Author Contributions
Conceptualization, M.D.S.; methodology, M.D.S.; validation, V.C., G.V. and L.R.; formal analysis, F.V. and F.I. investigation, M.D.S. and D.G.; resources, M.D.S., F.I. and V.S.; data curation, C.R., G.D.O. and M.C.; outlining the drafts D.G.; writing—original draft preparation, M.D.S.; writing—review and editing, M.D.S., F.I. and M.L.S.; supervision, L.R. All authors have read and agreed to the published version of the manuscript.
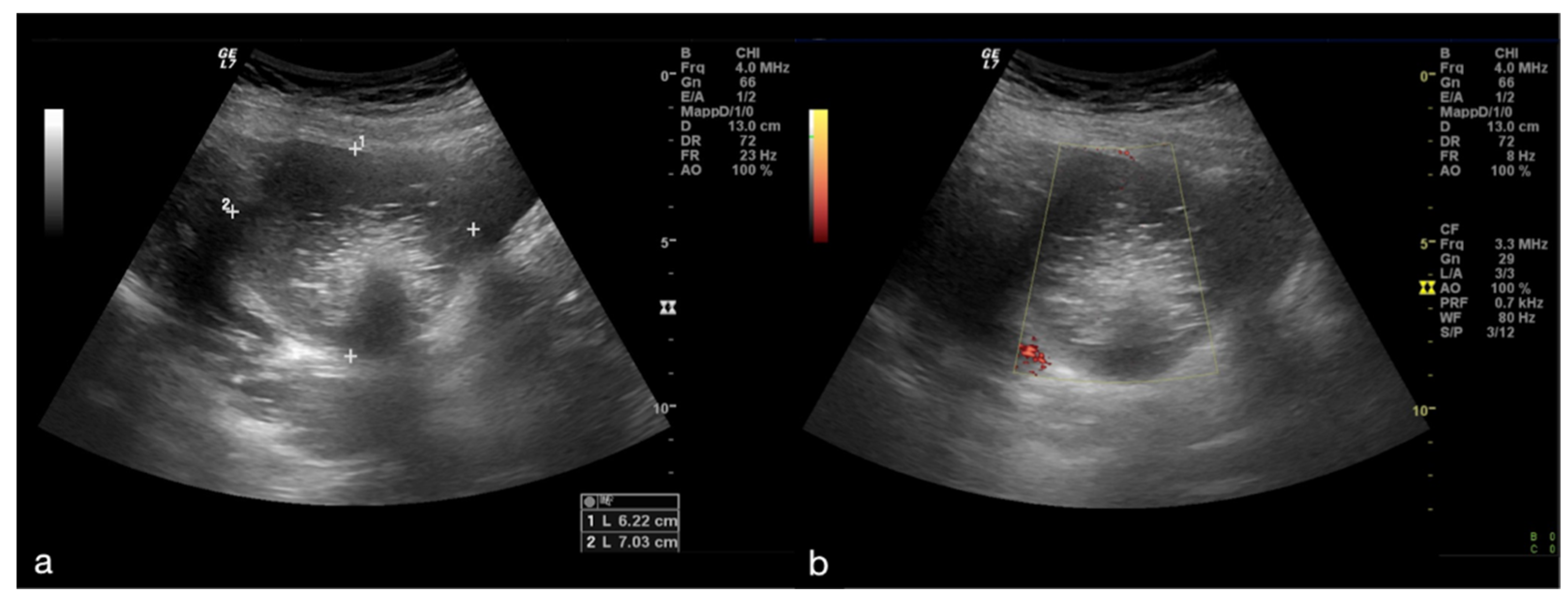
Figure 1.
