ–AI | 0.619 (0.514–0.732) | ≥1.33 | 0.42 | 0.80 | 0.69 | 0.58 | 2.10 |
| Whole image SVC VD | 0.676 (0.557–0.770) | ≤35.8% | 0.58 | 0.70 | 0.66 | 0.62 | 1.93 |
| Whole image DVC VD | 0.719 (0.616–0.804) | ≤42.5% | 0.74 | 0.61 | 0.66 | 0.69 | 1.90 |
| FD–300 | 0.601 (0.500–0.700) | ≤42.82% | 0.49 | 0.71 | 0.64 | 0.58 | 1.69 |
| Cutoff | BCVA < 70 (n) | BCVA ≥ 70 (n) | GEE p-value a | Cutoff | LLVA < 70 (n) | LLVA ≥ 70 (n) | GEE p-Value a |
|---|---|---|---|---|---|---|---|
| FAZ area ≥ 0.64 mm2 | 10 | 14 | <0.001 | FAZ area ≥ 0.6 mm2 | 24 | 6 | 0.001 |
| FAZ area < 0.64 mm2 | 7 | 84 | FAZ area < 0.6 mm2 | 33 | 50 | ||
| Perimeter ≥ 3.49 mm | 11 | 26 | 0.002 | Perimeter ≥ 3.59 mm | 25 | 7 | 0.002 |
| Perimeter < 3.49 mm | 6 | 72 | Perimeter < 3.59 mm | 32 | 49 | ||
| FAZ–AI ≥ 1.42 | 6 | 11 | 0.010 | FAZ–AI ≥ 1.33 | 24 | 11 | 0.012 |
| FAZ–AI < 1.42 | 11 | 87 | FAZ–AI < 1.33 | 33 | 45 | ||
| wi SVC VD > 37.7% | 3 | 43 | 0.051 | wi SVC VD > 35.8% | 24 | 39 | 0.003 |
| wi SVC VD ≤ 37.7% | 14 | 55 | wi SVC VD ≤ 35.8% | 33 | 17 | ||
| wi DVC VD > 41.9% | 1 | 53 | 0.004 | wi DVC VD > 42.5% | 15 | 34 | 0.001 |
| wi DVC VD ≤ 41.9% | 16 | 45 | wi DVC VD ≤ 42.5% | 42 | 22 | ||
| FD–300 > 42.82% | 5 | 64 | 0.007 | FD–300 > 42.82% | 29 | 40 | 0.017 |
| FD–300 ≤ 42.82% | 12 | 34 | FD–300 ≤ 42.82% | 28 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, W.-S.; Thottarath, S.; Gurudas, S.; Sen, P.; Pearce, E.; Giani, A.; Chong, V.; Cheung, C.M.G.; Sivaprasad, S. Correlation of Optical Coherence Tomography Angiography Characteristics with Visual Function to Define Vision-Threatening Diabetic Macular Ischemia. Diagnostics 2022, 12, 1050. https://doi.org/10.3390/diagnostics12051050
Tsai W-S, Thottarath S, Gurudas S, Sen P, Pearce E, Giani A, Chong V, Cheung CMG, Sivaprasad S. Correlation of Optical Coherence Tomography Angiography Characteristics with Visual Function to Define Vision-Threatening Diabetic Macular Ischemia. Diagnostics. 2022; 12(5):1050. https://doi.org/10.3390/diagnostics12051050
Chicago/Turabian StyleTsai, Wei-Shan, Sridevi Thottarath, Sarega Gurudas, Piyali Sen, Elizabeth Pearce, Andrea Giani, Victor Chong, Chui Ming Gemmy Cheung, and Sobha Sivaprasad. 2022. "Correlation of Optical Coherence Tomography Angiography Characteristics with Visual Function to Define Vision-Threatening Diabetic Macular Ischemia" Diagnostics 12, no. 5: 1050. https://doi.org/10.3390/diagnostics12051050
APA StyleTsai, W.-S., Thottarath, S., Gurudas, S., Sen, P., Pearce, E., Giani, A., Chong, V., Cheung, C. M. G., & Sivaprasad, S. (2022). Correlation of Optical Coherence Tomography Angiography Characteristics with Visual Function to Define Vision-Threatening Diabetic Macular Ischemia. Diagnostics, 12(5), 1050. https://doi.org/10.3390/diagnostics12051050