Adnexal torsion of giant mature cystic right teratoma. Axial TSA-US image (a) shows a mostly echogenic mass (caliper) with some sound attenuation. On power Doppler (b) no adnexal vascularization is detected.
Figure 1.
Adnexal torsion of giant mature cystic right teratoma. Axial TSA-US image (a) shows a mostly echogenic mass (caliper) with some sound attenuation. On power Doppler (b) no adnexal vascularization is detected.
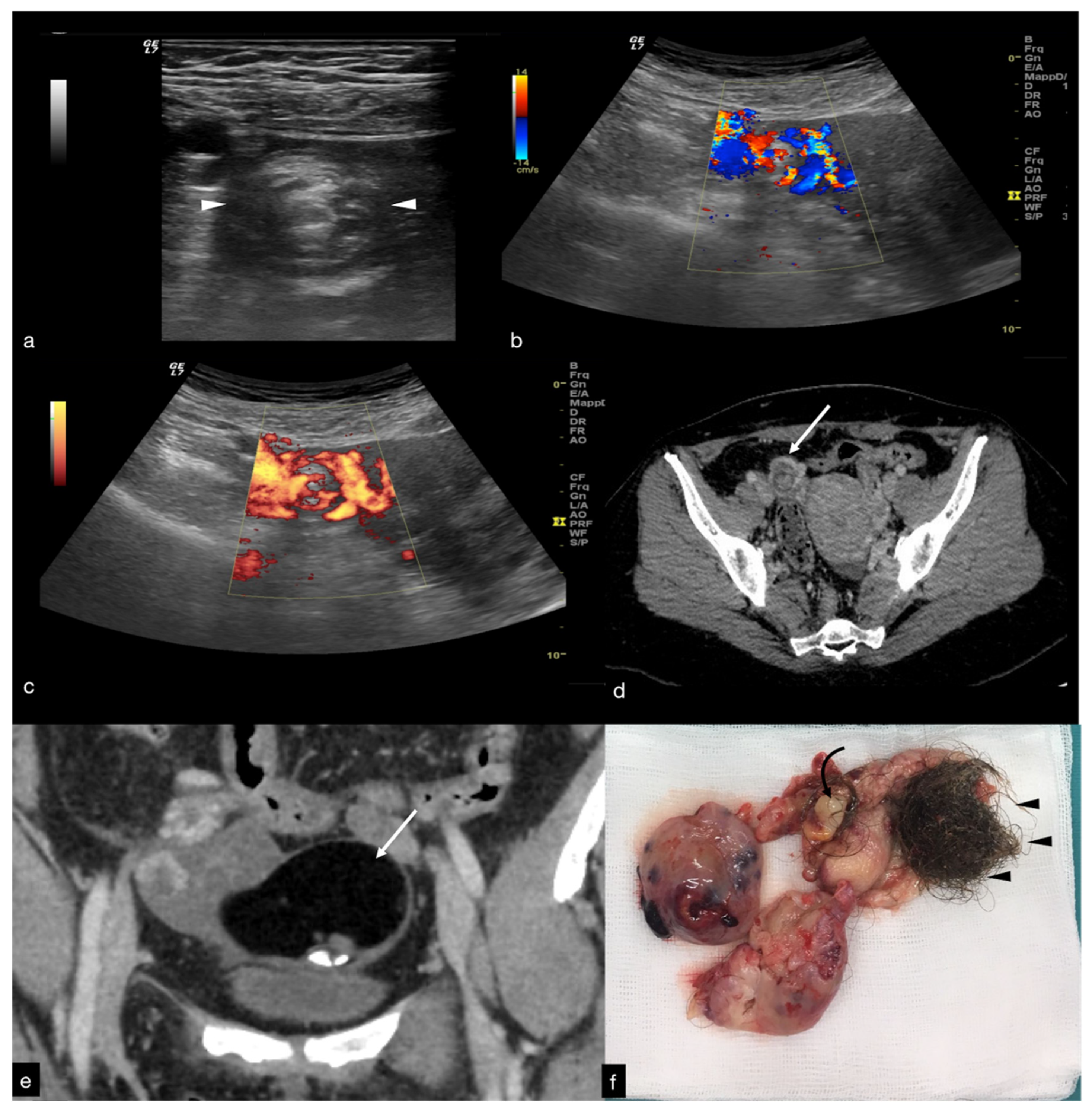
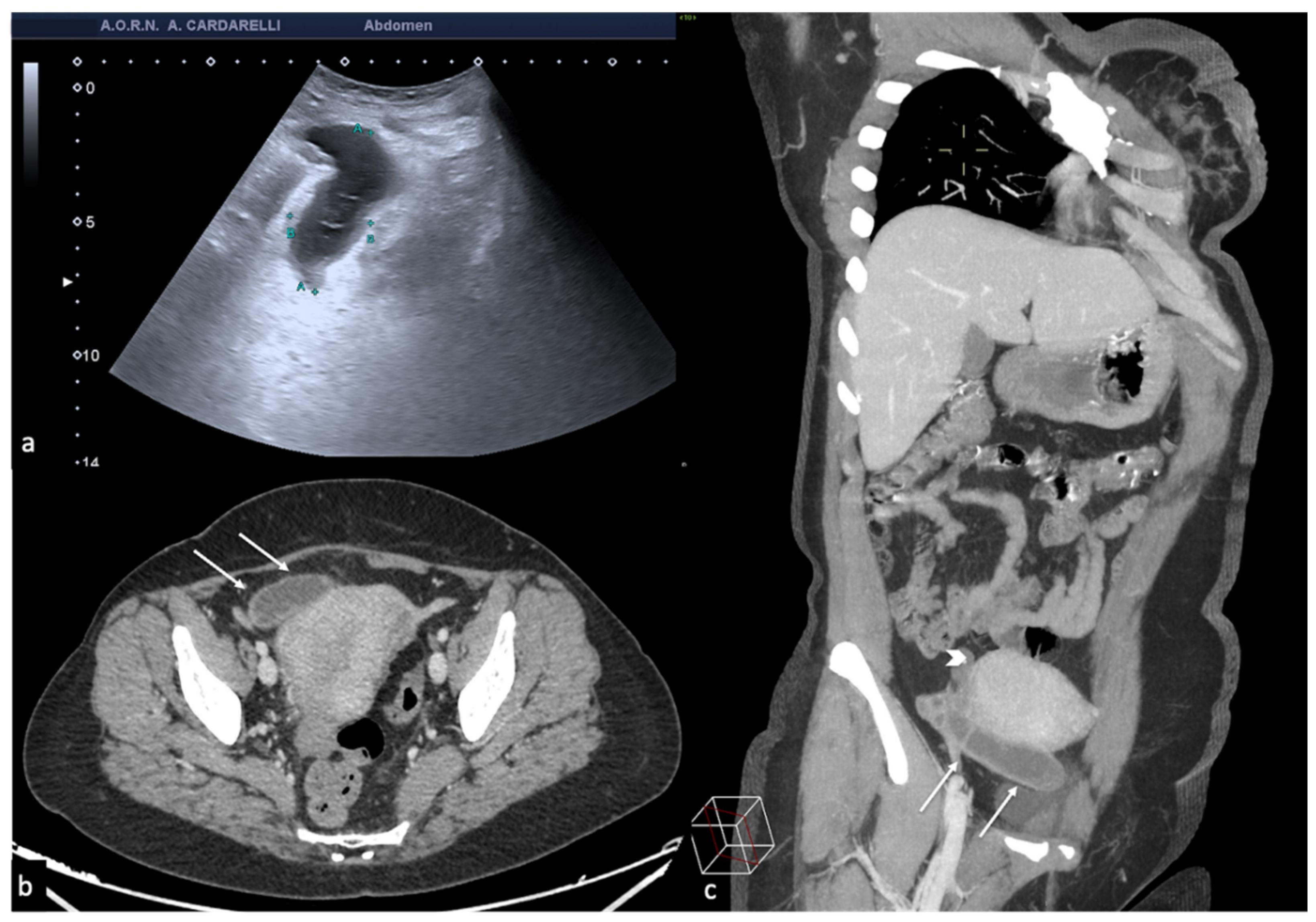
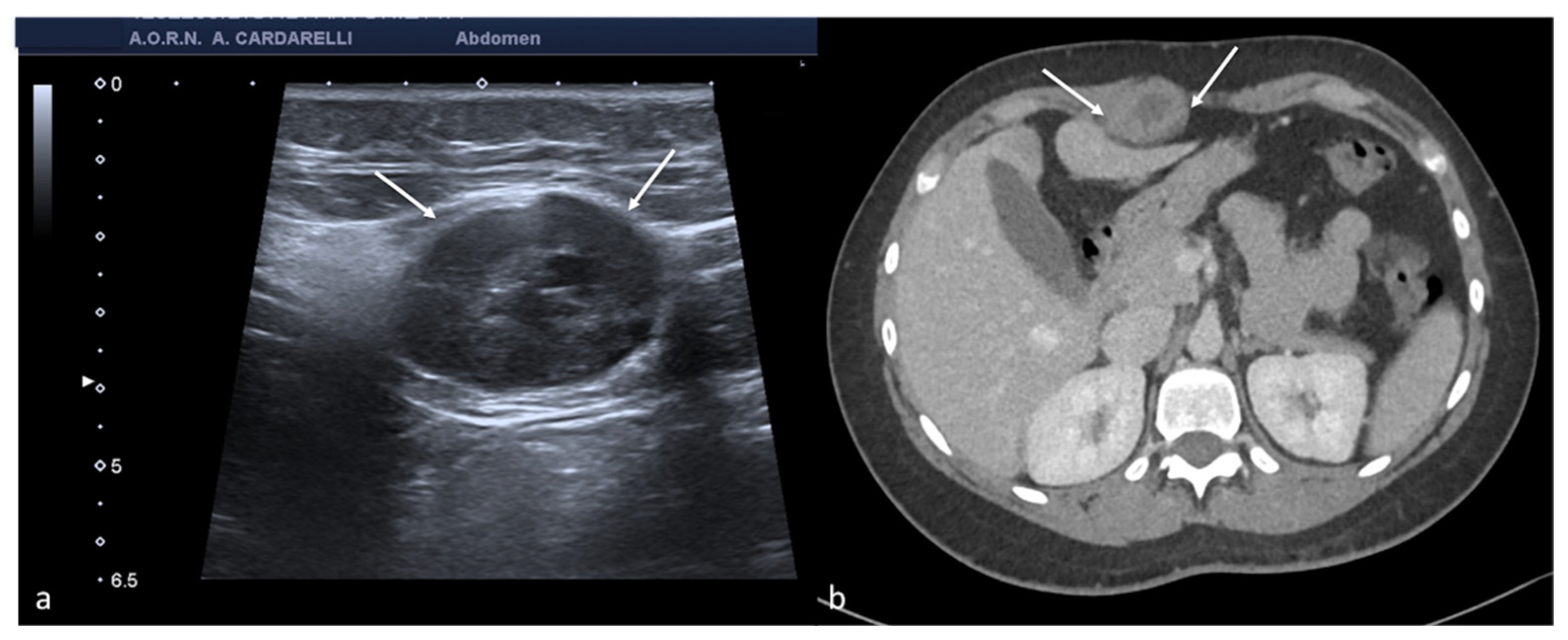
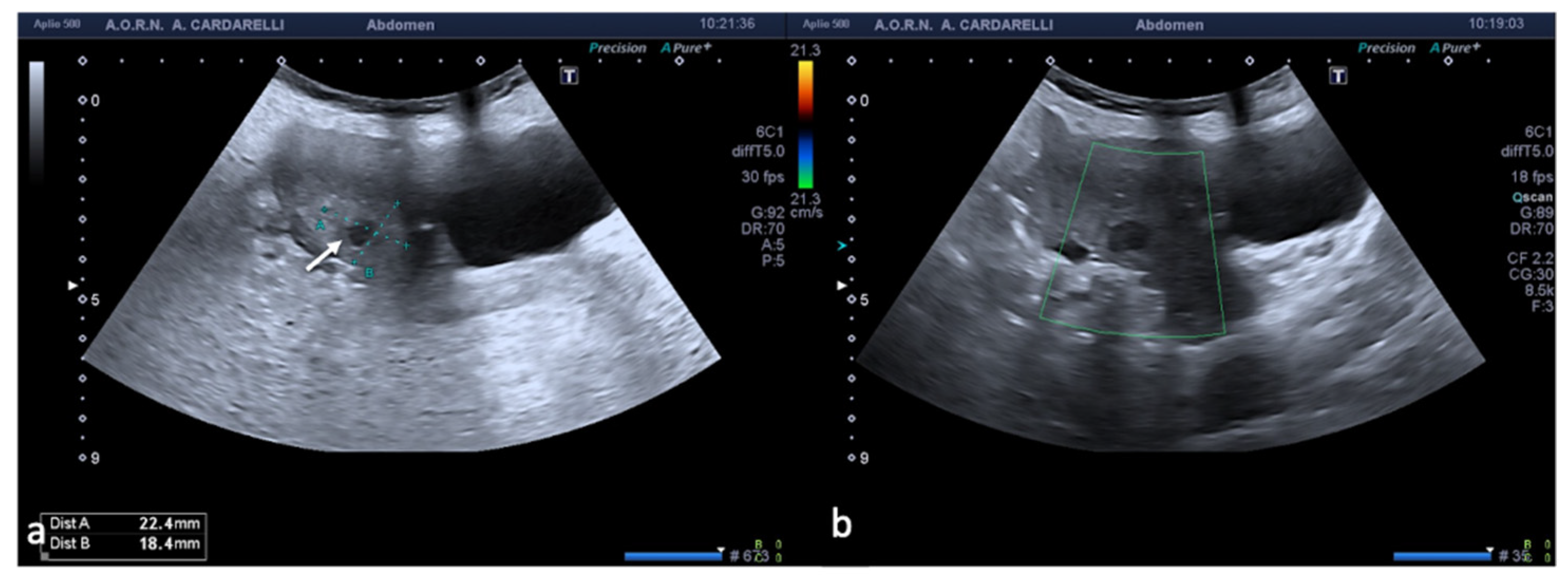
Figure 2.
Same patient of
Figure 1. Axial TSA–US image with high frequency probe (
a) shows a twisted vascular peduncle (“whirl” sign) from the side of the right adnexal mass of above
Figure 1. The twisted adnexal peduncle is better seen at colour-Doppler (
b) and power-Doppler evaluation (
c) and confirmed also at enhanced CT ((
d) arrow), related with the presence of a large mature cystic teratoma ((
e) arrow). At the operative specimen, ovarian torsion is confirmed, and the right ovary with the cystic teratoma with its mixed content of hairs ((
f) arrowheads) and teeth ((
f) curved arrow) is shown. Reprinted with permission from [
2].
Figure 2.
Same patient of
Figure 1. Axial TSA–US image with high frequency probe (
a) shows a twisted vascular peduncle (“whirl” sign) from the side of the right adnexal mass of above
Figure 1. The twisted adnexal peduncle is better seen at colour-Doppler (
b) and power-Doppler evaluation (
c) and confirmed also at enhanced CT ((
d) arrow), related with the presence of a large mature cystic teratoma ((
e) arrow). At the operative specimen, ovarian torsion is confirmed, and the right ovary with the cystic teratoma with its mixed content of hairs ((
f) arrowheads) and teeth ((
f) curved arrow) is shown. Reprinted with permission from [
2].
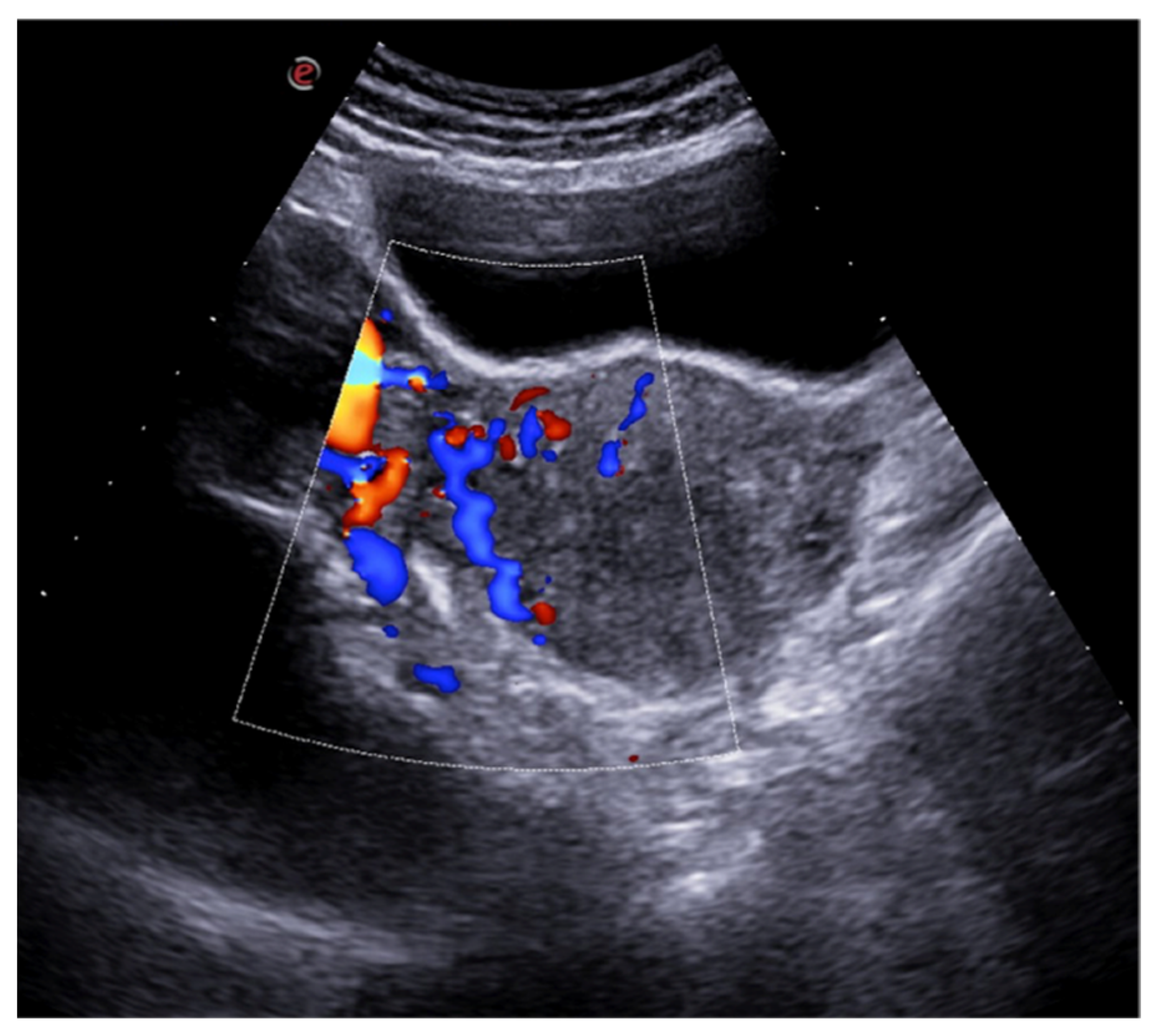
Figure 3.
Haemorrhagic left ovarian cyst. Axial TSA-US image (a) shows a hemorrhagic corpus luteum ((a) caliper) with blood deposits ((a) arrows). On colour-Doppler imaging (b) circumferential blood flow is also shown.
Figure 3.
Haemorrhagic left ovarian cyst. Axial TSA-US image (a) shows a hemorrhagic corpus luteum ((a) caliper) with blood deposits ((a) arrows). On colour-Doppler imaging (b) circumferential blood flow is also shown.
Figure 4.
Haemorrhagic left ovarian cyst. Axial US image (a) shows a hemorrhagic cyst ((a) arrow) with reticular structure. On colour-Doppler imaging (b) there is no blood flow of the reticular structure. At follow-up (c) a complete resorption of the cyst is observed ((c) arrow).
Figure 4.
Haemorrhagic left ovarian cyst. Axial US image (a) shows a hemorrhagic cyst ((a) arrow) with reticular structure. On colour-Doppler imaging (b) there is no blood flow of the reticular structure. At follow-up (c) a complete resorption of the cyst is observed ((c) arrow).
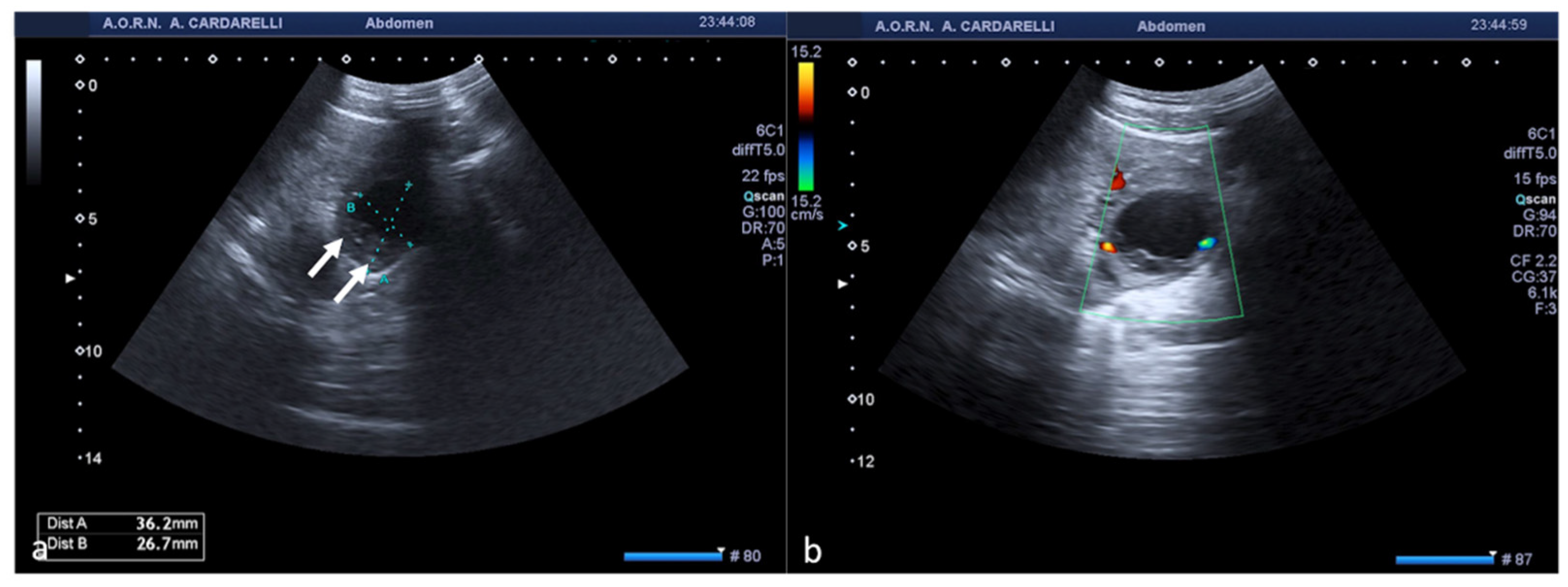
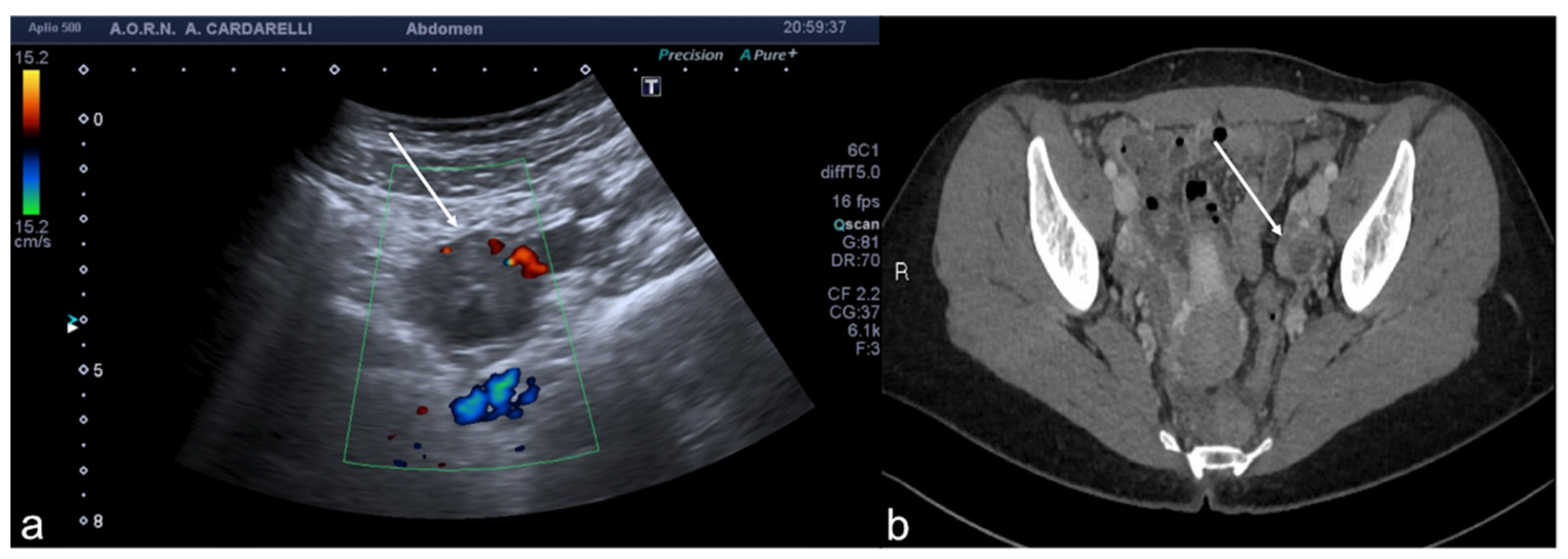
Figure 5.
Pelvic inflammatory disease. US scan of the right adnexa (a) shows an anechoic oblong structure ((a), caliper). Contrast-enhanced axial CT image (b) and maximum intensity projection (MIP) coronal-oblique reconstruction (c) show tortuous and tubular fluid filled structure seen in the right adnexa ((b,c) arrows).
Figure 5.
Pelvic inflammatory disease. US scan of the right adnexa (a) shows an anechoic oblong structure ((a), caliper). Contrast-enhanced axial CT image (b) and maximum intensity projection (MIP) coronal-oblique reconstruction (c) show tortuous and tubular fluid filled structure seen in the right adnexa ((b,c) arrows).
Figure 6.
Tubo-ovarian complex. Axial colour-Doppler TSA–US scan of the left adnexa (a) shows a complex cystic mass with peripheral signal colour Doppler ((a) arrow). Contrast-enhanced axial CT image (b) shows a thick enhancing left adnexa abscess wall ((b) arrows).
Figure 6.
Tubo-ovarian complex. Axial colour-Doppler TSA–US scan of the left adnexa (a) shows a complex cystic mass with peripheral signal colour Doppler ((a) arrow). Contrast-enhanced axial CT image (b) shows a thick enhancing left adnexa abscess wall ((b) arrows).
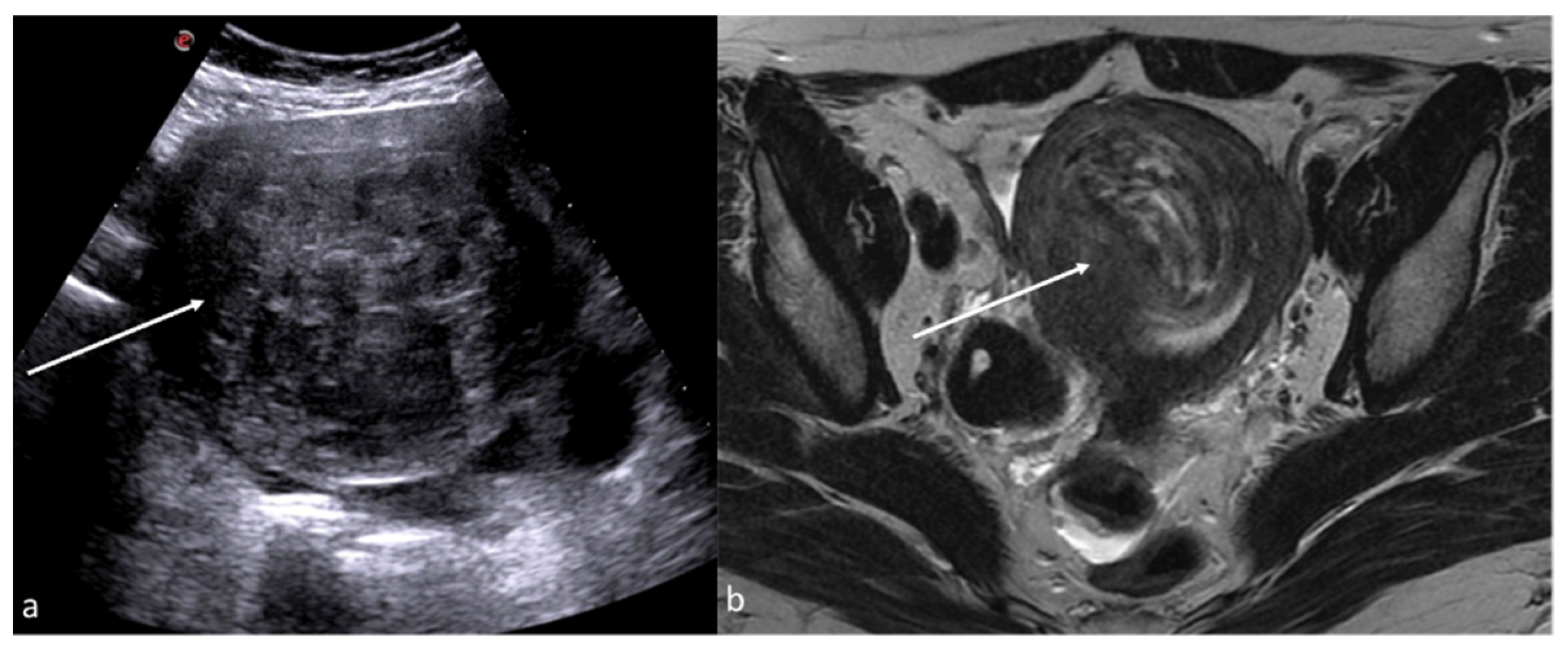
Figure 7.
Pyometra. TSA-US scan of the uterus (a) shows a distended uterine cavity containing complex fluid with echogenic foci (short arrows). Axial T2w MRI imaging (b) confirms the diagnosis showing pus collection into uterine cavity.
Figure 7.
Pyometra. TSA-US scan of the uterus (a) shows a distended uterine cavity containing complex fluid with echogenic foci (short arrows). Axial T2w MRI imaging (b) confirms the diagnosis showing pus collection into uterine cavity.
Figure 8.
Adnexal endometriomas. TSA–US axial scan (a) shows a left adnexal unilocular cyst with acoustic enhancement and diffuse homogeneous ground-glass echoes as a result of the hemorrhagic debris (arrows). On axial unenhanced CT scan, a hypodense mass in the left annex ((b) long arrow). Note the fluid-thick level ((b) short arrow). This latter finding appears clearly visible on T2w MRI ((c) arrow).
Figure 8.
Adnexal endometriomas. TSA–US axial scan (a) shows a left adnexal unilocular cyst with acoustic enhancement and diffuse homogeneous ground-glass echoes as a result of the hemorrhagic debris (arrows). On axial unenhanced CT scan, a hypodense mass in the left annex ((b) long arrow). Note the fluid-thick level ((b) short arrow). This latter finding appears clearly visible on T2w MRI ((c) arrow).
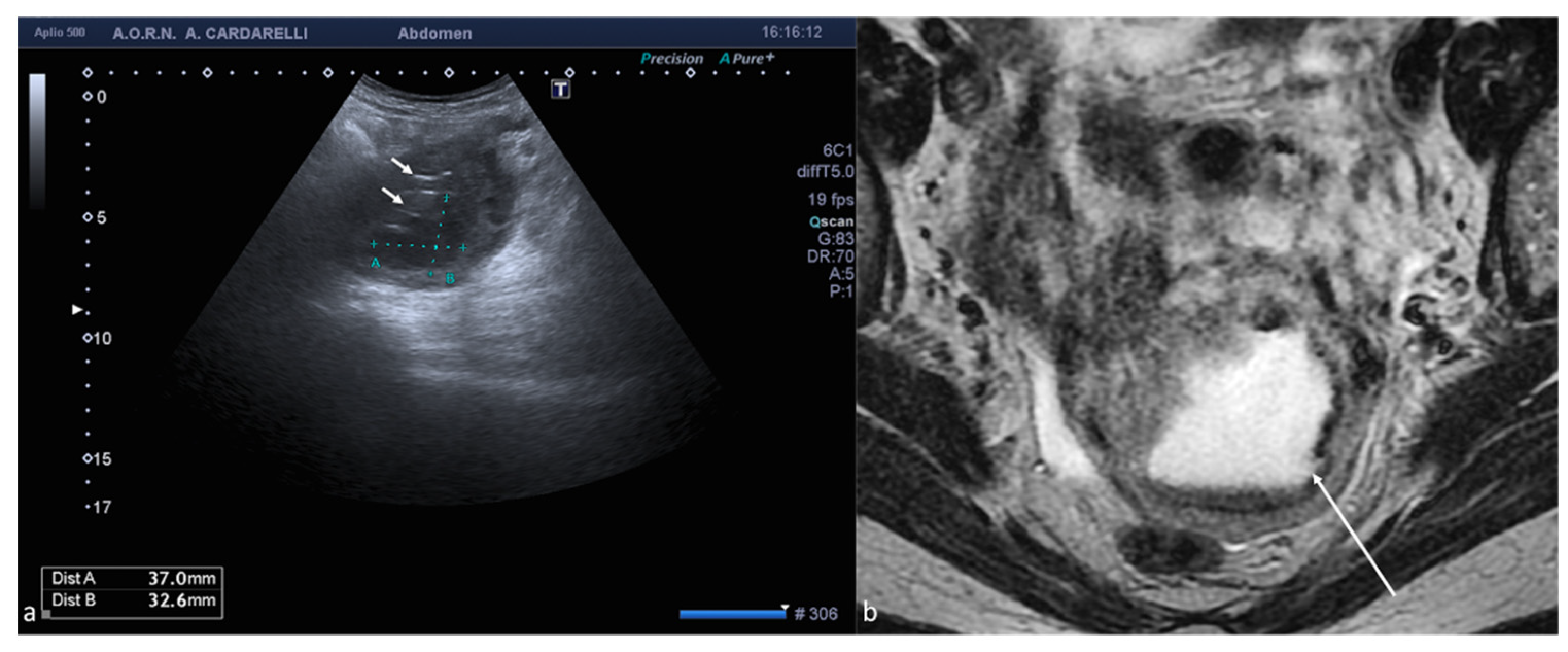
Figure 9.
Abdominal wall endometriosis nodule. TSA–US axial scan (a) of the abdominal wall shows a 35 mm abdominal wall endometriosis nodule with hypoechoic content and well-defined margins ((a), arrows). The nodule is enclosed in the muscular fascia along the right rectal abdomen. Contrast-enhanced axial CT image (b) shows heterogeneous enhanced mass in the right rectus sheath. The mass was subsequently proved to be abdominal endometriosis.
Figure 9.
Abdominal wall endometriosis nodule. TSA–US axial scan (a) of the abdominal wall shows a 35 mm abdominal wall endometriosis nodule with hypoechoic content and well-defined margins ((a), arrows). The nodule is enclosed in the muscular fascia along the right rectal abdomen. Contrast-enhanced axial CT image (b) shows heterogeneous enhanced mass in the right rectus sheath. The mass was subsequently proved to be abdominal endometriosis.
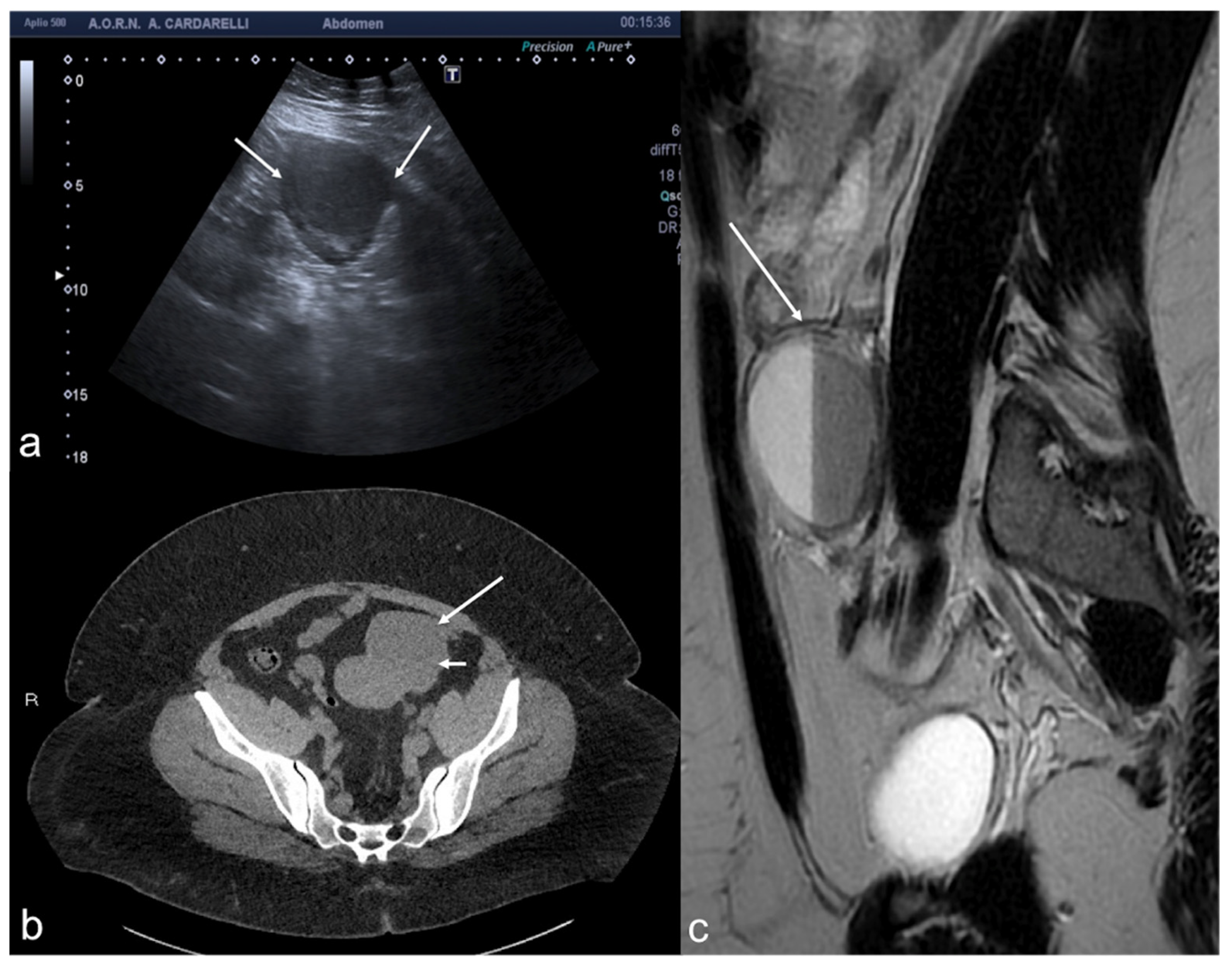
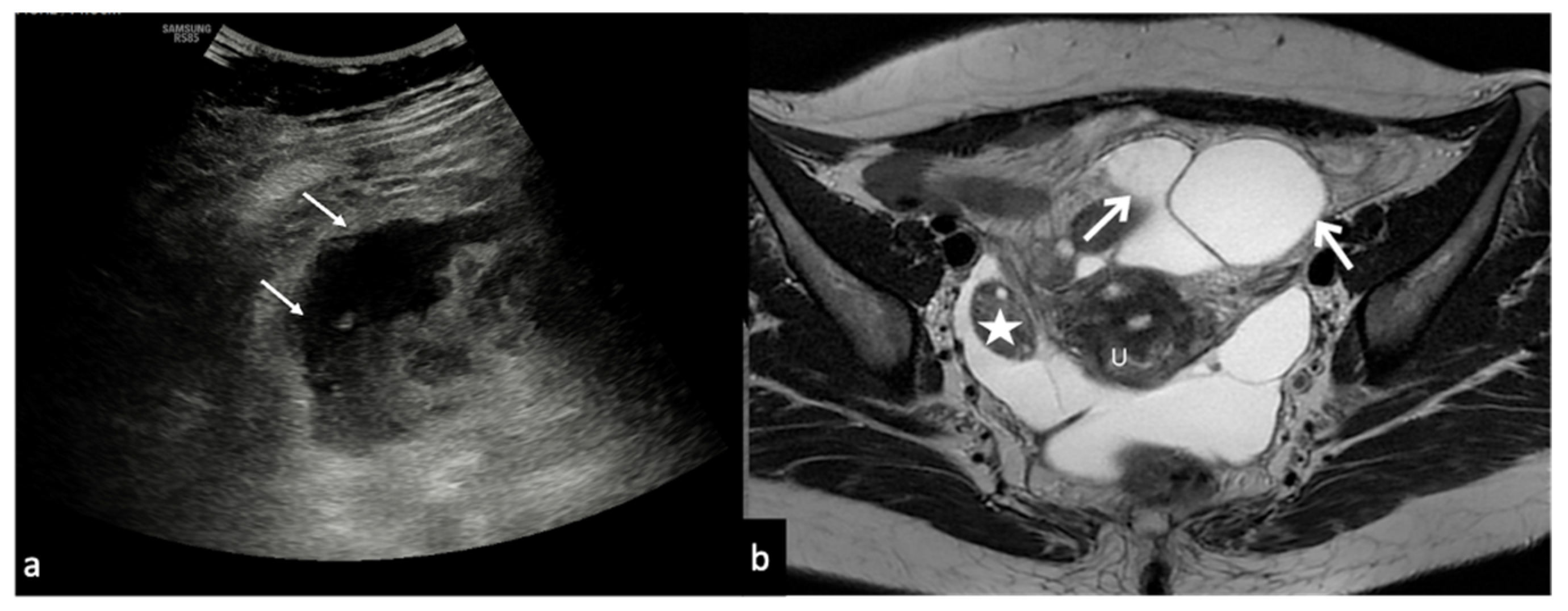
Figure 10.
Peritoneal inclusion cyst. TSA–US axial scan (a) shows an irregular, anechoic para-adnexal right fluid collection ((a) arrows). Axial T2w MRI (b) shows a large, well-defined multilocular cystic pelvic mass (arrows). Note several thin internal septa, radiating from the ovary, representing pelvic adhesions. The septa radiate from the ovary (star; (b)). U: uterus.
Figure 10.
Peritoneal inclusion cyst. TSA–US axial scan (a) shows an irregular, anechoic para-adnexal right fluid collection ((a) arrows). Axial T2w MRI (b) shows a large, well-defined multilocular cystic pelvic mass (arrows). Note several thin internal septa, radiating from the ovary, representing pelvic adhesions. The septa radiate from the ovary (star; (b)). U: uterus.
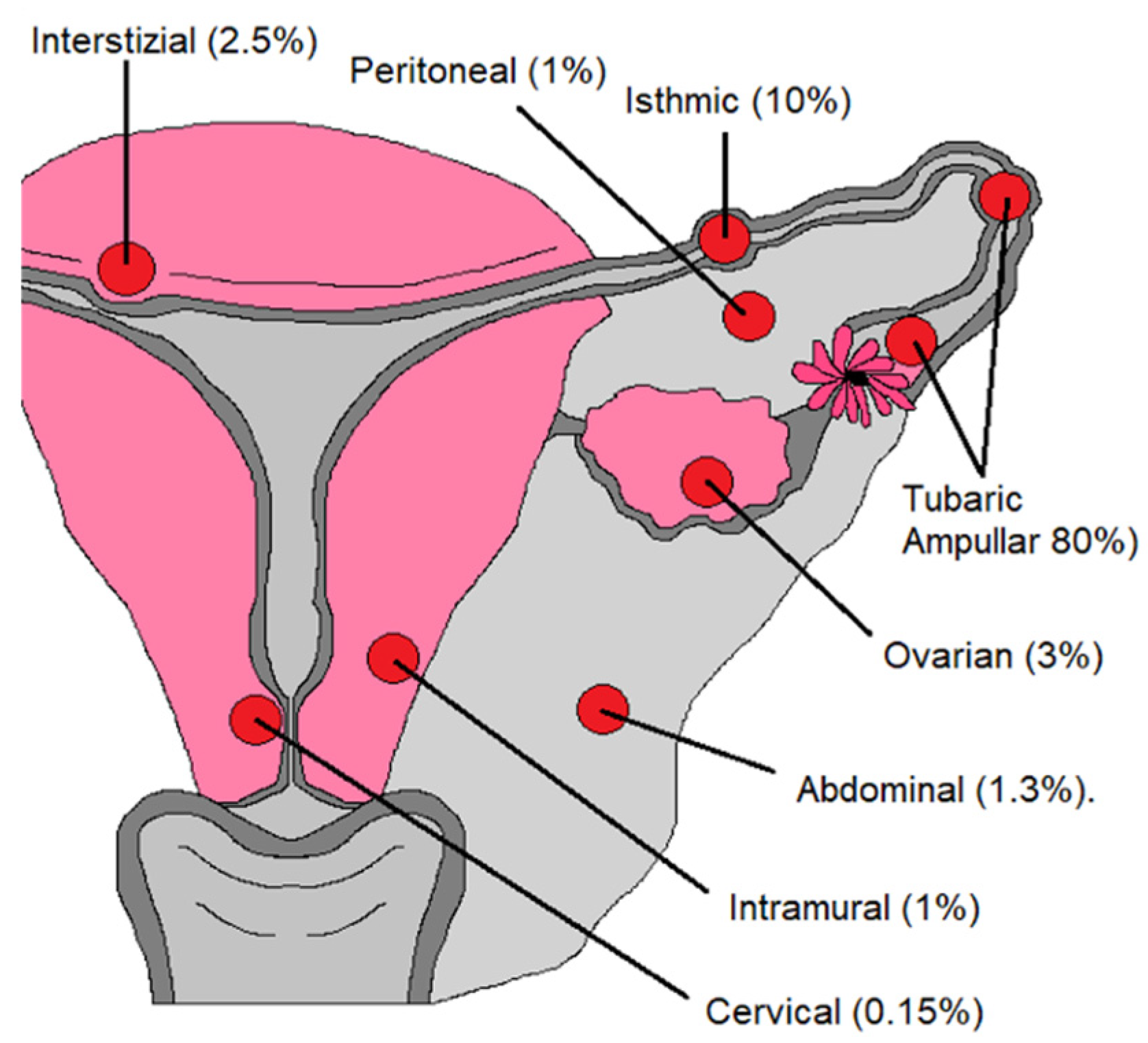
Figure 11.
Abnormal implantation sites for ectopic pregnancy.
Figure 11.
Abnormal implantation sites for ectopic pregnancy.
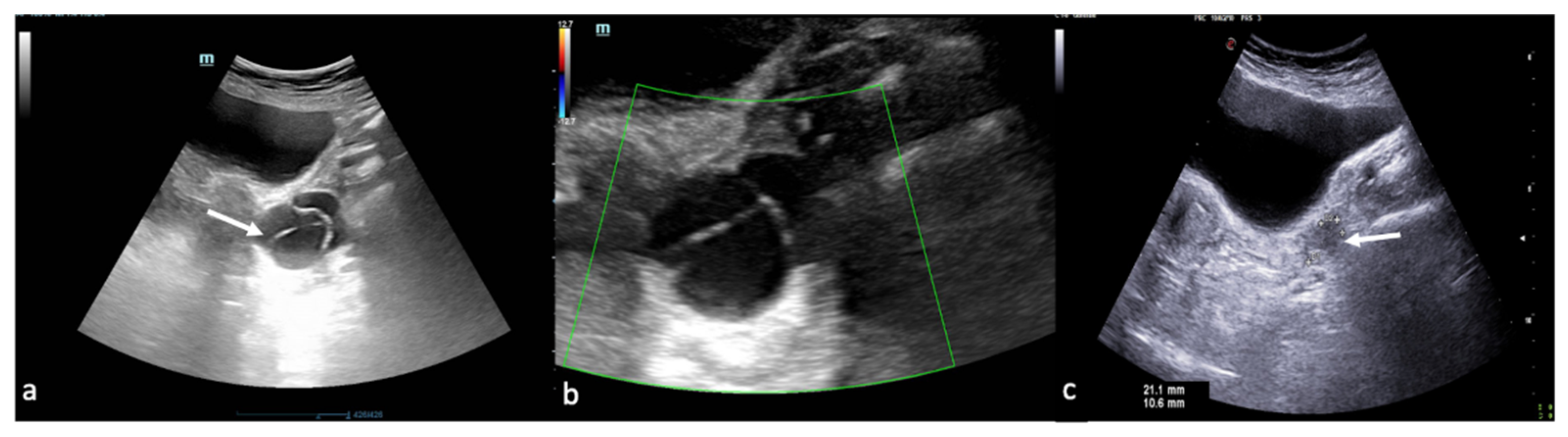
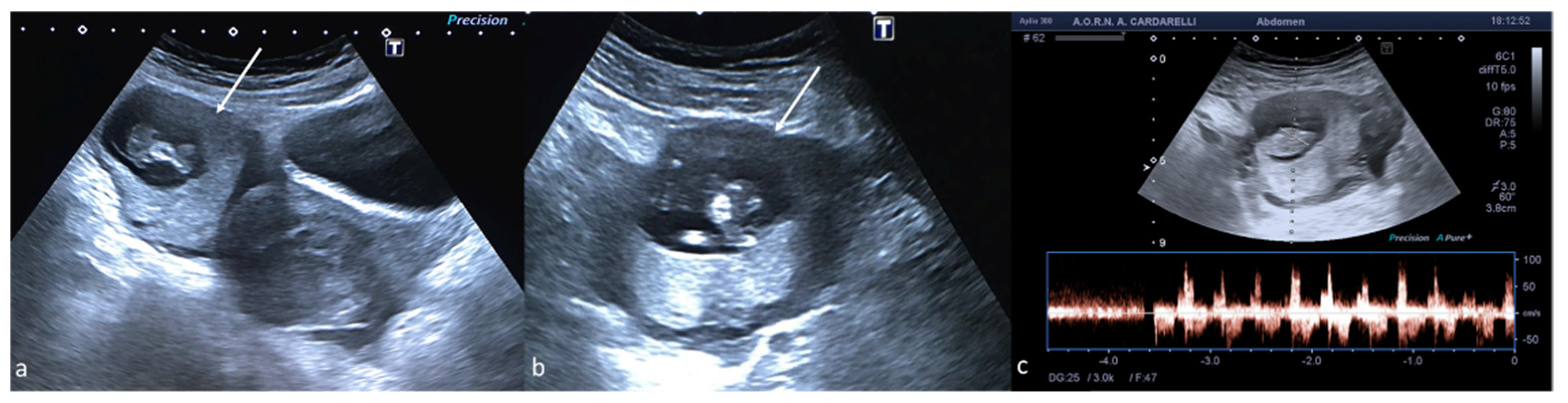
Figure 12.
Ectopic pregnancy. Axial (a) and longitudinal (b) TSA–US scans reveal an extrauterine adnexal gestational sac with a fetal body ((a,b), arrow). On pulsed Doppler (c) a heartbeat is also detected.
Figure 12.
Ectopic pregnancy. Axial (a) and longitudinal (b) TSA–US scans reveal an extrauterine adnexal gestational sac with a fetal body ((a,b), arrow). On pulsed Doppler (c) a heartbeat is also detected.
Figure 13.
Fibroid degeneration. Axial (a) TSA–US scan shows a heterogeneous uterine mass ((a), caliper) with cystic areas inside ((a) arrow). On colour Doppler (b) no vascular signal is detected.
Figure 13.
Fibroid degeneration. Axial (a) TSA–US scan shows a heterogeneous uterine mass ((a), caliper) with cystic areas inside ((a) arrow). On colour Doppler (b) no vascular signal is detected.
Figure 14.
Post-embolization fibroid necrosis. Axial TSA-US (a) scan shows a heterogeneous fibroid ((a) arrow) in necrotic evolution. Axial T2w MRI (b) confirmed the diagnosis (arrow).
Figure 14.
Post-embolization fibroid necrosis. Axial TSA-US (a) scan shows a heterogeneous fibroid ((a) arrow) in necrotic evolution. Axial T2w MRI (b) confirmed the diagnosis (arrow).
Figure 15.
Pelvic congestion syndrome. US axial scan with colour-Doppler mode shows dilated veins in the right adnexa with reversed venous flow after Valsalva maneuver.
Figure 15.
Pelvic congestion syndrome. US axial scan with colour-Doppler mode shows dilated veins in the right adnexa with reversed venous flow after Valsalva maneuver.
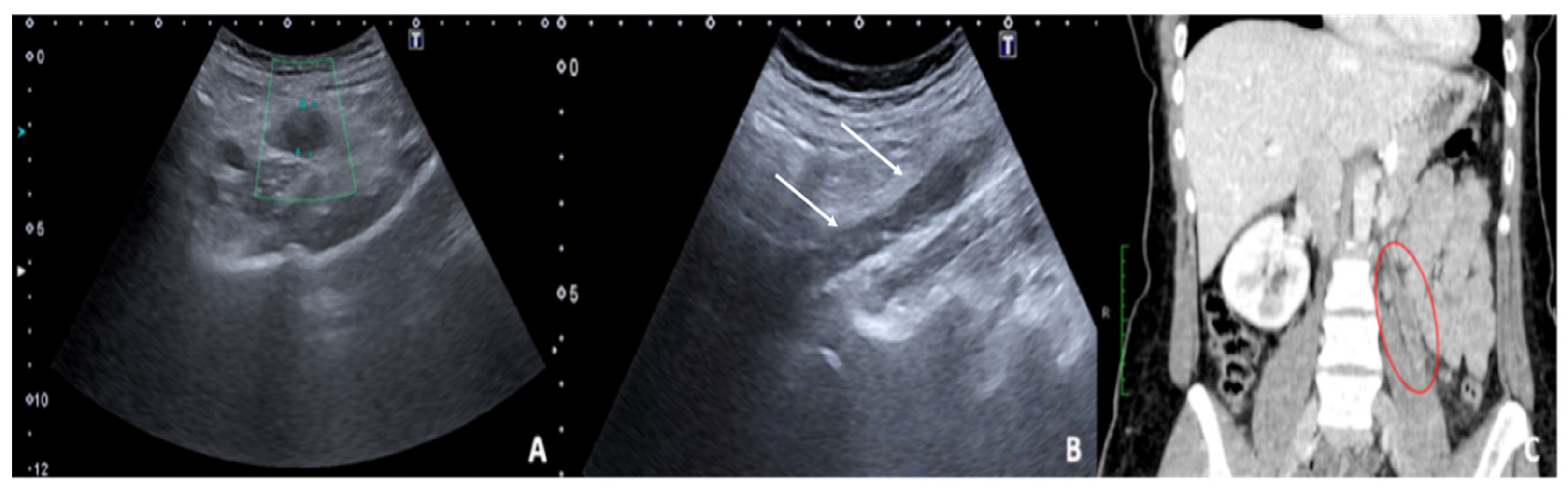
Figure 16.
Thrombosis of the right gonadal vein. TSA–US scan of the left ovarian vein in axial (
A) and longitudinal (
B) view before delivery show the ovarian vein as a tubular structure with heterogeneous hypoechoic echotexture ((
B) arrows), located superiorly to the ovary and anteriorly to the psoas muscle. Contrast-enhanced CT coronal image (
C) was performed after delivery and confirmed the left gonadic vein thrombosis (red circle). Reprinted with permission from [
46].
Figure 16.
Thrombosis of the right gonadal vein. TSA–US scan of the left ovarian vein in axial (
A) and longitudinal (
B) view before delivery show the ovarian vein as a tubular structure with heterogeneous hypoechoic echotexture ((
B) arrows), located superiorly to the ovary and anteriorly to the psoas muscle. Contrast-enhanced CT coronal image (
C) was performed after delivery and confirmed the left gonadic vein thrombosis (red circle). Reprinted with permission from [
46].
Table 1.
TSA–US and TSV–US in comparison.
Table 1.
TSA–US and TSV–US in comparison.
| US Methods | Probe | Protocol | Utility | Limits |
|---|
| Transabdominal sonography (TSA–US) | Low-frequency probe (convex probe 3–5 MHz). | The standard protocol for examining the female pelvis involves an initial TAS with the urinary bladder completely full, so it can act as an acoustic window. Following bladder emptying, the patient assumes a lithotomy position, and TVS is performed. The two imaging techniques are complementary and often provide different diagnostic information.
The protocol often also calls for the execution of Doppler, power Doppler, and pulsed wave Doppler flowmetry depending on the clinical situation and pathology that emerge from grayscale imaging. | TAS offers a wider field of view than TVS and allows better visualisation of the superficial and distal structures of the vagina by bringing the probe closer to the target organs. | |
| Transvaginal sonography (TVS–US) | High-frequency probe (endocavitary probe > 7 MHz). | TVS approach requires a greater penetration depth to avoid the attenuating soft tissues that cover the pelvic organs. Therefore, it requires the use of a higher frequency probe, which, in turn, provides greater resolution of the anatomical details of the uterus, ovary, and adnexal structures. | Limited field of view. Should not be performed on patients who are unable or unwilling to consent to the procedure, as well as on most virgin patients and for those in which the insertion of the probe produces marked discomfort. It is contraindicated in some obstetric patients in the 2nd and 3rd trimester of pregnancy due to the risk of active bleeding or membrane rupture.
|
Table 2.
Pelvic pain in reproductive age: causes, symptoms, and occurrence.
Table 2.
Pelvic pain in reproductive age: causes, symptoms, and occurrence.
| Causes of Pelvic Pain | Occurrence | Pain Characteristics |
|---|
| • Adnexal |
| Adnexal torsion | 3% of gynecologic emergencies | Acute persistent (complete torsion) or intermittent (intermittent torsion) right/left pelvic pain |
| Ruptured or bleeding ovarian cysts | The incidence is difficult to estimate. A broader estimate calculates about 7% of women worldwide experience a symptomatic cyst during their lifetime | Acute right/left pelvic pain |
| Pelvic inflammatory disease | No specific international data are available for PID incidence worldwide. A study reports a prevalence of self-reported lifetime PID of 4.4% | Chronic pelvic pain with reacutization |
| Endometritis | Pregnancy-related endometritis with an incidence of 1–3% after a vaginal delivery and of 13–90% following cesarean delivery, and endometritis unrelated to pregnancy that may occur in up to 70–90% of documented cases of PID | Chronic pelvic pain with reacutization |
| Endometriosis | It affects up to 10% of women of reproductive age | Asymptomatic/poorly symptomatic/chronic pelvic pain with reacutization during menses |
| Peritoneal inclusion cysts | Approximately 3–5% occur in women of childbearing age following invasive pelvic surgery, infection, or cancer | Asymptomatic/poorly symptomatic/chronic pelvic pain with reacutization (especially if complicated) |
| Ectopic pregnancy | 1–2% of all pregnancies | Acute pelvic pain |
| • Uterine |
| Fibroids: degeneration, rupture, and torsion | A study examining the incidence of degeneration of leiomyoma in patients referred for uterine fibroid embolisation underwent MRI found an incidence of 5.1%.
Torsion and rupture are a rare entity (reported incidence for torsion of less than 0.25%) | Acute pelvic pain |
| Post-embolisation syndrome | Occurs in about 40% of women undergoing uterine artery embolisation | Pelvic pain of variable entity |
| • Vascular |
| Pelvic congestion syndrome | In patients with presenting complaints of chronic pelvic pain, the prevalence of PCS is nearly 30% | Chronic pelvic pain |
| Thrombosis of the gonadal veins | Referred incidence of about 0.18% of the general population | Acute pelvic pain of variable entity |
Table 3.
Adnexal torsion US diagnostic clue.
Table 3.
Adnexal torsion US diagnostic clue.
| Adnexal Causes of Pelvic Pain | US Diagnostic Clue | US Limits |
|---|
| Adnexal torsion | Twisted vascular peduncle (whirl sign) with absent flows or with increase in resistance indices if represented | Transabdominal US may be limited in obese patients or when the ovaries are masked by intestinal meteorism.
Endovaginal US may be limited in cases of large ovarian masses causing cranial displacement of the ovary, hindering the exploration of the ovarian vessels. |
Table 4.
Ruptured or bleeding ovarian cysts US diagnostic clue.
Table 4.
Ruptured or bleeding ovarian cysts US diagnostic clue.
| Adnexal Causes of Pelvic Pain | US Diagnostic Clue | US Limits |
|---|
| Ruptured or bleeding ovarian cysts | Cystic mass with an inhomogeneous echo structure in relation to hemoglobin degradation often with evidence of haematic sediment or in an advanced phase with relief of thin internal echoes arranged in a “fishing net” or fibrin bundles not vascularized by colour-Doppler or CEUS (differential diagnosis with tumor mass). | US cannot detect and quantify the active bleeding |
Table 5.
Inflammatory disease US diagnostic clue.
Table 5.
Inflammatory disease US diagnostic clue.
| Adnexal Causes of Pelvic Pain | US Diagnostic Clue | US Limits |
|---|
| Pelvic inflammatory disease | The fallopian tubes with thickened and hyperemic walls, dilated in the presence of pyosalpinx with occluded ovarian fimbria, echogenic intraluminal sediments, and echoes stratified by exudate. The inflamed fallopian tube appears adjacent to or adhering to the ovary with the formation, in more advanced cases, of an ovarian tube abscess represented on US by an inflammatory mass that engulfs the ovary and the fallopian tube, no longer making the ovary distinguishable. | US may suffer from limited panoramicity and, in cases of extensive adhesions, can be difficult to discriminate each anatomical structure. |
Table 6.
Endometritis US diagnostic clue.
Table 6.
Endometritis US diagnostic clue.
| Adnexal Causes of Pelvic Pain | US Diagnostic Clue | US Limits |
|---|
| Endometritis | Thickened endometrium with an irregular profile and the presence of more or less echogenic fluid or pus in the uterine cavity (pyometra) | At US, may be difficult to distinguish severe endometritis from cancer |
Table 7.
Endometriosis US diagnostic clue.
Table 7.
Endometriosis US diagnostic clue.
| Adnexal Causes of Pelvic Pain | US Diagnostic Clue | US Limits |
|---|
| Endometriosis | Unilocular swellings, which are often bilateral and multiple, with a thick capsule, regular margins, and homogeneously echogenic content, with fine internal echoes, due to the blood cells flaking off the walls, resulting in a “ground glass” appearance. Useful monitoring for differential diagnosis with hemorrhagic ovarian cyst (persistence to follow-up of the endometriotic cyst addresses the diagnosis) | At US, may be difficult to detect millimetric foci of ovarian endometriosis and to detect retrocervical or ligaments thickening, as well as possible intestinal or nerve involvement. |
Table 8.
Peritoneal inclusion cyst US diagnostic clue.
Table 8.
Peritoneal inclusion cyst US diagnostic clue.
| Adnexal Causes of Pelvic Pain | US Diagnostic Clue | US Limits |
|---|
| Peritoneal inclusion cyst | Trapped ovary inside a cyst, surrounded by septa and fluid. The fluid is usually anechoic but may contain echoes in some compartments due to haemorrhage or protein-rich fluid | At US, can be difficult to differentiate the cystic origin. |
Table 9.
Ectopic pregnancy US diagnostic clue.
Table 9.
Ectopic pregnancy US diagnostic clue.
| Adnexal Causes of Pelvic Pain | US Diagnostic Clue | US Limits |
|---|
| Ectopic pregnancy | When the b-hCG value is below the cut-off value of 2000 mIU/mL (IRP) and there is no intrauterine gestational sac, the diagnosis could be an early intrauterine pregnancy, a miscarriage, or an ectopic pregnancy, and therefore follow-up is indicatedUterine findings: gestational pseudosac (differential diagnosis with gestational sac: double echogenic wall versus single echogenic wall of the pseudosac). Adnexal findings: echogenic tubal ring or ectopic gestational sac containing the yolk sac or the embryo (with or without cardiac activity)
| Related with difficulties in exploring the adnexa and in detecting early pregnancy as well as active bleeding in case of rupture |
Table 10.
Fibroid torsion US diagnostic clue.
Table 10.
Fibroid torsion US diagnostic clue.
| Uterine Causes of Pelvic Pain | US Diagnostic Clue | US Limits |
|---|
| Fibroids torsion | Hypovascular or avascular mass distinct from the ovary and with a twisted or “pointed” peduncle | Difficulties in exploring the whole uterus in cases of multiple fibromas and in detecting the twisted pedicle |
Table 11.
Post-embolisation syndrome US diagnostic clue.
Table 11.
Post-embolisation syndrome US diagnostic clue.
| Uterine Causes of Pelvic Pain | US Diagnostic Clue | US Limits |
|---|
| Post-embolisation syndrome | Fibroid with internal echoes and reverberation artifacts in the context of a poorly defined mass following infarction and tissue necrosis | Difficulties in exploring the whole uterus in cases of multiple fibromas |
Table 12.
Pelvic congestion syndrome US diagnostic clue.
Table 12.
Pelvic congestion syndrome US diagnostic clue.
| Vascular Causes of Pelvic Pain | US Diagnostic Clue | US Limits |
|---|
| Pelvic congestion syndrome | Multiple pelvic veins with a diameter greater than 5 mm and a venous reflux greater than 1 s | Possible difficulties in sampling the vessel and correctly evaluate the blood flow, and in detecting eventual complications such as thrombosis. |
Table 13.
Thrombosis of the gonadal veins US diagnostic clue.
Table 13.
Thrombosis of the gonadal veins US diagnostic clue.
| Vascular Causes of Pelvic Pain | US Diagnostic Clue | US Limits |
|---|
| Thrombosis of the gonadal veins | Avascular structure with a tortuous tubular appearance, with adjacent anechoic or hypoechoic areas without any flow detection on the colour Doppler evaluation. | Possible limits in panoramicity that hinder the detection of thrombosed vessels. Difficulties in Doppler evaluations. |